Abstract
Advancing age is the most significant risk factor for the development of Alzheimer's disease (AD), however the age-related changes that underlie this effect remain unclear. In men, one normal consequence of aging is a robust decline in circulating and brain levels of the sex steroid hormone testosterone. Testosterone depletion leads to functional impairments and increased risk of disease in androgen-responsive tissues throughout the body, including brain. In this review we discuss the relationship between age-related testosterone depletion and the development of AD. Specifically, we focus on androgen regulation of β-amyloid protein (Aβ), the accumulation of which is a key initiating factor in AD pathogenesis. Emerging data suggest that the regulatory actions of androgens on both Aβ and the development of AD support consideration of androgen therapy for the prevention and treatment of AD.
Keywords: Alzheimer’s disease, β-amyloid, androgen, dihydrotestosterone, estrogen, testosterone
1. Introduction
Alzheimer’s disease (AD) is a devastating neurodegenerative disease that affects over four and one half million people in the United States alone, a number that is projected to grow with the aging of the population (Brookmeyer, et al., 1998, Hebert, et al., 2003). Advancing age is the most significant risk factor for the development of AD (Evans, et al., 1989, Jorm, et al., 1987, Rocca, et al., 1986), however what age-related changes underlie this effect remain uncertain. In this review, we discuss recent work from our laboratory and others suggesting that one normal age-related change that may contribute to the risk of AD in men is testosterone loss. Further, we suggest that the mechanism by which androgen depletion increases risk of AD in men involves recent evidence that androgens regulate accumulation of β-amyloid (Aβ), perhaps the key event in AD pathogenesis.
1.1 Androgen depletion during normal male aging
With advancing age, men experience a significant decrease in circulating levels of testosterone (Morley, et al., 1997, Swerdloff and Wang, 1993). The decline in total testosterone levels begins in the thirties and progresses at an annual rate between 0.2%–1% (Feldman, et al., 2002, Gray, et al., 1991). Due to an age-related increase in sex hormone binding globulin (SHBG) at an annual rate of 1.1%–1.6% (Feldman, et al., 2002, Gray, et al., 1991, Harman, et al., 2001, Purifoy, et al., 1981, Vermeulen, et al., 1996), levels of bioavailable testosterone decrease at a higher rate (2%–3% per year) than total testosterone (Feldman, et al., 2002, Gray, et al., 1991, Muller, et al., 2003). While age changes in circulating DHT levels are not consistently reported (Feldman et al., 2002, Gray et al., 1991), decreases in other androgens including DHEA and androstendione are observed with increasing age in men (Feldman, et al., 2002, Gray, et al., 1991, Muller, et al., 2003, Vermeulen, et al., 1996). The age-associated decrease in testosterone is not paralleled by changes in estradiol levels, which decrease slightly or not at all in aging men (Davidson, et al., 1983, Muller, et al., 2003, Vermeulen, et al., 1996).
Normal age-related testosterone depletion has been associated with functional impairments in androgen-responsive tissues, including bone, muscle, and heart (Baumgartner, et al., 1999, Burger, et al., 1998, Ferrando, et al., 2002, Jones, et al., 2003, Meier, et al., 1987, Sheffield-Moore and Urban, 2004). Dysfunction and disease due to age-related testosterone loss has been collectively recognized as a clinical syndrome termed ‘androgen deficiency in aging males’ (Morley, 2001). Since the brain is also an androgen responsive tissue, it may be susceptible to age-related androgen loss. Although studies have reported alterations in mood, libido, and cognition resulting from androgen depletion (Gooren, 2003, Kaufman and Vermeulen, 2005, Morley, 2001, Swerdloff and Wang, 1993, Swerdloff and Wang, 2002), the full range of consequences of age-related testosterone loss on the brain remain incompletely defined.
Circulating levels of hormones generally parallel tissue levels, however factors such as sex hormone binding globulin, hormone transport across the blood brain barrier, and the presence of steroid converting enzymes in brain suggest that brain levels of hormones may vary from what is observed in blood (Manni, et al., 1985, Pardridge, 1985, Pardridge, 1986). Recent work from our lab was the first to examine age-related changes in brain levels of testosterone in men. Using neuropathologically normal human postmortem tissue we found a robust decrease in brain levels of testosterone with advancing age that appeared to reach minimal values in men over 80 years of age (Rosario et. al., 2004). Consistent with prior observations in circulating levels, we observed no significant change in brain levels of estradiol with increasing age in men (Rosario et al., 2004). The decrease in brain levels of androgens suggest the possibility that beneficial neural actions of androgens may be compromised during aging, resulting in increased risk of neural dysfunction and disease, including AD.
1.2 Androgen actions in the brain
Testosterone and its active metabolite dihydrotestosterone (DHT) have several important actions in the brain. Androgen actions are mediated in part via activation of androgen receptors (AR), which are localized in many brain areas including regions important for learning and memory such as hippocampus and amygdala (Kerr, et al., 1995, Simerly, et al., 1990, Tohgi, et al., 1995). Beneficial actions of androgens in the brain include stimulation of neuronal differentiation, maintenance of neuronal morphology, and promotion of synaptic density (Beyer and Hutchison, 1997, Leranth, et al., 2004, Marron, et al., 2005, Matsumoto, 1997). For example, studies of hippocampus in male rats show a significant decrease in the density of spine synapses following gonadectomy (GDX) (Kovacs, et al., 2003, Leranth, et al., 2003), an effect reversed by replacement with either testosterone or DHT (Kovacs, et al., 2003, Leranth, et al., 2003). In addition to androgen actions in neurons, testosterone has also been found to down-regulate astrogliosis (Day, et al., 1998).
