Abstract
6-β-Naltrexol is the major active metabolite of naltrexone, NTX, a potent μ-opioid receptor antagonist used in the treatment of alcohol dependence and opioid abuse. Compared to naloxone, NTX has a longer duration of action largely attributed to 6-β-naltrexol. This study was carried out in order to determine percutaneous absorption of a transdermal codrug of naltrexol, 6-β-naltrexol-hydroxybupropion codrug (CB-NTXOL-BUPOH), in hairless guinea pigs as well as to evaluate the safety of 6-β-naltrexol for development as a transdermal dosage form. This codrug may be useful in the simultaneous treatment of alcohol dependence and tobacco addiction. The carbonate codrug traversed the skin at a faster rate than 6-β-naltrexol. 6-β-naltrexol equivalent steady state plasma concentrations of 6.4 ng/ml were obtained after application of the codrug as compared to 1.2 ng/ml from 6-β-naltrexol base. The steady state plasma concentration of hydroxybupropion after codrug application was 6.9 ng/ml. Skin sensitization and irritation tested in the hairless guinea pigs using the Buehler method revealed that 6-β-naltrexol had no skin sensitizing potential. The method was validated with a known sensitizer, p-phenylenediamine, which induced sensitization in 90% of the animals. 6-β-Naltrexol caused only mild transient skin irritation after the initial application of the patch. During subsequent applications, erythema was slightly increased but no skin damage was observed. In conclusion, a transdermal codrug of 6-β-naltrexol could be a viable alternative treatment for alcohol and opiate abuse.
Keywords: transdermal, codrug, hairless guinea pig, 6-β-naltrexol, bioconversion, sensitization
1. Introduction
The therapeutic efficacy of a drug for transdermal delivery, following its application on the skin, mainly depends on its ability to penetrate the skin fast enough to provide the required plasma concentrations for eliciting the desired pharmacological activity (Bonina et al., 2001). One of the strategies used to enhance skin permeation of poorly permeable drugs is the codrug approach (Kiptoo et al., 2006). A codrug or a mutual prodrug is a chemically modified therapeutic agent, which consists of two synergistic drugs covalently linked together in order to improve the drug delivery properties of one or both drugs. The main advantage of using a transdermal codrug that can undergo bioconversion to the active parent drugs is that the skin irritation and allergenic potential of a codrug should only mirror the profile of the active parent drugs, without the added toxicities of penetration enhancers or active transport devices. Because the skin and plasma have an abundance of esterase enzymes, codrugs with esterase-susceptible linkages can be cleaved by these enzymes to release the active parent drugs in tissue and plasma.
Naltrexone, NTX, a potent μ-opioid receptor antagonist is used in the treatment of alcohol dependence as well as to help maintain opioid addicts in a drug-free state (McCaul et al., 2000; Volpicelli et al., 1992). Compared with naloxone, NTX has a longer duration of action, allowing for once daily dosing. This long duration of action is largely considered to be due, in part, to its major metabolite, 6-β-naltrexol (Verebey et al., 1976; Volpicelli, 1995). In humans, NTX undergoes extensive first-pass metabolism with an oral bioavailability of 5-40% and the primary metabolite, 6-β-naltrexol, is formed via reduction of the 6-keto group in NTX. 6-β-Naltrexol has a longer half-life of about 12 h, as compared with the 4 h half-life for NTX (Meyer et al., 1984). Data from a study of alcoholics showed a significant negative correlation between high plasma levels of 6-β-naltrexol and a lower reported number of drinks per month (McCaul et al., 2000). 6-β-Naltrexol is expected to have a more predictable biotransformation profile and may be useful in providing a more precise control over dosing compared to the variability experienced with NTX oral dosing (Ferrari et al., 1998; McCaul et al., 2000; Verebey et al., 1976). Also, 6-β-naltrexol may be better tolerated than NTX in recovering opioid addicts, due to its neutral antagonist profile and potential decreased withdrawal effects (Raehal et al., 2005).
Transdermal delivery of 6-β-naltrexol via a codrug strategy could provide controlled drug release rates, avoid the first-pass effect in hepato-compromised alcoholics, improve patient compliance (Rothenberg et al., 2002; Volpicelli et al., 1997), and reduce gastrointestinal side-effects associated with oral therapy (Kranzler et al., 2000). This unique concept of a codrug or mutual prodrug has been utilized to improve the delivery of several other drug combinations (Cynkowska et al., 2005; Faull et al., 1995; Otagiri et al., 1999).
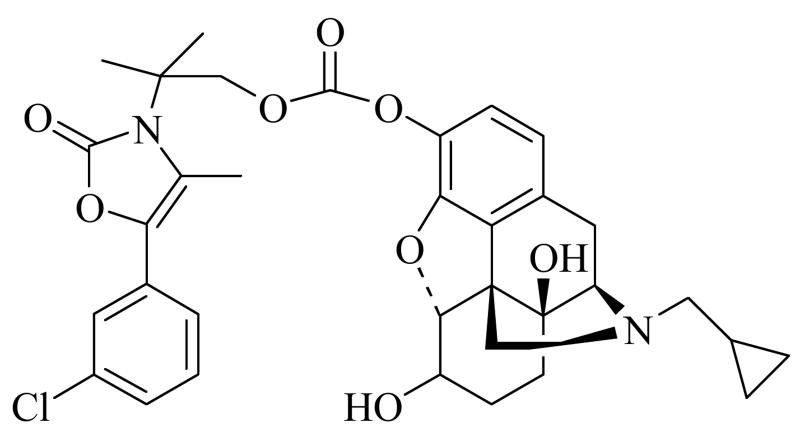
Simultaneous treatment of alcohol dependence and tobacco addiction would be beneficial because of the high prevalence of cigarette and alcohol co-abuse (Frosch et al., 2000). Bupropion is an aminoketone used as an antidepressant and non-nicotine aid to smoking cessation (Johnston et al., 2001). It is extensively metabolized in vivo, and less than 10% of a bupropion dose is excreted unchanged (Schroeder, 1983). In humans, the pharmacological activity of bupropion is likely due to substantial contributions from its major active metabolite, hydroxybupropion (Belson and Kelley, 2002; Schroeder, 1983). Both bupropion and hydroxybupropion have excellent physicochemical properties that would allow for transdermal delivery, and conjugation of either of these drugs to the phenolic hydroxy group of 6-β-naltrexol via a covalent linkage would improve the skin permeability characteristics of 6-β-naltrexol. However, attempts to conjugate bupropion with 6-β-naltrexol afforded mixed reaction products which were either extremely difficult to isolate or were chemically too unstable (Hamad et al., 2006). Previously, a novel codrug consisting of 6-β-naltrexol linked to hydroxybupropion via a carbonate ester linkage, CB-NTXOL-BUPOH (Fig. 1), was synthesized (Hamad et al., 2006) and successfully tested using human skin in vitro (Kiptoo et al., 2006). The goal of this current study was twofold. First, in vivo studies were carried out in a hairless guinea pig model to determine the percutaneous absorption of a transdermal codrug of 6-β-naltrexol. Second, skin irritation and sensitization studies in guinea pigs were conducted in order to evaluate the safety of 6-β-naltrexol as a transdermal dosage form. Preclinical skin irritation and sensitization studies in animals serve the purpose of limiting risks whenever a new active substance is to be used as a clinical product.
