Abstract
A wide variety of α-diazo-β-ketoesters can be prepared in good overall yields via a two-step sequence involving addition of ethyl lithiodiazoacetate to aliphatic, aromatic and conjugated aldehydes followed by mild oxidation with the Dess-Martin periodinane.
Keywords: ethyl diazoacetate, Dess-Martin periodinane, oxidation
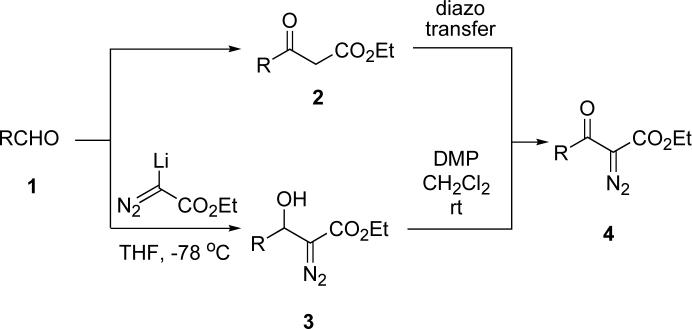
In the course of some natural product syntheses currently ongoing in these labs, we needed to convert an aldehyde 1 into an α-diazo-β-ketoester 4 and two reasonable sequences seemed possible (Scheme 1). The most common methodology for this transformation would involve first homologating 1 to the corresponding β-ketoester 2, followed by a Regitz diazo transfer step to produce 4.1 Alternatively, a potentially more attractive route would be to initially add ethyl lithiodiazoacetate to aldehyde 1 to produce an α-diazo-β-hydroxyester 3, which would be oxidized to the desired diazo compound 4. Although the addition of metallated ethyl diazoacetate to aldehydes is well precedented,2 examples of the oxidation of adducts 3 to the corresponding ketones 4 are rare. In the few extant cases, the β-hydroxy-α-diazoesters 3 are derived only from aromatic or conjugated aldehydes. Moreover, the only reagents which have been used for alcohol oxidation in these few examples are limited to manganese dioxide,3 IBX,4 or barium permanganate.5
Scheme 1.
Since we were particularly interested in effecting this sequence starting with aliphatic aldehydes, and also required a mild oxidant for the second step which is compatible with sensitive functionality, we have explored this methodology further, particularly with regard to the oxidation process. Since there was good literature precedent that α-diazo-β-ketoesters are stable towards Dess-Martin periodinane (DMP),6 we chose to investigate this reagent for oxidation of substrates like 3.
As listed in Table 1, several α-diazo-β-hydroxyesters 3 were prepared from a variety of aliphatic, aromatic and unsaturated aldehydes and ethyl lithiodiazoacetate in THF using the experimental procedure of Padwa, et al.2b It was found that exposure of these compounds to Dess-Martin periodinane in methylene chloride at room temperature indeed led to the formation of the corresponding α-diazo-β-ketoesters 4. An interesting observation was that addition of excess pyridine (∼12 equiv) to the oxidation reaction resulted in higher product yields in a few cases. This improvement may be due to the pyridine minimizing decomposition of the diazo compounds by adventitious acid.7 A number of examples of this oxidation are listed in the Table along with the isolated yields of diazoketones 4. Thus, Dess-Martin periodinane appears to be a general, superior oxidant for conversion of all types of α-diazo-β-hydroxyesters to α-diazo-β-ketoesters and thereby expands the scope of this two-step procedure for synthesizing the latter class of compounds.
Table 1.