Another beneficial neural action of androgens is regulation of neuron viability during developmental apoptosis (Lund, et al., 2000, Nordeen, et al., 1985, Nuñez, et al., 2000) and in adult brain following toxic challenge. Neuronal cell culture studies have revealed neuroprotective effects of androgens against serum deprivation (Brooks, et al., 1998, Hammond, et al., 2001), Aβ toxicity (Pike, 2001, Zhang, et al., 2004, Nguyen et al., 2005), and oxidative stress (Ahlbom, et al., 2001). In animal models, testosterone and DHT have been found to accelerate the rate of cranial nerve regeneration (Yu, 1982, Yu and Srinivasan, 1981) and attenuate motor neuron loss following axotomy (Yu, 1989). Similarly, following facial nerve crush in male hamsters, testosterone increased the rate of axonal growth and functional recovery (Kujawa, et al., 1991, Kujawa, et al., 1989). In addition, androgens have also been found to protect against toxic insult in hippocampus, a brain region vulnerable to neurodegenerative effects of AD. For example, Azocitia et al. (2001) found that androgen depletion resulting from GDX of adult male rodents increased neuron loss in the hilus of the dentate gyrus following excitotoxic lesion, an effect that was significantly attenuated by acute treatment with testosterone but not DHT. However, Ramsden et al. (2003a) found that extended DHT treatment in GDX male rats significantly blocked the increased hippocampal neuron death caused by the excitotoxin kainate. Because AR levels decrease following GDX, prolonged rather than acute androgen exposure may be necessary for neuroprotection since it allows for restoration of AR expression and consequently AR-dependent signaling (Ramsden et al., 2003a). Although acute treatment with the DHT metabolite 3α-androstanediol can protect against excitotoxin-induced seizures (Frye and Reed, 1998), we found that neuroprotection afforded by long-term DHT treatment was not associated with a decrease in either the latency or severity of kainate-induced seizure (Ramsden, et al., 2003a). Thus, available evidence suggests that androgens can afford neuroprotection through a variety of pathways, including both estrogen and androgen pathways.
2. Androgens and Alzheimer’s disease
2.1 Androgens and cognition
Androgens are known to affect some aspects of cognition including spatial abilities (Gouchie and Kimura, 1991, Janowsky, et al., 1994) and verbal fluency (Alexander, et al., 1998). Low levels of androgens have been associated with impaired cognitive performance in some but not all studies (Haren, et al., 2005, Moffat, et al., 2002). Men with a relatively higher free testosterone index performed better on visual and verbal memory tasks and exhibited better long-term memory (Barrett-Connor, et al., 1999) while those with low free testosterone showed decreased visual memory, visuomotor scanning, verbal memory, and visuospatial processing (Moffat, et al., 2002). In men with low testosterone androgen therapy may improve some cognitive abilities. For example, verbal fluency was increased in hypogonadal and eugonadal men treated with either intramuscular injections of testosterone enanthate, which has the addition of an ester group allowing for longer action than testosterone, or oral administration of sublingual testosterone cyclodextrin, which is surrounded by a carbohydrate ring facilitating absorption of T through the oral mucosa (Alexander, et al., 1998). In a small clinical study of men recently diagnosed with AD, testosterone treatment resulted in improved performance on both the mini-mental status exam and the clock drawing test (CDT) (Tan and Pu, 2003). Overall, findings with testosterone treatment in cases with AD and mild cognitive impairments have shown mixed results, with beneficial results in some but not all studies (Cherrier, et al., 2005, Lu, et al., 2006, Tan and Pu, 2003).
Since testosterone levels tend to positively correlate with at least some aspects of cognition, pharmacological depletion of androgens and inhibition of androgen signaling in men may be predicted to yield deleterious cognitive consequences. Consistent with this possibility, anti-androgen therapies used for the treatment of prostate cancer (e.g., leuprolide, cyproterone acetate) have been associated with cognitive impairments (Green, 2002, Salminen, et al., 2004). Conversely, the discontinuation of anti-androgen therapy was reported to restore cognitive performance, in particular verbal memory (Almeida and Papadopoulos, 2003). Similarly, a study evaluating the effect of cycling anti-androgen therapy found that androgen blockade negatively affected spatial memory (Cherrier, et al., 2003). Together, this literature suggests that androgens significantly modulate specific aspects of cognition, and that androgen depletion – either through normal aging or pharmacological action – can result in specific cognitive impairments.
2.2 Androgen depletion and risk for Alzheimer’s disease in men
Since androgens decrease with age and have several beneficial neural actions, low androgen levels may place the brain at increased risk for dysfunction and the development of disease. In agreement with this prediction, accumulating data from our group and others indicate that one consequence of normal, age-related androgen depletion in men is an increased risk for AD (Pike et al., 2006).
Several studies now confirm that circulating levels of testosterone are significantly lower in men with AD in comparison to age-matched, non-demented men, a relationship that appears strongest in men younger than 80 years of age (Hogervorst, et al., 2003, Hogervorst, et al., 2002, Hogervorst, et al., 2001, Rasmuson, et al., 2002, Watanabe, 2004, Paoletti, et al., 2004). In contrast to these studies, some studies have not found differences in testosterone levels between AD cases and controls (Pennanen, et al., 2004, Twist, et al., 2000), however small sample size may be contributing to the conflicting results. A variety of factors may affect the relationship between AD and low testosterone levels. For example, the apolipoprotein ε4 allele risk factor for AD may interact with testosterone. Hogervorst and colleagues (2002) found not only that low circulating levels of testosterone in men are associated with AD, but also that cases with at least one apolipoprotein ε4 allele had significantly lower levels of testosterone than those cases without the ε4 allele (Hogervorst, et al., 2002). It remains unclear how apolipoprotein ε4 interacts with testosterone in modulating the risk for AD. In mice genetically engineered to express human apolipoprotein ε4, female animals showed deficits in learning and memory that was prevented by androgen treatment whereas males showed deficits upon antagonism of the androgen receptor (Raber, et al., 2002). Interestingly, a recent study examining the relationship between testosterone levels, apolipoprotein ε4 and cognition in aged men found that in non- ε4 carriers, higher levels of testosterone were associated with better cognitive outcomes. In contrast, in individuals with an ε4 allele, higher levels of testosterone were not associated with better overall cognition (Burkhardt, et al., 2006).
Although several studies identified a relationship between low testosterone in aging men and AD, they did not clearly determine whether low testosterone is a result of the disease process or rather contributes to its development. In a prospective longitudinal study using subjects from the Baltimore Longitudinal Study on Aging, men that developed AD were observed to exhibit lower testosterone levels 5–10 years prior to clinical diagnosis of AD (Moffat, et al., 2004). In complementary work from our lab, we found that testosterone depletion appeared to occur before the development of AD neuropathology. Using samples of human postmortem brain tissue, we purified and quantified brain levels of sex steroid hormones in men neuropathologically diagnosed as either normal (Braak stage 0–1), mild neuropathological changes (Braak stage 2–3), or advanced AD (Braak stage 5–6) (Rosario et. al., 2004). We found significantly lower brain levels of testosterone in cases with advanced AD in comparison to neuropathologically normal cases, thereby confirming in brain the relationship between low testosterone and AD. Importantly, we also observed significantly lower brain levels of testosterone in men with mild neuropathological changes. This result indicates that brain levels of testosterone are reduced prior to neuropathological development of AD, suggesting that low testosterone is a factor that contributes to rather than results from the development of AD (Rosario et. al., 2004).