Figure 1.
Chemical structure of the carbonate codrug, CB-NTXOL-BUPOH, consisting of 6-β-naltrexol covalently linked to structurally modified form of hydroxybupropion, via a carbonate ester linkage.
2. Experimental
2.1. Materials
Hanks' balanced salts modified powder, sodium bicarbonate, and the internal standard, naloxone, were purchased from Sigma (St. Louis, MO). p-Phenylenediamine, ammonium acetate, ethyl acetate, trifluoroacetic acid (TFA), triethylamine (TEA), 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), propylene glycol (PG), and acetonitrile (ACN) were obtained from Fisher Scientific (Fairlawn, NJ). Ammonium citrate was obtained from Alfa Aesar (Ward Hill, MA). ARcare® 7396 (pressure-sensitive tape with MA-38 medical grade acrylic adhesive and 60# Kraft release paper) was a gift from Adhesive Research, Inc. (Glen Rock, PA). MEDIFLEX® 1502 (backing membrane; pigmented metalized polyester) was a gift from Mylan Technologies Inc. (St. Albans, VT). SCOTCHPAK™ 9742, a fluoropolymer release liner and CoTran™ 9715, a 3 mil ethylene vinyl acetate (EVA) copolymer with 19% vinyl acetate were gifts from 3M™ Drug Delivery Systems (St. Paul, MN). Hill Top Chambers® were obtained from Hill Top Research, Inc. (Cincinnati, OH). Water was purified using a Barnstead nanopure Diamond Ultrapure water system (Barnstead International, Dubuque, IA).
2.2. Synthetic procedures
The detailed synthetic procedures for the preparation of 6-β-naltrexol, hydroxybupropion, and the carbonate codrug, CB-NTXOL-BUPOH, have been reported elsewhere (Hamad et al., 2006; Kiptoo et al., 2006). Synthesis and characterization of a transient intermediate, observed during chemical or enzymatic hydrolysis of the codrug to hydroxybupropion, have also been reported previously (Hamad et al., 2006).
2.3. Plasma hydrolysis of the carbonate codrug
Guinea pig plasma, which was stored at -20°C, was thawed to room temperature. Known and sub-saturated amounts of the codrug (50 nmol) were added to approximately 2.0 ml of the guinea pig plasma and vortexed. Two-hundred microliter aliquot parts of the spiked plasma were distributed into sealed vials, and incubated at 37°C. Samples were withdrawn at predetermined time intervals; i.e. 0, 0.5, 1, 2, 4, 6, 9, 24, 30, and 48 h. Five-hundred microliters of ACN was added to each sample to precipitate plasma protein. Each sample was vortexed and centrifuged at 10,000 × g for 15 min. The supernatant was separated and evaporated under nitrogen at room temperature, and the residue was reconstituted in ACN for high-pressure liquid chromatography (HPLC) analysis. A standard curve was prepared from drug-spiked plasma samples processed by the same method. The amounts of the codrug remaining, or parent drugs released, were plotted against time. These hydrolytic profiles were fit to previously developed mathematical models using nonlinear least-squares regression analysis (SCIENTIST®, Micromath Inc., Salt Lake City, UT) as described by the equations below:
| (1) |
| (2) |
| (3) |
| (4) |
Where k1 and k2 are rate constants, CA0 is the initial concentration of the codrug, and CA, CB, CC, and CD are the concentrations of the codrug, intermediate, hydroxybupropion, and 6-β-naltrexol at any time t, respectively.
2.4. Preparation of the gel formulation
A hydroxyethyl cellulose-based gel was prepared for topical application. First, either CB-NTXOL-BUPOH or 6-β-naltrexol (∼25 mg each) was dissolved in 1 ml of PG:Hanks' buffer, pH 7.4 (2:3, v/v) solution using a magnetic stirrer. Hydroxyethyl cellulose (2%, w/w) was dispersed into these mixtures, sonicated, vortexed, and allowed to form a gel at room temperature. Each formulation was a saturated suspension.
2.5. Percutaneous absorption studies in guinea pigs in vivo
All animal studies were approved by the University of Kentucky IACUC. Male and female Hairless IAF guinea pigs (Charles River) weighing 345-482 g were used for topical studies (n = 5 to 6 per treatment group). Prior to surgery, the animals were treated with glycopyrrolate and buprenorphine (to induce analgesia), and then ketamine (100 mg/kg, i.p.) and xylazine (8 mg/kg, i.m.) were used for anesthetic purposes. Catheters were surgically implanted into the jugular vein. A “blank” baseline blood sample was drawn from each animal immediately before drug treatment. Five-hundred microliters of the gel containing the drug was applied onto the dorsal region of the guinea pig with a syringe and subsequently spread to cover an area of 7.25 cm2. A rubber ring fitted with a drug-impermeable backing laminate (MEDIFLEX® 1502) was used to enclose the area of gel application. ARcare® acrylic adhesive was used to ensure that the ring maintained contact with the skin. Additionally, Bioclusive® tape was placed over the patches to further secure the formulation on the guinea pig skin. Drug formulations were applied on both sides of each guinea pig to afford a total application area of 14.5 cm2. Blood samples were obtained at predetermined time intervals, which included 48 h while the formulation was in contact with the animal, and for another 48 h after patches and formulations were removed. Blood samples were immediately centrifuged at 10,000 × g for 3 min, and the plasma was separated. Plasma samples were stored at -70°C until analysis by liquid chromatographic mass spectrometry (LC-MS).
2.6. Plasma sample extraction procedure
Exactly 500 μl of acetonitrile: ethyl acetate (1:1, v/v) was added to 100 μl of plasma sample in a 1.5 ml microcentrifuge tube, and the mixture was vortexed for 30 s and then centrifuged at 10,000 × g for 20 min. The supernatant was decanted into a clean test tube and evaporated under nitrogen at 30°C. The residue was reconstituted with 100 μl of acetonitrile, vortexed, and sonicated for 5 min. The clear solution was placed into an HPLC vial containing low volume inserts, and 20 μl of the sample was injected onto the LC-MS column. The extraction efficiencies were 78.5 ± 2.1%, 87.0 ± 3.5%, and 81.6 ± 2.2% for CB-NTXOL-BUPOH, hydroxybupropion, and 6-β-naltrexol, respectively. Data were corrected for the respective extraction efficiencies.