Preparation of representative α-diazo-β-ketoesters
| entry | aldehyde substrate | α-diazo-β-hydroxyester 3 | α-diazo-β-ketoester 4 |
|---|---|---|---|
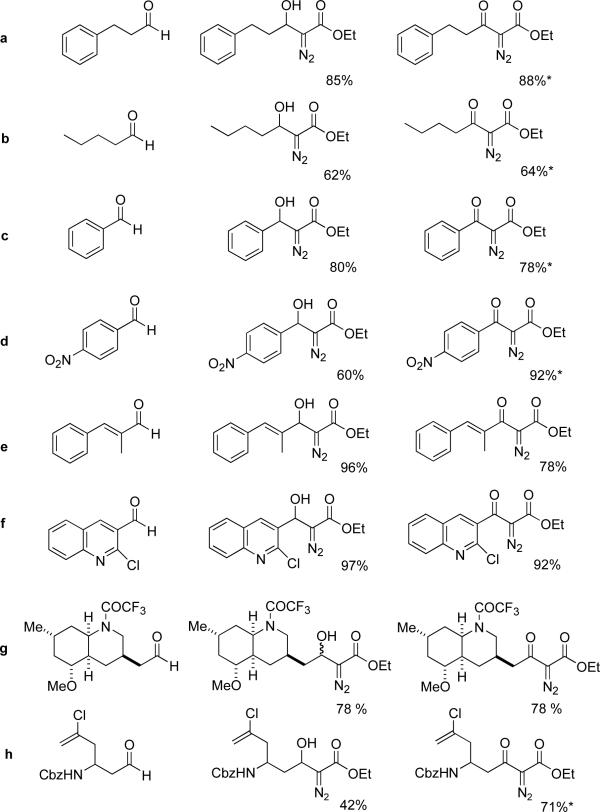 | |||
*∼12 equiv pyridine added to reaction mixture
General procedure for oxidation of α-diazo-β-hydroxyesters 3 to α-diazo-β-ketoesters 4
To a solution of alcohol 3 (0.51 mmol) in CH2Cl2 (7.5 mL) was added DMP (0.76 mmol). The heterogeneous mixture was stirred at rt until the complete consumption of the starting material was observed by TLC (∼1−3 h). The reaction mixture was diluted with a 1:1 mixture of NaHCO3(aq) and Na2S2O3(aq). The organic layer was separated and the aqueous layer was extracted with CH2Cl2. The combined organic layers were dried with Na2SO4 and the solvent was removed in vacuo. The resulting crude residue was purified by flash column chromatography on silica gel using a mixture of ethyl acetate and hexanes. Isolated yields of products 4 are shown in Table 1.
Acknowledgment
We are grateful to the National Institutes of Health (CA-034303) for financial support of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.a Regitz M. Angew. Chem. Int. Ed. 1967;6:733. For reviews and lead references see. [Google Scholar]; b Kurti L, Czako B. Strategic Applications of Named Reactions in Organic Synthesis. Elsevier; London: 2005. p. 376. [Google Scholar]
- 2.a Moody CJ, Taylor RJ. Tetrahedron Lett. 1987;28:5351. See inter alia. [Google Scholar]; b Padwa A, Kulkarni YS, Zhang Z. J. Org. Chem. 1990;55:4144. [Google Scholar]; c Moody CJ, Morfitt CN. Synthesis. 1998:1039. [Google Scholar]; d Hasegawa K, Arai S, Nishida A. Tetrahedron. 2006;62:1390. [Google Scholar]; e Varala R, Engula R, Nuvula S, Adapa SR. Tetrahedron Lett. 2006;47:877. [Google Scholar]; f Kantam ML, Chakrapani L, Ramani T. Tetrahedron Lett. 2007;48:6121. [Google Scholar]
- 3.Deng G, Xu B, Wang J. Tetrahedron. 2005;61:10811. [Google Scholar]
- 4.a Bagley MC, Hind SL, Moody CJ. Tetrahedron Lett. 2000;41:6897. [Google Scholar]; b Davies JR, Kane PD, Moody CJ, Slawin AMZ. J. Org. Chem. 2005;70:5840. doi: 10.1021/jo050303h. [DOI] [PubMed] [Google Scholar]; c Davies JR, Kane PD, Moody CJ. J. Org. Chem. 2005;70:7305. doi: 10.1021/jo0509760. [DOI] [PubMed] [Google Scholar]
- 5.a Moody CJ, Taylor RJ. Tetrahedron. 1990;46:6525. [Google Scholar]; b Padwa A, Dean DC, Osterhout MH, Precedo L, Semones MA. J. Org. Chem. 1994;59:5347. [Google Scholar]
- 6.Hodgson DM, Bailey JM, Villalonga-Barber C, Drew MGB, Harrison T. J. Chem. Soc., Perkin Trans. 2000;1:3432. [Google Scholar]
- 7.Dess DB, Martin JC. J. Org. Chem. 1983;48:4156. Pyridine has been used previously as an additive in Dess-Martin oxidations. [Google Scholar]