2.3 Relationship between luteinizing hormone and Alzheimer’s disease
Recently, elevated gonadotropin levels, specifically luteinizing hormone (LH), have also been linked to an increased risk of AD in men (Bowen, et al., 2000, Short, et al., 2001). Some have suggested that age-related increases in LH may be more relevant to AD pathogenesis than the associated decline in testosterone levels (Casadesus et al., 2004). The interactions between age-related LH increase, testosterone depletion, and increased risk for AD in men remain to be fully elucidated. However, one possibility is that the LH relationship with AD may be most important in late stages of male aging. Significant increases in circulating LH levels appear to occur rather late in normal male aging, often not becoming significant until age 80 (Morley, et al., 1997). In a study of men with a mean age of 75 years, AD was associated with low testosterone but not with either LH or FSH levels (Hogervorst, et al., 2003). In contrast, in the study by Bowen et al. (2000) which found an association between elevated LH and AD, the mean age of the men was 85 years. In our studies, we find that brain levels of testosterone are significantly lower in AD cases in men aged 60–80 years (Rosario et al., 2004), but that there is no further decline in testosterone levels in men over the age of 80 and no significant relationship between testosterone and AD in this older age group (unpublished observations). Given the current knowledge on this subject, we speculate that both testosterone and LH may be relevant to AD pathogenesis, and that the testosterone relationship may be more important in early and middle stages of male aging.
3. Androgen regulation of β-amyloid
With a relationship between age-related testosterone depletion in men and increased risk for AD reasonably well established, a critical issue is how androgen loss promotes AD pathogenesis. Perhaps the most likely possibility is through regulation of β-amyloid (Aβ) accumulation, which is widely believed to be the critical initiating step in AD pathogenesis (Hardy and Selkoe, 2002). As discussed below, evidence from our lab and others suggest that androgens function as negative endogenous regulators of Aβ, and thus the age-related loss of androgens is predicted to increase brain levels of Aβ and consequentially the risk of AD.
3.1 Androgens correlate with Aβ levels in men
Some of the initial evidence linking androgen action with regulation of Aβ came from studies of men undergoing anti-androgen therapy for prostate cancer. Upon combined treatment with the AR antagonist flutamide and the GnRH agonist leuprolide acetate, men exhibited decreased circulating levels of estrogen, testosterone, and gonadotropins as well as increased circulating levels of Aβ (Almeida and Papadopoulos, 2003, Gandy, et al., 2001). A relationship between androgens and Aβ has also been observed during normal male aging in which circulating levels of Aβ were inversely associated with circulating levels of testosterone but unrelated to estradiol levels (Gillett, et al., 2003). Using human postmortem tissue, we compared the relationship between brain levels of soluble Aβ and sex steroid hormone levels in aging men. In cases with mild neuropathological changes (Braak stage 2–3), we found that brain levels of testosterone significantly and inversely correlated with brain levels of soluble Aβ (unpublished observations), suggesting that age-related testosterone depletion may result in elevated brain levels of Aβ and thereby increase the risk of AD.
3.2 Androgen regulation of Aβ in cell culture studies
Perhaps the first association between androgens and Aβ regulation came from cell culture studies reporting that Aβ levels in cultures of murine neuroblastoma N2a cells and rat primary neurons were reduced by testosterone treatment (Gouras, et al., 2000). Because this study utilized testosterone at rather high concentrations and for extended time periods, it raised the possibility that the observed testosterone effect was mediated via aromatization to estradiol, a previously established pathway of Aβ regulation (Xu, et al., 1998). In fact, subsequent work found that the ability of testosterone to decrease Aβ levels in a different culture paradigm was blocked in the presence of aromatase inhibitors (Goodenough, et al., 2000). Regardless of whether the mechanism involves estrogen and or androgen pathways, these initial culture data suggest that androgens can regulate neural Aβ levels.
3.3 Androgen regulation of Aβ in rodent models
Consistent with predictions from cell culture studies, recent work from our lab demonstrated that androgens regulate brain levels of Aβ in adult male rodents (Ramsden, et al., 2003b). We reasoned that if androgens are endogenous negative regulators of Aβ, then androgen depletion should increase Aβ levels in brain. Consistent with this prediction, we found that after six weeks of androgen depletion in adult male rats induced by gonadectomy (GDX), soluble brain levels of Aβ were significantly increased in comparison to sham GDX rats (Ramsden, et al., 2003b). Suggesting an androgen rather than estrogen mechanism of action, we also found that treatment of GDX male rats for four weeks with the non-aromatized androgen DHT completely prevented the increase in brain Aβ levels caused by GDX (Ramsden, et al., 2003b). In ongoing studies, we are investigating the extent to which normal, age-related androgen depletion in male rodents affects Aβ similarly to GDX-induced androgen depletion.
The studies discussed thus far, ranging from human analyses to animal models to cell culture paradigms, consistently predict a relationship between low testosterone in men and increased risk of AD through a mechanism involving at least in part regulation of Aβ. To directly test this idea, we recently investigated how androgen status affects the progression of AD-like pathology in a triple transgenic mouse model of AD (3xTg-AD). We observed that the age-dependent accumulation of Aβ in the subiculum, hippocampus CA1, and amygdala of male 3xTg-AD mice was significantly accelerated following GDX-induced androgen depletion in adulthood (Rosario et. al., 2006). Importantly, DHT treatment at the time of GDX prevented the acceleration of Aβ accumulation (Fig. 1), suggesting an androgen receptor (AR)-dependent mechanism of Aβ regulation. We also found that androgen status regulated the development of memory deficits in this model. Specifically, we observed that GDX worsened the performance of male 3xTg-AD mice in a spontaneous alternation behavior in the Y-maze, a hippocampal-dependent task of working memory (Rosario et. al., 2006). DHT treatment of GDX 3xTg-AD mice rescued their behavioral deficit. Because androgen status did not affect spontaneous alternation behavior in wild-type mice of the 3xTg-AD background strain (Rosario et. al., 2006), these data suggest that androgens regulated behavioral deficits via underlying effects on pathology (e.g., Aβ accumulation) rather than direct actions on behavior.
Figure 1.