2.7. Quantitative analysis
2.7.1. HPLC conditions
A modified HPLC assay from Hussain et al (Hussain et al., 1988) was used for the determination of the hydrolytic profile of CB-NTXOL-BUPOH. The HPLC system consisted of a Waters (Milford, MA) 717 Plus Autosampler, 1525 Binary Pumps, and a 2487 dual wavelength UV absorbance detector with Breeze™ software. A Brownlee C18 reversed-phase Spheri5 column (220 × 4.6 mm, 5 μm) with a C18 reversed-phase guard column (15 × 3.2 mm, 7 μm) was used with the UV/VIS detector set at a wavelength of 215 nm. The mobile phase consisted of acetonitrile (ACN): 0.1% TFA buffer adjusted with TEA to pH 3.0 (55:45). The flow rate of the mobile phase was 1.5 ml/min with 100 μl sample injections. Retention times on the column were found to be 2.3, 4.6, 5.2, and 10.5 min for 6-β-naltrexol, hydroxybupropion, intermediate (BUPOH-CB), and CB-NTXOL-BUPOH, respectively. Standards were analyzed with each set of hydrolysis samples and linear regression of standard curves exhibited excellent linearity over the entire concentration range (50-1000 ng/ml) employed in the assays. The assay sensitivity was at least 20 ng/ml or better for all the drugs. Both intra-day and inter-day assays had small coefficients of variation (<10% CV), indicating that the method was reproducible, accurate, and precise.
2.7.2. LC-MS conditions
LC-MS was employed in the analysis of guinea pig plasma samples to determine percutaneous absorption in hairless guinea pigs in vivo. Chromatography was performed on a Waters Symmetry® C18 (2.1 × 150 mm, 5 μm) column at 35°C with a mobile phase consisting of ammonium acetate (2 mM) containing 0.01 mM ammonium citrate:acetonitrile (35:65 v/v) at a flow rate of 0.25 ml/min. A Waters Symmetry® C18 (2.1 × 10 mm, 3.5 μm) guard column was used. The volume of injection was 20 μl. The MS detection was performed using electrospray ionization (ESI) for ion production. Selected ion monitoring (SIM) was performed in the positive mode for CB-NTXOL-BUPOH, m/z 650 (dwell time, 0.30 s), 6-β-naltrexol, m/z 344, and BUPOH, m/z 238. Naloxone (m/z 324) was used as the internal standard. The capillary voltage was 4.5 kV and the cone voltage was 30 V. The source block and desolvation temperatures were 120°C and 250°C, respectively. Nitrogen was used as a nebulization and drying gas at flow rates of 50 and 450 L/h, respectively. The retention times for 6-β-naltrexol, hydroxybupropion, naloxone (internal standard), and CB-NTXOL-BUPOH were 3.27 ± 0.11, 4.32 ± 0.20, 6.26 ± 0.23, and 16.60 ± 0.28 min, respectively.
2.8. Pharmacokinetic analysis
Topical administration data were analyzed by non-compartmental analysis to determine mean steady-state plasma concentration (CSS), lag time to steady-state (tlag), and area under the curve from 0 to 48 h, AUC0-48. The steady-state plasma concentration of the drug after application of the gel formulation containing either the codrug or 6-β-naltrexol was calculated by using the following equation:
| (5) |
2.9. Skin irritation and sensitization of 6-β-naltrexol in hairless guinea pigs
2.9.1. 6-β-Naltrexol and p-phenylenediamine formulations
A saturated drug solution (24 mg/ml) of 6-β-naltrexol base, prepared in 3:1 (v/v) propylene glycol:buffer pH 7.4 was used for induction, challenge, and rechallenge. p-Phenylenediamine, prepared as a 2% solution in 80% aqueous ethanol was used for the induction phase, and as a 2% solution in acetone for challenge and rechallenge phases.
2.9.2. Fabrication of transdermal systems of 6-β-naltrexol and p-phenylenediamine
The transdermal patches of 6-β-naltrexol base (7.25 cm2) were fabricated by sandwiching a drug reservoir between a drug-impermeable backing laminate (MEDIFLEX®1502) and a rate-controlling EVA membrane (CoTran™9715) with ARcare®7396 adhesive. A release slip composed of SCOTCHPAK™ 9742 was used to leave a small opening into the reservoir of the empty device. The membrane/adhesive laminate was then heat sealed to the metallized polyester backing membrane. The slip was removed to form a small port, and 500 μL of the saturated drug solution (24 mg/ml) of 6-β-naltrexol in 3:1 (v/v) propylene glycol: buffer pH 7.4 was injected into the reservoir. After injecting the drug solution into the reservoir, the port was heat sealed. Molded plastic Hill Top Chambers® (25 mm diameter) were used for p-phenylenediamine studies. A non-woven cotton Webril® pad was placed in each chamber to hold the test material. Hill Top Chambers® were then inserted into the patch by cutting a hole in the rate-controlling membrane so that the Webril® pad would be in direct contact with the skin. The patches were made with the same materials as described for 6-β-naltrexol. Five-hundred microliters of the drug solution was added to the Webril® pad just before patch application on the guinea pig.
2.9.3. Animal studies
Sensitization studies were carried out according to the Buehler (Buehler, 1965; Buehler, 1994) method in hairless guinea pigs without restraint. p-Phenylenediamine was used as the positive control substance because it has been shown to have strong sensitizing potential by the Buehler method (Buehler, 1965). Five guinea pigs were used in both the control group and in the test group.
During induction, challenge, and rechallenge phases, two patches were applied to the dorsal region of the hairless guinea pigs. Bioclusive® tape was placed over the patches to ensure intimate contact with the skin. p-Phenylenediamine and 6-β-naltrexol patches were kept on the animals for 6 h and 24 h, respectively, during all the phases of the study. Previously, 6-β-naltrexol was reported to have a lag time longer than 6 h (Paudel et al., 2005), and thus the need for a longer period of exposure. All the readings during the induction, challenge, and rechallenge phases were taken on both flanks of the animal.
2.9.3.1 Induction phase
Three induction exposures (one/wk for 3 wk) of 6-β-naltrexol and p-phenylenediamine were carried out on the same selected area on the flank of the test guinea pigs. The skin irritation potential of the drugs was evaluated by taking erythema and trans-epidermal water loss (TEWL) readings at the induction sites as a relative measure of redness and skin damage, respectively. Readings were taken at 5 min and thereafter at 1, 2, 24 and 48 h after removing the patches and gently dabbing the site with a gauze pad. Both chromameter and TEWL readings were taken before patch applications as well. Changes in color on the treated sites were measured against each animal's own baseline standard.
Erythema from topical drug treatments was quantitated with a Konica Minolta colorimeter (Sutinen et al., 2000) (ChromaMeter CR-400, Konica Minolta, Japan). The color reflectance was recorded on a three-dimensional scale L*a*b* (6). The L* value (luminance) expresses the relative brightness of the color ranging from total black (L* = 0) to pure white (L* = 100). The term a* is the red-green axis with +100 expressing full red and -100 full green, b* is the yellow-blue axis. Theoretically, skin irritation should decrease the L* value and increase the a* value. The colorimeter was calibrated each day against a white plate provided by Konica Minolta. The head of the instrument was gently placed on the skin area where the patch was applied in order to record the color reflectance. TEWL was measured using an Evaporimeter EP-1 (Servomed, Sweden). The TEWL is calculated automatically and displayed digitally in g/m2·h. Colorimeter and TEWL readings were taken on both sides of the guinea pig and are presented as the mean value.
2.9.3.2. Challenge and rechallenge phase
There was a 2-week rest period between the last induction exposure and the primary challenge for the test group of 6-β-naltrexol and p-phenylenediamine. During the challenge phase, the test animals and the non-sensitized control animals received drug patches at a naive skin site. The test animals were rechallenged one week later at the remaining new site. The resulting reaction at 24 and 48 h after patch removal of the test drugs and chemical in the test group during the challenge and the rechallenge phases was compared with the original (challenge) control group. Reactions were visually graded for erythema at 24 and 48 h after patch removal by the same investigator according to a 5-point grading scale (0, ± 0.5, 1, 2, 3). The grades 1, 2, and 3 denote increasing severity of erythema with grades ≥1 considered positive. The “0.5 or (±)” grade was used for equivocal reactions and were considered negative.
Colorimeter and TEWL readings were taken on both flanks of the control and the test guinea pigs at 24 and 48 h during the challenge and the rechallenge phases. Readings from both flanks were presented as the mean value. To evaluate the incidence of sensitization in an animal, the higher visual score among the two sites was taken into account. Digital photo recording was also performed at these time points.
2.10. Statistical analysis
Statistical analysis of the pharmacokinetic parameters obtained after the topical application and skin sensitization studies were computed with a one-way ANOVA followed by Tukey's post hoc analysis using SIGMA-STAT (SPSS, Chicago, IL). Data were reported as mean ± S.D and were considered to be significant at p<0.05.
3. Results and discussion
3.1. In vitro enzymatic hydrolysis
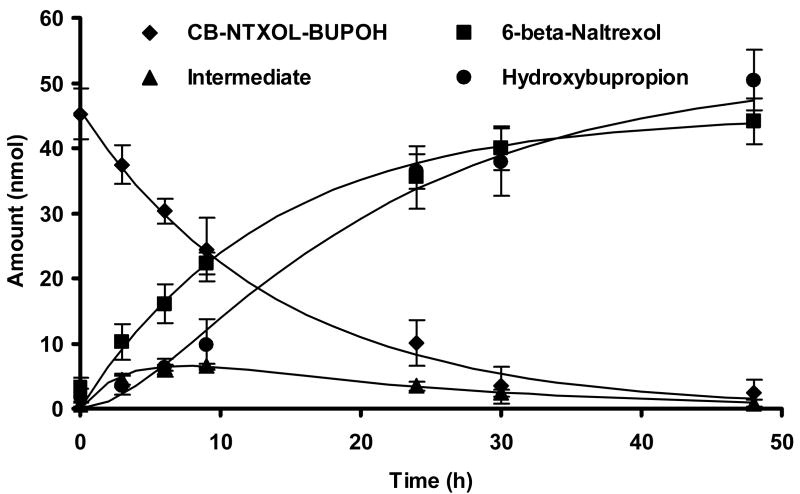
Esterases are ubiquitously found in most tissues and plasma, and have been reported to be resistant to the stresses of freezing, thawing, and storage (Hewitt et al., 2000). It is expected that the codrug under investigation should be capable of being converted to the parent drugs by chemical and/or enzymatic hydrolysis. Figure 2 illustrates the hydrolytic profile of the carbonate codrug, and the time course of formation of the two active parent drugs, hydroxybupropion and 6-β-naltrexol, in guinea pig plasma. The degradation half-life of the codrug in guinea pig plasma was approximately 8.7 hr. We expected the release of 6-β-naltrexol from the codrug to involve a one-step reaction, and this was confirmed by the appearance of 6-β-naltrexol with a similar half-life of formation to that of the disappearance of the codrug. Compared to the previously reported codrug half-life of 29 hrs in isotonic pH 7.4 phosphate buffer (Kiptoo et al., 2006) (see Table 1), the codrug undergoes an almost three-fold faster hydrolysis in guinea pig plasma in vitro, largely attributed to more rapid enzymatic hydrolysis by plasma esterase enzymes. However, the release of hydroxybupropion proceeded through a two-step process and therefore the rate of formation of hydroxybupropion was slower compared to 6-β-naltrexol. Although the formation of the two parent drugs occurred through different hydrolytic pathways, molar equivalent concentrations of 6-β-naltrexol and hydroxybupropion (as the parent drug itself and the intermediate) species were obtained. In vitro enzymatic hydrolysis data not only confirms that hydrolysis of the codrug would release the two active parent drugs in the skin and/or in the body, but helps to evaluate the relative impact of these rates of bioconversion on the transport of the drugs across the skin.
Figure 2.
Hydrolytic profile of the carbonate codrug, CB-NTXOL-BUPOH, in guinea pig plasma at 37°C.
Table 1.
Permeation properties of 6-β-naltrexol, hydroxybupropion, and the codrug, CB-NTXOL-BUPOH. Data is represented as mean (± S.D.). n =3 for the parent drugs and n = 4 for CB-NTXOL-BUPOH.
| Drug | Light mineral oil solubility
(mM)‡ |
Half-life in buffer, pH 7.4
(h) ‡ |
Guinea pig plasma half-life
(h) |
In vitro flux
(nmol cm-2 h-1) ‡ |
In vitro lag time
(h) ‡ |
|---|---|---|---|---|---|
| 6-β-naltrexol | 0.03 ± 0.01 | stable | stable | 0.36 ± 0.15 | 15.72 ± 1.76 |
| CB-NTXOL-BUPOH | 2.88 ± 0.09 | 28.875 | 8.72 | 1.34 ± 0.35 | 12.10 ± 0.77 |
| Hydroxybupropion | 4.04 ± 0.04 | stable | stable | 25.89 ± 6.01 | 7.83 ± 1.73 |
Values obtained from (Kiptoo et al., 2006). Data are from human skin studies.