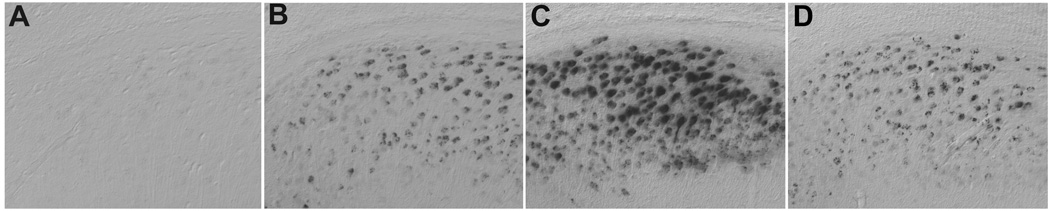
Androgens regulate accumulation of Aβ in the triple transgenic model of AD (3xTg-AD). Immunostaining with anti-Aβ antibodies in adult (age 7 mo) male mice shows absent Aβ immunoreactivity in subiculum of wild-type mice (A), but significant intracellular accumulation in 3xTg-AD mice (B). 3xTg-AD mice that were androgen depleted by gonadectomy (GDX) at age 3 mo show a robust increase in Aβ accumulation at age 7 mo (C), an effect prevented by DHT treatment beginning immediately after GDX (D).
4. Mechanism of androgen regulation of Aβ
Beneficial actions of androgens such as neuron viability and modulation of Aβ levels support the hypothesis that age-related androgen depletion may increase the risk of developing AD. As previously discussed, several studies have identified androgens as endogenous regulators of Aβ (Gandy et al., 2001, Gouras et al., 2000, Gillett, et al., 2003, Ramsden, et al., 2003b, Rosario et. al., 2006). The mechanism(s) by which androgens regulate Aβ is not known, but presumably involves one or more of three general pathways; direct actions through AR-dependent pathways, indirect actions through estrogen pathways via testosterone aromatization to estradiol, indirect actions through gonadotropin pathways via testosterone modulation of the hypothalamic-pituitary-gonadal axis.
4.1 Androgen regulation of Aβ through direct androgen pathways
Testosterone has been shown to regulate levels of Aβ in cell culture, rodent models, and human brain (as reviewed in Pike et al., 2006). Work from our lab indicates that androgen, not estrogen, pathways are responsible for the regulation of Aβ levels in males (Ramsden, et al., 2003b). Aβ levels were significantly increased following GDX, and this effect was completely blocked by DHT treatment (Ramsden, et al., 2003b). Because DHT is not aromatized to estradiol, these data would appear to suggest that the mechanism is independent of estrogen receptors (ER) and likely involves AR. However, recent observations demonstrate that the DHT metabolite 5α-androstan-3β, 17 β-diol can act as an agonist for ERβ (Lund et al., 2006), leaving open the possibility that DHT actions may be mediated indirectly through ERβ. However, in the Ramsden et al., (2003b) study, treatment of GDX male rats with estradiol did not reverse the GDX-induced elevation in Aβ, suggesting that androgen rather than estrogen pathways regulate Aβ levels in this model. In our recent study of androgen regulation of neuropathology in male 3xTg-AD mice, we also found that DHT blocked increased Aβ accumulation resulting from GDX (Rosario et. al., 2006). Initial evidence suggests that estradiol treatment of GDX male 3xTg-AD mice can partially reduce Aβ accumulation in a region-specific manner, but that estradiol is generally less effective than either DHT or testosterone (unpublished observations). In aging men, circulating levels of androgens but not estrogen were correlated with Aβ (Gillett, et al., 2003). Recent cell culture studies in our lab have identified a novel AR-dependent mechanism in which androgens reduce Aβ levels by increasing expression of neprilysin, an important Aβ-catabolizing enzyme (unpublished observations). Thus, available evidence is consistent with a direct androgen mechanism but does not exclude potential roles of indirect androgen actions through estrogen and perhaps gonadotropin pathways.
4.2 Androgen regulation of Aβ through estrogen pathways
Similar to androgens, estrogen has also been found to be an endogenous regulator of Aβ. In cell culture models, 17β-estradiol has been shown to reduce Aβ levels directly by assay of soluble Aβ and or indirectly by demonstration of increased levels of soluble APPα (sAPPα), a proteolytic product of non-amyloidogenic APP metabolism (Jaffe, et al., 1994, Xu, et al., 1998, Chang, et al., 1997, Vincent and Smith, 2000). These studies indicate that estrogen regulation of Aβ is likely mediated by regulation of APP processing and or APP trafficking (Greenfield, et al., 2002), perhaps through activation of mitogen-activated protein kinase-signaling (MAPK) pathway (Manthey, et al., 2001). Although the precise mechanism remains to be fully elucidated, the cell culture studies all suggest that estrogen modulates Aβ levels by regulating its production from APP.
Estrogen regulation of Aβ levels has also been observed in wild-type female rodents (Petanceska, et al., 2000) and in some but not all rodent models of AD (Zheng, et al., 2002, Levin-Allerhand, et al., 2002, Heikkinen, et al., 2004, Yue, et al., 2005, Green et al., 2005). It is unclear why estrogen apparently fails to regulate Aβ levels in some mouse models of AD, but there are several potentially important differences across these studies, including mouse strains and experimental parameters such as age, dose of estrogen, method of Aβ assay, and duration of hormonal manipulation. In those in vivo studies that reported estrogen regulation of Aβ, the mechanism was not clear. In contrast to cell culture studies, there is some evidence that estrogen may not regulate sAPPα levels (Petanceska, et al., 2000, Savage, et al., 1998). Interestingly, one report indicates that estrogen may increase the activity of the Aβ-catabolizing enzyme neprilysin (Huang, et al., 2004), suggesting a possible effect of estrogen on Aβ clearance rather than on Aβ production.
4.3 Androgen regulation of Aβ through gonadotropin pathways
In addition to direct activation of AR-dependent signaling and indirect activation of estrogen pathways, androgens may affect Aβ levels indirectly by regulation of the hypothalamic-pituitary-gonadal axis and the gonadotropin luteinizing hormone (LH). Because testosterone loss results in diminished negative feedback on the hypothalamic-pituitary-gonadal axis, it also results in elevated LH levels, which some have theorized might increase risk of AD (Casadesus et al., 2004). Perhaps consistent with this idea are recent data demonstrating that treatment of female mice with the GnRH agonist leuprolide acetate, which presumably suppressed LH levels, resulted in reduced levels of Aβ (Bowen, et al., 2004). In a transgenic mouse model of AD, 3 mo treatment with leuprolide acetate resulted in decreased Aβ accumulation and a reduction in cognitive impairments (Casadesus, et al., 2006). Further, LH treatment in a neuroblastoma cell line increased levels of secreted Aβ and decreased levels of sAPPα, suggesting LH may promote amyloidogenic processing of APP (Bowen, et al., 2004). Gonadotropins may also play a role in regulation Aβ levels through changing expression of presenilins, which are important mediators of Aβ production (for review see Barron, et al., 2006). Although accumulating data continue to implicate androgens as endogenous regulators of Aβ, the relative roles of the various direct and indirect mechanisms of androgen action in this effect remain to be established.