3.2. Percutaneous absorption in hairless guinea pigs in vivo
A slightly modified version of a previously described LC-MS assay described (Valiveti et al., 2004) was used to quantify the amounts of CB-NTXOL-BUPOH, hydroxybupropion, and 6-β-naltrexol in guinea pig plasma following in vivo transdermal application of CB-NTXOL-BUPOH or 6-β-naltrexol (control). CB-NTXOL-BUPOH, hydroxybupropion, and 6-β-naltrexol peaks were well resolved and free of interference from endogenous compounds in the plasma. No significant matrix effect was observed for the analytes in the plasma samples. Results of the intra-day and inter-day validation assays indicated that the accuracy of the assay was >92%, and the coefficient of variation did not exceed 10%. The lower limit of quantification (LLOQ) was 0.5 ng/ml for CB-NTXOL-BUPOH and hydroxybupropion, and 0.75 ng/ml for 6-β-naltrexol. Post-preparative stability studies indicated that the stabilities of CB-NTXOL-BUPOH, hydroxybupropion, and 6-β-naltrexol were greater than 94% for at least 48 h at 12°C, autosampler temperature.
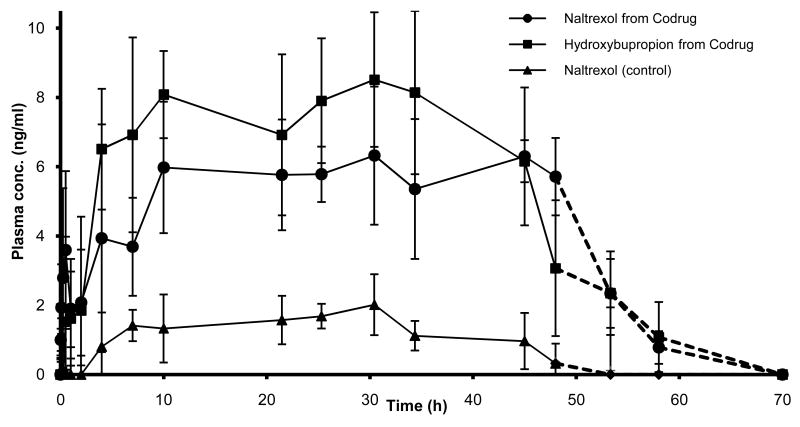
The plasma concentration profiles of the analytes following topical applications of gel formulations containing either CB-NTXOL-BUPOH or 6-β-naltrexol in hairless guinea pigs are shown in Fig. 3. and the pharmacokinetic parameters, including Cmax, Tmax, CSS, AUC0-48, and Tlag, are given in Table 2. 6-β-Naltrexol equivalent steady state plasma concentrations of 6.40 ± 0.93 ng/ml after application of the codrug were maintained for 48 h, as compared to 1.25 ± 0.51 ng/ml observed for the control animals. Increased percutaneous absorption of 6-β-naltrexol after application of the codrug is further demonstrated by the significantly higher AUC value (p<0.05). Trace amounts of the codrug and the intermediate (m/z 282), formed during the hydrolysis of the codrug into hydroxybupropion, were observed in some of the plasma samples, but no further effort was made to quantify these amounts. Overall, a significant five-fold (p<0.05) enhancement in the delivery of 6-β-naltrexol was obtained via the codrug strategy compared to the control. As illustrated in Fig. 3, no significant skin reservoir effect was observed in guinea pigs, since plasma levels of each of the studied drugs declined on removal of the formulation at rates similar to their systemic elimination rates. Also, the observed in vivo lag times were significantly shorter than the lag times observed in vitro (Kiptoo et al., 2006). Often, flow-through diffusion cells utilized in in vitro studies artificially add extra time to observed lag times in vivo. On the other hand, short in vivo lag times may be attributable to significant contributions from the shunt routes through the stratum corneum for the somewhat larger codrug molecule, as compared to the in vitro rate of the carbonate linked codrug. In this respect, large and/or polar drugs can be carried through the stratum corneum via paths of least resistance provided by the shunt routes. These routes avoid the tortuous pathway of the stratum corneum lipid bilayers, and thus exhibit minimal lag times (Scheuplein, 1967; Wallace and Barnett, 1978). Additionally, reduced lag times may be due to an increased in vivo bioconversion rate of the carbonate linked codrug. The more lipophilic codrug exhibits increased partitioning into the stratum corneum (Kiptoo et al., 2006), and once in the skin, reverts back either chemically or enzymatically to the more hydrophilic parent drugs, hydroxybupropion and 6-β-naltrexol. These more hydrophilic parent drug forms should have the capability to traverse the viable epidermis faster than the larger lipophilic codrug, which may result in shorter lag times. The pharmacokinetic parameters of hydroxybupropion after codrug application were Cmax, 9.23±2.11 ng/ml; Tmax, 18.72±13.75 h; CSS, 6.92±1.2 ng/ml; AUC0-48, 335±56 ng/ml*h; and Tlag, 5.5±1.7 h.
Figure 3.
Mean (± S.D.) plasma concentration profiles in guinea pigs after topical application of a gel formulation containing either CB-NTXOL-BUPOH (n = 6) or 6-β-naltrexol (control, n = 5). The dotted line (----) indicates the plasma concentration obtained after the removal of the formulation.
Table 2.
Pharmacokinetic parameters of 6-β-naltrexol (NTXOL) after application of a gel formulation containing either CB-NTXOL-BUPOH or 6-β-naltrexol base (control). Data is represented as mean (±S.D.). n = 5 for NTXOL and n = 6 for CB-NTXOL-BUPOH
| Parameter | NTXOL | NTXOL from CB-NTXOL-BUPOH |
|---|---|---|
| AUC0-48(ng/ml*h) | 66.4 ± 7.9 | 282.0 ± 14.5 |
| Cmax(ng/ml) | 1.5 ± 0.2 | 6.6 ± 0.4 |
| Tmax (h) | 28.1 ± 1.5 | 10.1 ± 0.9 |
| Tlag (h) | 5.1 ± 0.7 | 5.0 ± 1.1 |
| Observed CSS(ng/ml) | 1.3 ± 0.5 | 6.4 ± 0.9 |
| Predicted CSS(ng/ml)* | 0.2 ± 0.1 | 0.7 ± 0.3 |
| Enhancement factor | 1 | 5.3 |
Predicted from in vitro human skin diffusion data
3.3. Skin irritation and sensitization
3.3.1. Induction phase (Skin irritation)
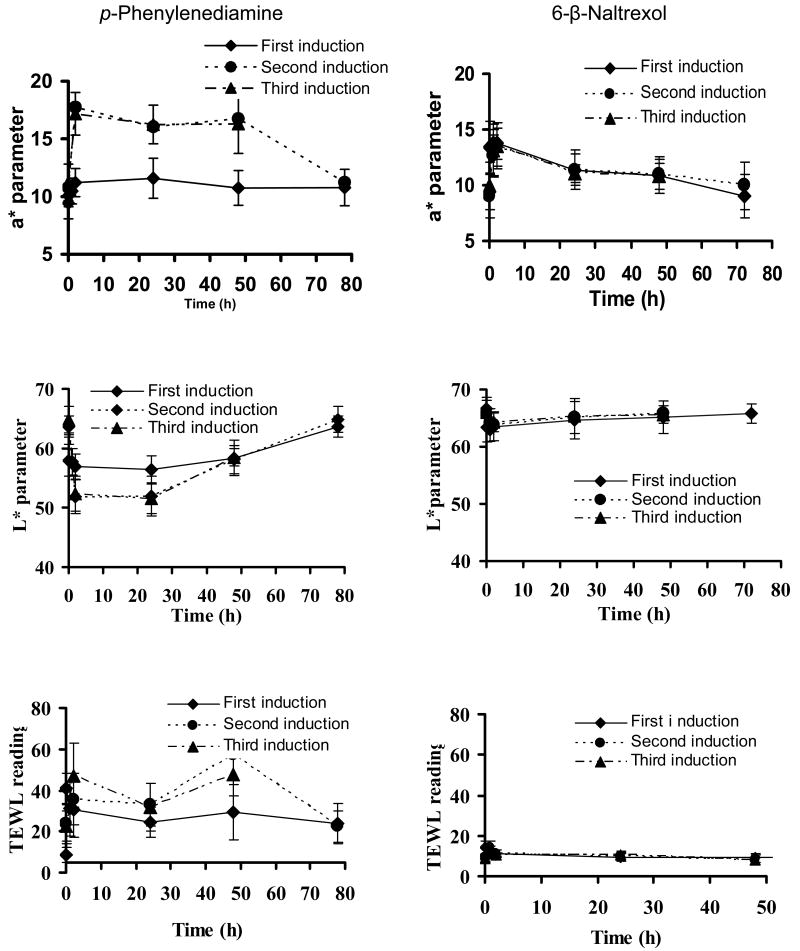
Skin irritation was evaluated after 6 h of patch treatment for p-phenylenediamine and after 24 h of patch treatment for 6-β-naltrexol. Results are summarized in figure 4. All the patches were in close contact with the skin.
Figure 4.
Colorimeter (a*), (L*) and TEWL readings of sites treated with p-phenylenediamine and 6-β-naltrexol in hairless guinea pigs during the induction phase. Data represent mean ± SD. (n = 10 for p-phenylenediamine test group; n=5 for 6-β-naltrexol test group).
3.3.2. p-Phenylenediamine
p-Phenylenediamine induced significant skin irritation in hairless guinea pigs, as was reflected by the significant redness and barrier damage after patch removal. During the first induction phase of p-phenylenediamine, the redness parameter (a* value of the colorimeter) reached a peak at 2 h after patch removal as shown in Figure 4. During the second and third induction, the redness increased to a higher extent, and decreased slowly but never declined to the original baseline level. Brightness parameter (L* value) decreased at 5 min after patch removal during the first induction phase and more so during subsequent inductions. TEWL increased to a maximum at 5 min after patch removal, and then remained constant for up to 78 h. During subsequent inductions, the TEWL increased gradually and did not return to the original baseline level within 78 h.
3.3.3. 6-β-Naltrexol
NTXOL produced mild irritation (as reflected in a* and L* values) during the first induction phase which peaked at 5 min and lasted for about 2 h as shown in Figure 4. Erythema was slightly increased during the second and third induction phases but the TEWL reading remained similar indicating no skin damage.
3.3.4. Codrug
In this study, 500 μL of the gel containing the codrug was applied on the dorsal region of both sides of each guinea pig. After 48 h of contact, where the drug remained occluded on the skin for two days, the formulation was removed. No sign of redness or irritation was observed on either side of the guinea pig skin immediately afterwards or in the days following the removal of the formulation. From these observations, it is clear that the codrug can be considered a non-irritant and potentially safe for transdermal delivery. We could not carry out the sensitization studies on the codrug itself because of the limited amount of the synthesized drug to do these studies. However, since the irritation and sensitization potentials of 6-β-naltrexol have not been reported previously, this drug was independently studied to prove the nonsensitizing and nonirritating behavior of one component of the codrug.
3.3.5. Challenge and rechallenge (sensitization)
Sensitization responses were evaluated by two indices as described previously by Buehler (Buehler, 1965), indices for incidence and severity were determined for both test and control animals. The results of sensitization are summarized in Table 3. Ninety percent of the animals were sensitized to p-phenylenediamine compared to none in the control group. The sensitized group had more than a three-fold higher severity index than the control group. No incidence of sensitization was observed for 6-β-naltrexol. The severity indices for 6-β-naltrexol exposure in both control and test animals were less than (p<0.05) that for p-phenylenediamine exposure. Nevertheless, 6-β-naltrexol patches generally performed better than some of the commercially available opioid patches, where irritation has been observed up to 72 h after patch removal (Schmid-Grendelmeier et al., 2006); no other topical adverse events were observed.
Table 3.
Sensitization potential of p-phenylenediamine and 6-β-naltrexol in hairless guinea pigs.