5. Conclusions and future directions
In this review, we have discussed recent evidence from a number of different research groups, including our own, that collectively indicate a significant relationship between normal, age-related testosterone depletion in men and increased risk for the development of AD. Because androgens exert a variety of beneficial actions in brain, androgen loss may negatively impact the aging brain and increase its vulnerability to age-related neurodegenerative diseases by a variety of mechanisms, including regulation of neuron viability, neuronal plasticity, and glial activation. Importantly, because beneficial neural actions of androgens affect many general features of neural health and functioning, we speculate that androgen loss may be relevant not only to AD but also several other neurodegenerative disorders. Perhaps most relevant to the discussion of AD is evidence that androgens can reduce levels of Aβ, the accumulation of which is thought to initiate and drive AD pathogenesis. Available evidence highlights three general pathways by which androgens may regulate Aβ levels and vulnerability to AD: directly through AR-dependent pathways, indirectly through estrogen pathways after testosterone conversion to 17β-estradiol or DHT conversion to 5α-androstan-3β,17 β-diol, and indirectly through gonadotropin actions as a consequence of androgen regulation of the hypothalamic-pituitary-gonadal axis (Fig. 2). Although currently there is insufficient available data to determine the relative importance of these different androgen pathways, recent data from our laboratory is most consistent with a primary role of direct AR-dependent signaling pathways resulting in increased Aβ clearance. As these novel and important findings are pursued in future studies, it will be important to address the role of aging, which results not only in altered hormone levels but often in diminished hormone responsiveness.
Figure 2.

Androgen regulation of Aβ may involve three general pathways. Aging decreases testosterone, which can reduce Aβ levels directly by androgen receptor (AR)-dependent regulation of the Aβ-catabolizing enzyme neprilysin (NEP) and indirectly by aromatization to 17β-estradiol, which has been shown to reduce amyloidogenic processing of the Aβ precursor protein (APP). Through regulation of the hypothalamic-pituitary-gonadal axis, age-related testosterone depletion also elevates LH levels, which have been associated with increased Aβ by an incompletely defined mechanism that may include the LH receptor (LHR)
The obvious and clinically important implication from these data is that androgen therapy may provide an effective and relatively safe means to prevent and or treat AD in aging men with low testosterone. There are potential adverse risks associated with androgen therapy, including prostatic carcinoma, atherosclerosis, breast carcinoma, and hypertension (for review see Kaufman and Vermeulen, 2005). The principal concern is the development of prostate cancer since the majority of prostate cancers are androgen sensitive, at least initially (Goldenberg, et al., 1995). Despite these potential risks, there is little direct evidence that androgen therapy is associated with increased incidence of prostate cancer (Raynaud, 2006, Kaufman and Vermeulen, 2005). For example, in a recent clinical trial, short-term (6 months) androgen therapy was effective in regulating serum levels of androgens in hypogonadal men but had no adverse effects on prostate tissue (Marks, et al., 2006). Because extended androgen therapy will likely be required for maximal efficacy in preventing AD, long-term safety is an issue that must be addressed. Future research should continue to elucidate the mechanisms underlying neural actions of androgens and begin to utilize these advances in the rational development of selective androgen receptor modulators that ideally will mimic beneficial androgen actions and minimize those linked to adverse outcomes. Importantly, future studies must also address the role of aging, which often results not only in altered hormone levels but also diminished hormone responsiveness.
Acknowledgement
This work was supported by grants from the NIA (AG23739; CJP) and NINDS (NS52143; ERR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Swerdloff RS, Wang C, Davidson T, McDonald V, Steiner B, Hines M. Androgen-behavior correlations in hypogonadal men and eugonadal men. II. Cognitive abilities. Horm Behav. 1998;33:85–94. doi: 10.1006/hbeh.1998.1439. [DOI] [PubMed] [Google Scholar]
- Almeida TA, Papadopoulos N. Progression model of prostate cancer. Methods Mol Biol. 2003;222:211–222. doi: 10.1385/1-59259-328-3:211. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurobiol. 2001;47:318–329. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84:3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- Barron AM, Verdile G, Martins RN. The role of gonadotropins in Alzheimer's disease: potential neurodegenerative mechanisms. Endocrine. 2006;29:257–269. doi: 10.1385/ENDO:29:2:257. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–136. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- Beyer C, Hutchison JB. Androgens stimulate the morphological maturation of embryonic hypothalamic aromatase-immunoreactive neurons in the mouse. Dev. Brain. Res. 1997;98:74–81. doi: 10.1016/s0165-3806(96)00170-8. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Isley JP, Atkinson RL. An association of elevated serum gonadotropin concentrations and Alzheimer disease? J Neuroendocrinol. 2000;12:351–354. doi: 10.1046/j.1365-2826.2000.00461.x. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J Biol Chem. 2004;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BP, Merry DE, Paulson HL, Lieberman AP, Kolson DL, Fischbeck KH. A cell culture model for androgen effects in motor neurons. J Neurochem. 1998;70:1054–1060. doi: 10.1046/j.1471-4159.1998.70031054.x. [DOI] [PubMed] [Google Scholar]
- Burkhardt MS, Foster JK, Clarnette RM, Chubb SA, Bruce DG, Drummond PD, Martins RN, Yeap BB. Interaction between testosterone and apolipoprotein E epsilon4 status on cognition in healthy older men. J Clin Endocrinol Metab. 2006;91:1168–1172. doi: 10.1210/jc.2005-1072. [DOI] [PubMed] [Google Scholar]
- Burger H, de Laet CE, van Daele PL, Weel AE, Witteman JC, Hofman A, Pols HA. Risk factors for increased bone loss in an elderly population: the Rotterdam Study. Am J Epidemiol. 1998;147:871–879. doi: 10.1093/oxfordjournals.aje.a009541. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA. Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta. 2006;1762:447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Zhu X, Atwood CS, Webber KN, Perry G, Bowen RL, Smith MA. Curr Drug Targets CNS Neurol Dis. 2004;3:28–285. doi: 10.2174/1568007043337265. [DOI] [PubMed] [Google Scholar]
- Chang D, Kwan J, Timiras PS. Estrogens influence growth, maturation, and amyloid beta-peptide production in neuroblastoma cells and in a beta-APP transfected kidney 293 cell line. Adv Exp Med Biol. 1997;429:261–271. doi: 10.1007/978-1-4757-9551-6_19. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Rose AL, Higano C. The effects of combined androgen blockade on cognitive function during the first cycle of intermittent androgen suppression in patients with prostate cancer. J Urol. 2003;170:1808–1811. doi: 10.1097/01.ju.0000091640.59812.83. [DOI] [PubMed] [Google Scholar]
- Davidson JM, Chen JJ, Crapo L, Gray GD, Greenleaf WJ, Catania JA. Hormonal changes and sexual function in aging men. J Clin Endocrinol Metab. 1983;57:71–77. doi: 10.1210/jcem-57-1-71. [DOI] [PubMed] [Google Scholar]
- Day JR, Frank AT, O'Callaghan JP, Jones BC, Anderson JE. The effect of age and testosterone on the expression of glial fibrillary acidic protein in the rat cerebellum. Exp Neurol. 1998;151:343–346. doi: 10.1006/exnr.1998.6801. [DOI] [PubMed] [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer's disease in a community population of older persons Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–E607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- Frye CA, Reed TA. Androgenic neurosteroids: antiseizure effects in an animal model of epilepsy. Psychoneuroendocrinology. 1998;23:385–399. doi: 10.1016/s0306-4530(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Gandy S, Almeida OP, Fonte J, Lim D, Waterrus A, Spry N, Flicker L, Martins RN. Chemical andropause and amyloid-beta peptide. JAMA. 2001;285:2195–2196. doi: 10.1001/jama.285.17.2195-a. [DOI] [PubMed] [Google Scholar]
- Gillett MJ, Martins RN, Clarnette RM, Chubb SA, Bruce DG, Yeap BB. Relationship between testosterone, sex hormone binding globulin and plasma amyloid beta peptide 40 in older men with subjective memory loss or dementia. J Alzheimers Dis. 2003;5:267–269. doi: 10.3233/jad-2003-5401. [DOI] [PubMed] [Google Scholar]
- Goldenberg SL, Bruchovsky N, Gleave ME, Sullivan LD, Akakura K. Intermittent androgen suppression in the treatment of prostate cancer: a preliminary report. Urology. 1995;45:839–844. doi: 10.1016/s0090-4295(99)80092-2. [DOI] [PubMed] [Google Scholar]
- Goodenough S, Engert S, Behl C. Testosterone stimulates rapid secretory amyloid precursor protein release from rat hypothalamic cells via the activation of the mitogen-activated protein kinase pathway. Neurosci Lett. 2000;296:49–52. doi: 10.1016/s0304-3940(00)01622-0. [DOI] [PubMed] [Google Scholar]
- Gooren L. Androgen deficiency in the aging male: benefits and risks of androgen supplementation. J Steroid Biochem Mol Biol. 2003;85:349–355. doi: 10.1016/s0960-0760(03)00206-1. [DOI] [PubMed] [Google Scholar]
- Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;16:323–334. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Xu H, Gross RS, Greenfield JP, Hai B, Wang R, Greengard P. Testosterone reduces neuronal secretion of Alzheimer's beta-amyloid peptides. Proc Natl Acad Sci USA. 2000;97:1202–1205. doi: 10.1073/pnas.97.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- Green HJ. Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing analogues and cyproterone acetate: a randomized controlled trial. BJU Int. 2002;90:427–432. doi: 10.1046/j.1464-410x.2002.02917.x. [DOI] [PubMed] [Google Scholar]
- Green PS, Bales K, Paul S, Bu G. Estrogen therapy fails to alter amyloid deposition in the PDAPP model of Alzheimer's disease. Endocrinology. 2005;146:2774–2781. doi: 10.1210/en.2004-1433. [DOI] [PubMed] [Google Scholar]
- Greenfield JP, Leung LW, Cai D, Kaasik K, Gross RS, Rodriguez_Boulan E, Greengard P, Xu H. Estrogen lowers Alzheimer beta-amyloid generation by stimulating trans-Golgi network vesicle biogenesis. J Biol Chem. 2002;277:12128–12136. doi: 10.1074/jbc.M110009200. [DOI] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Haren MT, Wittert GA, Chapman IM, Coates P, Morley JE. Effect of oral testosterone undecanoate on visuospatial cognition, mood and quality of life in elderly men with low-normal gonadal status. Maturitas. 2005;50:124–133. doi: 10.1016/j.maturitas.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Kalesnykas G, Rissanen A, Tapiola T, Iivonen S, Wang J, Chaudhuri J, Tanila H, Miettinen R, Puolivali J. Estrogen treatment improves spatial learning in APP + PS1 mice but does not affect beta amyloid accumulation and plaque formation. Exp Neurol. 2004;187:105–117. doi: 10.1016/j.expneurol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Combrinck M, Smith AD. Testosterone and gonadotropin levels in men with dementia. Neuroendocrinol Lett. 2003;24:203–208. [PubMed] [Google Scholar]
- Hogervorst E, Lehmann DJ, Warden DR, McBroom J, Smith AD. Apolipoprotein E epsilon4 and testosterone interact in the risk of Alzheimer's disease in men. Int J Ger Psych. 2002;17:938–940. doi: 10.1002/gps.714. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Barnetson L, Combrinck M, Smith AD. Serum total testosterone is lower in men with Alzheimer's disease. Neuroendocrinol Lett. 2001;22:163–168. [PubMed] [Google Scholar]