| Group | Response grade | Incidence | Severity | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | 3 | ||||
| p-Phenylenediamine | Control | 0 | 5 | 0 | 0 | 0 | 0/5 | 0.8 |
| Test | 0 | 1 | 4 | 5 | 0 | 9/10 | 2.65 | |
| NTXOL | Control | 5 | 0 | 0 | 0 | 0 | 0/5 | 0 |
| Test | 5 | 0 | 0 | 0 | 0 | 0/5 | 0 | |
3.4. Predicting plasma concentrations from in vitro permeation studies
To predict the plasma concentration of 6-β-naltrexol after application of the gel formulation containing either the codrug or the control formulation, the following equation was used:
| (4) |
where CSS is the predicted steady state plasma concentration (ng/ml); JSS is the steady-state flux obtained from previous in vitro permeation studies (Table 1); A is the area of the skin in contact with the formulation (14.5 cm2); and Cl is the total body clearance. Pharmacokinetic parameters such as total body clearance were previously investigated after intravenous administration of 6-β-naltrexol (50 mg/kg) in guinea pigs (Paudel et al., 2005). In the current study, the predicted steady state plasma concentrations of 6-β-naltrexol equivalents were 0.7 ± 0.3 ng/ml and 0.2 ± 0.1 ng/ml following topical application of CB-NTXOL-BUPOH and 6-β-naltrexol, respectively. These significantly lower (p<0.05) predicted steady-state plasma concentrations of 6-β-naltrexol following topical application of CB-NTXOL-BUPOH and 6-β-naltrexol can largely be attributed to the use of human skin in the in vitro studies. In previous experiments with 6-β-naltrexol, flux and other in vitro permeation parameters were found to be 5.6-fold greater in guinea pig skin than in human skin (Paudel et al., 2005). If the 5.6-fold factor is taken into account in the plasma level prediction, then the plasma levels from 6-β-naltrexol would be predicted to be 1.1 ng/ml (i.e. quite close to the observed value of 1.2 ng/ml) and the plasma levels from CB-NTXOL-BUPOH would be 4.2 ng/ml (significantly different than the observed value of 6.4 ng/ml). The inconsistency observed with the in vitro/in vivo correlation for CB-NTXOL-BUPOH could be due to the added complications of potential interspecies differences in bioconversion rates. Additionally, in vitro experiment bioconversion rates can sometimes be slower than bioconversion rates in vivo at maximum viability.
The increased flux of CB-NTXOL-BUPOH through guinea pig skin in vivo can be explained by the increased hydrophobicity and rapid bioconversion of the codrug when compared to 6-β-naltrexol treatment (control). As shown in Table 1, CB-NTXOL-BUPOH had a higher oil solubility compared to 6-β-naltrexol (2.88 ± 0.09 mM vs 0.03 ± 0.01 mM). Since the lipophilicity of the lipid bilayer domain in the stratum corneum is much higher than that of water, a lipophilic compound would partition preferentially into the stratum corneum (Scheuplein and Bronaugh, 1983). A preferentially oil soluble drug should partition with ease into the stratum corneum, however the drug may have difficulty leaving the stratum corneum and permeating through the viable tissue. As such, once CB-NTXOL-BUPOH becomes available in the skin following partitioning into the stratum corneum, it is rapidly converted into the more hydrophilic parent drugs, hydroxybupropion and 6-β-naltrexol. These more hydrophilic parent drug products can cross the viable epidermis and dermis, meeting less resistance than the more lipophilic codrug. Simultaneous transport and metabolism in the skin are related by a diffusion-bioconversion constant expressed as , where k is the metabolic rate constant for the codrug bioconversion, and D is the diffusivity of the drug in the viable tissue (Yu et al., 1979). It is possible that the in vivo environment may possess a higher level of enzymatic activity, which in turn would afford a higher diffusion-bioconversion constant, subsequently resulting in a higher flux.
4. Conclusion
The present investigation describes an in vivo evaluation of a codrug of 6-β-naltrexol linked to hydroxybupropion, CB-NTXOL-BUPOH, in hairless guinea pigs for the eventual purpose of increasing the therapeutic efficacy of 6-β-naltrexol and hydroxybupropion via a transdermal dosage form that can be used for the simultaneous treatment of alcoholism and tobacco dependence. The carbonate codrug was hydrolyzed on passing through skin, and appeared in plasma mainly as the parent drugs, 6-β-naltrexol and hydroxybupropion. Only trace amounts of the codrug were detected in plasma. The codrug traversed the skin at a faster rate than 6-β-naltrexol. 6-β-Naltrexol mean steady state plasma concentrations of 6.4 ng/ml were obtained after topical application of the codrug compared to 1.2 ng/ml from 6-β-naltrexol free base, representing a five-fold enhancement in the transdermal delivery of 6-β-naltrexol. Skin sensitization and irritation studies conducted according to the Buehler method revealed that 6-β-naltrexol had no sensitizing potential in hairless guinea pigs. The method was validated with a known sensitizer, p-phenylenediamine. p-Phenylenediamine induced sensitization in 90% of the animals. 6-β-naltrexol caused only mild temporary skin irritation during the first application of the patch. During subsequent applications, erythema was slightly increased but no skin damage was observed. Overall, this study demonstrated that a codrug strategy can be used to enhance transdermal delivery of 6-β-naltrexol and thereby improve the therapeutic efficacy 6-β-naltrexol as well as hydroxybupropion in simultaneous treatment of alcohol dependency and tobacco abuse.
Acknowledgments
This project was funded by NIH Grant R01AA013853. The authors would also like to thank Mylan Technologies (St. Albans, VT) for the gift of MEDIFLEX® 1502, 3M™ Drug Delivery Systems (St. Paul, MN) for providing the SCOTCHPAK™ 9742 and CoTran™ 9715, and Adhesives Research (Glen Rock, PA) for supplying ARcare®7396.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belson MG, Kelley TR. Bupropion exposures: clinical manifestations and medical outcome. J Emerg Med. 2002;23:223–230. doi: 10.1016/s0736-4679(02)00522-x. [DOI] [PubMed] [Google Scholar]
- Bonina FP, Puglia C, Barbuzzi T, de Caprariis P, Palagiano F, Rimoli MG, Saija A. In vitro and in vivo evaluation of polyoxyethylene esters as dermal prodrugs of ketoprofen, naproxen and diclofenac. Eur J Pharm Sci. 2001;14:123–134. doi: 10.1016/s0928-0987(01)00163-4. [DOI] [PubMed] [Google Scholar]
- Buehler EV. Delayed Contact Hypersensitivity In The Guinea Pig. Arch Dermatol. 1965;91:171–177. doi: 10.1001/archderm.1965.01600080079017. [DOI] [PubMed] [Google Scholar]
- Buehler EV. Occlusive patch method for skin sensitization in guinea pigs: the Buehler method. Food Chem Toxicol. 1994;32:97–101. doi: 10.1016/0278-6915(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Cynkowska G, Cynkowski T, Al-Ghananeem AA, Guo H, Ashton P, Crooks PA. Novel antiglaucoma prodrugs and codrugs of ethacrynic acid. Bioorg Med Chem Lett. 2005;15:3524–3527. doi: 10.1016/j.bmcl.2005.05.128. [DOI] [PubMed] [Google Scholar]
- Faull AW, Brewster AG, Brown GR, Smithers MJ, Jackson R. Dual-acting thromboxane receptor antagonist/synthase inhibitors: synthesis and biological properties of [2-substituted-4-(3-pyridyl)-1,3-dioxan-5-yl] alkenoic acids. J Med Chem. 1995;38:686–694. doi: 10.1021/jm00004a014. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Bertolotti M, Dell'Utri A, Avico U, Sternieri E. Serum time course of naltrexone and 6 beta-naltrexol levels during long-term treatment in drug addicts. Drug Alcohol Depend. 1998;52:211–220. doi: 10.1016/s0376-8716(98)00098-2. [DOI] [PubMed] [Google Scholar]
- Frosch DL, Shoptaw S, Nahom D, Jarvik ME. Associations between tobacco smoking and illicit drug use among methadone-maintained opiate-dependent individuals. Exp Clin Psychopharmacol. 2000;8:97–103. doi: 10.1037//1064-1297.8.1.97. [DOI] [PubMed] [Google Scholar]
- Hamad MO, Kiptoo PK, Stinchcomb AL, Crooks PA. Synthesis and hydrolytic behavior of two novel tripartate codrugs of naltrexone and 6beta-naltrexol with hydroxybupropion as potential alcohol abuse and smoking cessation agents. Bioorg Med Chem. 2006;14:7051–7061. doi: 10.1016/j.bmc.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Hewitt PG, Perkins J, Hotchkiss SA. Metabolism of fluroxypyr, fluroxypyr methyl ester, and the herbicide fluroxypyr methylheptyl ester. I: during percutaneous absorption through fresh rat and human skin in vitro. Drug Metab Dispos. 2000;28:748–754. [PubMed] [Google Scholar]
- Hussain MA, Aungst BJ, Koval CA, Shefter E. Improved buccal delivery of opioid analgesics and antagonists with bitterless prodrugs. Pharm Res. 1988;5:615–618. doi: 10.1023/a:1015958417047. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Fiedler-Kelly J, Glover ED, Sachs DP, Grasela TH, DeVeaugh-Geiss J. Relationship between drug exposure and the efficacy and safety of bupropion sustained release for smoking cessation. Nicotine Tob Res. 2001;3:131–140. doi: 10.1080/14622200110042852. [DOI] [PubMed] [Google Scholar]
- Kiptoo PK, Hamad MO, Crooks PA, Stinchcomb AL. Enhancement of transdermal delivery of 6-beta-naltrexol via a codrug linked to hydroxybupropion. J Control Release. 2006;113:137–145. doi: 10.1016/j.jconrel.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Modesto-Lowe V, Van Kirk J. Naltrexone vs. nefazodone for treatment of alcohol dependence. A placebo-controlled trial. Neuropsychopharmacology. 2000;22:493–503. doi: 10.1016/S0893-133X(99)00135-9. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Rohde C, Lee SM. Serum 6-beta-naltrexol levels are related to alcohol responses in heavy drinkers. Alcohol Clin Exp Res. 2000;24:1385–1391. [PubMed] [Google Scholar]
- Meyer MC, Straughn AB, Lo MW, Schary WL, Whitney CC. Bioequivalence, dose-proportionality, and pharmacokinetics of naltrexone after oral administration. J Clin Psychiatry. 1984;45:15–19. [PubMed] [Google Scholar]
- Otagiri M, Imai T, Fukuhara A. Improving the pharmacokinetic and pharmacodynamic properties of a drug by chemical conversion to a chimera drug. J Control Release. 1999;62:223–229. doi: 10.1016/s0168-3659(99)00041-3. [DOI] [PubMed] [Google Scholar]
- Paudel KS, Nalluri BN, Hammell DC, Valiveti S, Kiptoo P, Hamad MO, Crooks PA, Stinchcomb AL. Transdermal delivery of naltrexone and its active metabolite 6-beta-naltrexol in human skin in vitro and guinea pigs in vivo. J Pharm Sci. 2005;94:1965–1975. doi: 10.1002/jps.20398. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadee W, Bilsky EJ. In vivo characterization of 6-beta-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol Exp Ther. 2005;313:1150–1162. doi: 10.1124/jpet.104.082966. [DOI] [PubMed] [Google Scholar]
- Rothenberg JL, Sullivan MA, Church SH, Seracini A, Collins E, Kleber HD, Nunes EV. Behavioral naltrexone therapy: an integrated treatment for opiate dependence. J Subst Abuse Treat. 2002;23:351–360. doi: 10.1016/s0740-5472(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Scheuplein RJ. Mechanism of percutaneous absorption. II. Transient diffusion and the relative importance of various routes of skin penetration. J Invest Dermatol. 1967;48:79–88. [PubMed] [Google Scholar]
- Scheuplein RJ, Bronaugh RL. Percutaneous absorption. In: Goldsmith LA, editor. Biochemistry and physiology of the skin. Vol. 1. Oxford University Press; Oxford: 1983. pp. 1255–1294. [Google Scholar]
- Schmid-Grendelmeier P, Pokorny R, Gasser UE, Richarz U. A comparison of the skin irritation potential of transdermal fentanyl versus transdermal buprenorphine in middle-aged to elderly healthy volunteers. Curr Med Res Opin. 2006;22:501–509. doi: 10.1185/030079906X89829. [DOI] [PubMed] [Google Scholar]
- Schroeder DH. Metabolism and kinetics of bupropion. J Clin Psychiatry. 1983;44:79–81. [PubMed] [Google Scholar]
- Sutinen R, Paronen P, Saano V, Urtti A. Water-activated, pH-controlled patch in transdermal administration of timolol. II. Drug absorption and skin irritation. Eur J Pharm Sci. 2000;11:25–31. doi: 10.1016/s0928-0987(00)00083-x. [DOI] [PubMed] [Google Scholar]
- Valiveti S, Nalluri BN, Hammell DC, Paudel KS, Stinchcomb AL. Development and validation of a liquid chromatography-mass spectrometry method for the quantitation of naltrexone and 6-beta-naltrexol in guinea pig plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;810:259–267. doi: 10.1016/j.jchromb.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Verebey K, Volavka J, Mule SJ, Resnick RB. Naltrexone: disposition, metabolism, and effects after acute and chronic dosing. Clin Pharmacol Ther. 1976;20:315–328. doi: 10.1002/cpt1976203315. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR. Naltrexone in alcohol dependence. Lancet. 1995;346:456. doi: 10.1016/s0140-6736(95)91316-5. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O'Brien CP. Naltrexone and alcohol dependence. Role of subject compliance. Arch Gen Psychiatry. 1997;54:737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- Wallace SM, Barnett G. Pharmacokinetic analysis of percutaneous absorption: evidence of parallel penetration pathways for methotrexate. J Pharmacokinet Biopharm. 1978;6:315–325. doi: 10.1007/BF01060095. [DOI] [PubMed] [Google Scholar]
- Yu CD, Fox JL, Ho NF, Higuchi WI. Physical model evaluation of topical prodrug delivery-simultaneous transport and bioconversion of vidarabine-5′-valerate II: Parameter determinations. J Pharm Sci. 1979;68:1347–1357. doi: 10.1002/jps.2600681105. [DOI] [PubMed] [Google Scholar]