- Huang J, Guan H, Booze RM, Eckman CB, Hersh LB. Estrogen regulates neprilysin activity in rat brain. Neurosci Lett. 2004;367:85–87. doi: 10.1016/j.neulet.2004.05.085. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Toran_Allerand CD, Greengard P, Gandy SE. Estrogen regulates metabolism of Alzheimer amyloid beta precursor protein. J Biol Chem. 1994;269:13065–13068. [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- Jones RD, Pugh PJ, Hall J, Channer KS, Jones TH. Altered circulating hormone levels, endothelial function and vascular reactivity in the testicular feminised mouse. Eur J Endocrinol. 2003;148:111–120. doi: 10.1530/eje.0.1480111. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76:465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005 doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Kovacs EG, MacLusky NJ, Leranth C. Effects of testosterone on hippocampal CA1 spine synaptic density in the male rat are inhibited by fimbria/fornix transection. Neuroscience. 2003;122:807–810. doi: 10.1016/j.neuroscience.2003.08.046. [DOI] [PubMed] [Google Scholar]
- Kujawa KA, Emeric E, Jones KJ. Testosterone differentially regulates the regenerative properties of injured hamster facial motoneurons. J Neurosci. 1991;11:3898–3906. doi: 10.1523/JNEUROSCI.11-12-03898.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa KA, Kinderman NB, Jones KJ. Testosterone-induced acceleration of recovery from facial paralysis following crush axotomy of the facial nerve in male hamsters. Exp Neurol. 1989;105:80–85. doi: 10.1016/0014-4886(89)90174-x. [DOI] [PubMed] [Google Scholar]
- Lehmann DJ, Butler HT, Warden DR, Combrinck M, King E, Nicoll JA, Budge MM, de Jager CA, Hogervorst E, Esiri MM, Ragoussis J, Smith AD. Association of the androgen receptor CAG repeat polymorphism with Alzheimer's disease in men. Neurosci Lett. 2003;340:87–90. doi: 10.1016/s0304-3940(03)00069-7. [DOI] [PubMed] [Google Scholar]
- Lehmann DJ, Hogervorst E, Warden DR, Smith AD, Butler HT, Ragoussis J. The androgen receptor CAG repeat and serum testosterone in the risk of Alzheimer's disease in men. J Neurol Neurosurg Psych. 2004;75:163–164. [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J.Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Prange-Kiel J, Frick KM, Horvath TL. Low CA1 spine synapse density is further reduced by castration in male non-human primates. Cereb Cortex. 2004;14:503–510. doi: 10.1093/cercor/bhh012. [DOI] [PubMed] [Google Scholar]
- Levin-Allerhand JA, Lominska CE, Wang J, Smith JD. 17alpha-estradiol and 17beta-estradiol treatments are effective in lowering cerebral amyloid-beta levels in AbetaPPSWE transgenic mice. 2002;4:449–457. doi: 10.3233/jad-2002-4601. [DOI] [PubMed] [Google Scholar]
- Lu PH, Masterman DA, Mulnard R, Cotman C, Miller B, Yaffe K, Reback E, Porter V, Swerdloff R, Cummings JL. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Arch Neurol. 2006;63:177–185. doi: 10.1001/archneur.63.2.nct50002. [DOI] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Salyer DL, Fleming DE, Lephart ED. Pre- or postnatal testosterone and flutamide effects on sexually dimorphic nuclei of the rat hypothalamus. Dev Brain Res. 2000;120:261–266. doi: 10.1016/s0165-3806(00)00013-4. [DOI] [PubMed] [Google Scholar]
- Manni A, Pardridge WM, Cefalu W, Nisula BC, Bardin CW, Santner SJ, Santen RJ. Bioavailability of albumin-bound testosterone. J Clin Endocrinol Metab. 1985;61:705–710. doi: 10.1210/jcem-61-4-705. [DOI] [PubMed] [Google Scholar]
- Manthey D, Heck S, Engert S, Behl C. Estrogen induces a rapid secretion of amyloid beta precursor protein via the mitogen-activated protein kinase pathway. Eur J Biochem. 2001;268:4285–4291. doi: 10.1046/j.1432-1327.2001.02346.x. [DOI] [PubMed] [Google Scholar]
- Marks LS, Mazer NA, Mostaghel E, Hess DL, Dorey FJ, Epstein JI, Veltri RW, Makarov DV, Partin AW, Bostwick DG, Macairan ML, Nelson PS. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296:2351–2361. doi: 10.1001/jama.296.19.2351. [DOI] [PubMed] [Google Scholar]
- Marron TU, Guerini V, Rusmini P, Sau D, Brevini TA, Martini L, Poletti A. Androgen-induced neurite outgrowth is mediated by neuritin in motor neurones. J Neurochem. 2005;92:10–20. doi: 10.1111/j.1471-4159.2004.02836.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A. Hormonally induced neuronal plasticity in the adult motoneurons. Brain Res Bull. 1997;44:539–547. doi: 10.1016/s0361-9230(97)00240-2. [DOI] [PubMed] [Google Scholar]
- Meier DE, Orwoll ES, Keenan EJ, Fagerstrom RM. Marked decline in trabecular bone mineral content in healthy men with age: lack of association with sex steroid levels. J Am Geriatr Soc. 1987;35:189–197. doi: 10.1111/j.1532-5415.1987.tb02308.x. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- Morley JE. Androgens and aging. Maturitas. 2001;38:61–71. doi: 10.1016/s0378-5122(00)00192-4. [DOI] [PubMed] [Google Scholar]
- Morley JE. Testosterone replacement in older men and women. J Gend Specif Med. 2001;4:49–53. [PubMed] [Google Scholar]
- Morley JE, Kaiser FE, Perry HM, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metab Clin Exp. 1997;46:410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94:1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, Jurgens HA, Juraska JM. Androgens reduce cell death in the developing rat visual cortex. Dev. Brain Res. 2000;125:83–88. doi: 10.1016/s0165-3806(00)00126-7. [DOI] [PubMed] [Google Scholar]
- Paoletti AM, Congia S, Lello S, Tedde D, Orru M, Pistis M, Pilloni M, Zedda P, Loddo A, Melis GB. Low androgenization index in elderly women and elderly men with Alzheimer's disease. Neurology. 2004;62:301–303. doi: 10.1212/01.wnl.0000094199.60829.f5. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Transport of protein-bound hormones into tissues in vivo. Endo Rev. 1985;2:103–123. doi: 10.1210/edrv-2-1-103. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Serum bioavailability of sex steroid hormones. Clin Endocrinol Metabol. 1986;15:259–278. doi: 10.1016/s0300-595x(86)80024-x. [DOI] [PubMed] [Google Scholar]
- Pennanen C, Laakso MP, Kivipelto M, Ramberg J, Soininen H. Serum testosterone levels in males with Alzheimer's disease. J Neuroendocrinol. 2004;16:95–98. doi: 10.1111/j.0953-8194.2004.01133.x. [DOI] [PubMed] [Google Scholar]
- Petanceska SS, Nagy V, Frail D, Gandy S. Ovariectomy and 17beta-estradiol modulate the levels of Alzheimer's amyloid beta peptides in brain. Exp Gerontol. 2000;35:1317–1325. doi: 10.1016/s0531-5565(00)00157-1. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Rosario ER, Nguyen TV. Androgens, aging, and Alzheimer's disease. Endocrine. 2006;29:233–241. doi: 10.1385/ENDO:29:2:233. [DOI] [PubMed] [Google Scholar]
- Purifoy FE, Koopmans LH, Mayes DM. Age differences in serum androgen levels in normal adult males. Hum Biol. 1981;53:499–511. [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M, Nyborg AC, Murphy MP, Chang L, Stanczyk FZ, Golde TE, Pike CJ. Androgens modulate beta-amyloid levels in male rat brain. J Neurochem. 2003b;87:1052–1055. doi: 10.1046/j.1471-4159.2003.02114.x. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003a;122:573–578. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- Rasmuson S, Nasman B, Carlstrom K, Olsson T. Increased levels of adrenocortical and gonadal hormones in mild to moderate Alzheimer's disease. Dem Ger Cog Dis. 2002;13:74–79. doi: 10.1159/000048637. [DOI] [PubMed] [Google Scholar]
- Raynaud JP. Prostate cancer risk in testosterone-treated men. J Steroid Biochem Mol Biol. 2006;102:261–266. doi: 10.1016/j.jsbmb.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Amaducci LA, Schoenberg BS. Epidemiology of clinically diagnosed Alzheimer's disease. Ann Neurol. 1986;19:415–424. doi: 10.1002/ana.410190502. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer's disease. J Neurosci. 2006;26:13384–13389. doi: 10.1523/JNEUROSCI.2514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. JAMA. 2004;292:1431–1432. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- Salminen EK, Portin RI, Koskinen A, Helenius H, Nurmi M. Associations between serum testosterone fall and cognitive function in prostate cancer patients. Clin Cancer Res. 2004;10:7575–7582. doi: 10.1158/1078-0432.CCR-04-0750. [DOI] [PubMed] [Google Scholar]
- Savage MJ, Trusko SP, Howland DS, Pinsker LR, Mistretta S, Reaume AG, Greenberg BD, Siman R, Scott RW. Turnover of amyloid beta-protein in mouse brain and acute reduction of its level by phorbol ester. J Neurosci. 1998;18:1743–1752. doi: 10.1523/JNEUROSCI.18-05-01743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield-Moore M, Urban RJ. An overview of the endocrinology of skeletal muscle. Trends Endocrinol Metab. 2004;15:110–115. doi: 10.1016/j.tem.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Short RA, Bowen RL, O'Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc. 2001;76:906–909. doi: 10.4065/76.9.906. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Swerdloff RS, Wang C. Androgen deficiency and aging in men. West J Med. 1993;159:579–585. [PMC free article] [PubMed] [Google Scholar]
- Swerdloff RS, Wang C. Androgens and aging in men. Exp Gerontol. 2002;28:435–446. doi: 10.1016/0531-5565(93)90069-p. [DOI] [PubMed] [Google Scholar]
- Tan RS, Pu SJ. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer's disease. Aging Male. 2003;6:13–17. [PubMed] [Google Scholar]
- Tohgi H, Utsugisawa K, Yamagata M, Yoshimura M. Effects of age on messenger RNA expression of glucocorticoid, thyroid hormone, androgen, and estrogen receptors in postmortem human hippocampus. Brain Res. 1995;700:245–253. doi: 10.1016/0006-8993(95)00971-r. [DOI] [PubMed] [Google Scholar]
- Twist SJ, Taylor GA, Weddell A, Weightman DR, Edwardson JA, Morris CM. Brain oestradiol and testosterone levels in Alzheimer's disease. Neurosci Lett. 2000;286:1–4. doi: 10.1016/s0304-3940(00)01078-8. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J Clin Endocrinol Metab. 1996;81:1821–1826. doi: 10.1210/jcem.81.5.8626841. [DOI] [PubMed] [Google Scholar]
- Vincent B, Smith JD. Effect of estradiol on neuronal Swedish-mutated beta-amyloid precursor protein metabolism: reversal by astrocytic cells. Biochem Biophys Res Comm. 2000;271:82–85. doi: 10.1006/bbrc.2000.2581. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Koba S, Kawamura M, Itokawa M, Idei T, Nakagawa Y, Iguchi T, Katagiri T. Small dense low-density lipoprotein and carotid atherosclerosis in relation to vascular dementia. Metabolism. 2004;53:476–482. doi: 10.1016/j.metabol.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, Fried G, Jovanovic JN, Seeger M, Relkin NR, Liao F, Checler F, Buxbaum JD, Chait BT, Thinakaran G, Sisodia SS, Wang R, Greengard P, Gandy S. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nature Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- Xu H GP, Gandy S. Regulated formation of Golgi secretory vesicles containing Alzheimer beta-amyloid precursor protein. J Biol Chem. 1995;270:23243–23245. doi: 10.1074/jbc.270.40.23243. [DOI] [PubMed] [Google Scholar]
- Yu WH. Effect of testosterone on the regeneration of the hypoglossal nerve in rats. Exp Neurol. 1982;77:129–141. doi: 10.1016/0014-4886(82)90149-2. [DOI] [PubMed] [Google Scholar]
- Yu WH. Administration of testosterone attenuates neuronal loss following axotomy in the brain-stem motor nuclei of female rats. J Neurosci. 1989;9:3908–3914. doi: 10.1523/JNEUROSCI.09-11-03908.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Srinivasan R. Effect of testosterone and 5 alpha-dihydrotestosterone on regeneration of the hypoglossal nerve in rats. Exp Neurol. 1981;71:431–435. doi: 10.1016/0014-4886(81)90101-1. [DOI] [PubMed] [Google Scholar]
- Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer's disease animal model. Proc Natl Acad Sci USA. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh_Bohmer KA, Mayer LS, Steffens DC, Breitner JC. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1–42 toxicity through heat shock protein 70. J Neurosci. 2004;24:5315–5321. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Xu H, Uljon SN, Gross R, Hardy K, Gaynor J, Lafrancois J, Simpkins J, Refolo LM, Petanceska S, Wang R, Duff K. Modulation of A(beta) peptides by estrogen in mouse models. J Neurochem. 2002;80:191–196. doi: 10.1046/j.0022-3042.2001.00690.x. [DOI] [PubMed] [Google Scholar]


