Abstract
Background and objectives: Racial disparities in provision of healthcare are widespread in the United States but have not been specifically assessed in provision of chronic kidney disease (CKD) care.
Design, setting, participants, & measurements: We conducted a retrospective cohort study of the clinical database used in a Department of Defense (DOD) medical system. Beneficiaries studied were DOD-eligible beneficiaries with CKD stage 3 (n = 7729) and 4 (n = 589) using the modified Modification of Diet in Renal Disease (MDRD)-estimated GFR formula but requiring manual correction for Black race. Compliance with selected Kidney Disease Outcomes Quality Initiative (KDOQI) CKD recommended targets (monitoring of recommended laboratory data, prescription of recommended medications, and referral to nephrology) was assessed over a 12-mo period, stratified by CKD stage. Logistic regression analysis was used to assess whether race (White, Black, or other) was independently associated with provider compliance with targets, adjusted for demographic factors and burden of comorbid conditions.
Results: Among the targets, only monitoring of LDL cholesterol was significantly less common among Blacks. For all other measures, compliance was either not significantly different or significantly higher for Black compared with White beneficiaries. However, patients categorized as “Other” race were in general less likely to achieve targets than Whites, and at stage 3 CKD significantly less likely to achieve targets for monitoring of phosphorous, hemoglobin, and vitamin D.
Conclusions: In the DOD health system, provider compliance with selected CKD stage 3 and 4 targets was not significantly lower for Black beneficiaries than for Whites, with the exception of LDL cholesterol monitoring. Patients classified as Other race were generally less likely to achieve targets than Whites, in some patients significantly so.
Numerous studies have documented racial disparities in provision of health care for Black, as compared with White Americans. Blacks have been shown to experience higher mortality, less access to care, higher risk of renal disease progression, fewer referrals for renal transplantation, and shorter renal allograft survival than Whites (1–5). Some programs aimed at enhancing access to care have shown improvements in this health gap (6,7). The common theme of such interventions is the amelioration of financial, socioeconomic, and other (occasionally including transportation) barriers to access and care.
Medical care in the DOD direct care system is provided without cost and without the need for qualification based on existing conditions. If differences in care between Blacks and Whites observed in the United States are at least in part due to differences in insurance coverage or other financial factors, Black and White beneficiaries in the DOD health system should have less racial disparity in care provided by a similar group of primary care physicians and nephrologists than reported nationally.
Because previous reports on racial disparities in provided care have focused on Blacks and Whites, our objective was to perform a retrospective cohort study of a clinical/administrative healthcare database to assess the nephrology care provided to White and Black beneficiaries with CKD stages 3 and 4 in the DOD's National Capital Area (NCA) health system. Nephrology care was assessed per the National Kidney Foundation's (NKF) K/DOQI guidelines. Our null hypothesis was that there would be no significant difference in compliance with nationally recommended CKD care provided to Black and White beneficiaries in the DOD's NCA health system, including referral for nephrology consultation.
Concise Methods
Database
Data were obtained from the Composite Health Care System (CHCS) of the NCA. This database contains an integrated set of demographic data, laboratory results, prescriptions, and International Statistical Classification of Diseases and Related Health Problems (ICD-9) codes of all beneficiaries seen in the DOD's Tricare health system. All medications, laboratory tests, and consultations to other DOD providers ordered by authorized DOD healthcare providers are captured and visible throughout all DOD hospitals in the NCA. Demographic data extracted from the database included: age (determined at the end of the study, April 30, 2006), sex, and race (self-reported as White, Black, or Other). Patient height and weight were not available through the database during the years studied.
Patient Population
After permission was obtained from the Walter Reed Army Medical Center and National Naval Medical Center's institutional review boards, adults with CKD were identified from the CHCS database. Beneficiaries include active-duty military and their dependent family members as well as retired military personnel. DOD beneficiaries can select several types of medical care through the Tricare system. The data available to us was for beneficiaries who received care in the “direct care system”, i.e., care provided onsite at military treatment facilities, and not at civilian Tricare provider facilities. Active duty service members must use the direct care system except in the case of emergency care or in selected nonavailable care. For dependents or retirees, eligibility in the direct care system is voluntary. We therefore selected beneficiaries for whom we could demonstrate continuing use of the direct care system during the duration of the study. Study beneficiaries were identified using the following inclusion criteria: (1) Age >18 yr-old; (2) serum creatinine assessment from May 1, 2004 until April 30, 2005 and followed until April 30, 2006 (to assure a minimum of 1-yr observation time for all beneficiaries) (3) two estimated GFR (eGFR) between 15 and 60 cc/min per 1.73 m2, separated by an interval of at least 90 d, as per Patwardhan et al. (8). A total of 8318 beneficiaries met these criteria. The modified MDRD equation, five-variable formula (including BUN, Equation #3 (9)) was used to calculate eGFR. Since 2001 this formula was used to generate an automated report of eGFR visible to providers. However, because of technical factors, race was not automatically included in the equation but the result contained a message to multiply the reported eGFR by 1.18 (in comparison to the 1.21 for the four-point formula) if the patient was Black. Beneficiaries on dialysis were excluded based on diagnostic codes for ESRD or Current Procedural Terminology codes for dialysis.
Outcomes
Figure 1 shows the outcomes assessed during the study, which for purposes of uniformity are referred to collectively as targets. To avoid bias due to differences in follow-up among beneficiaries, in which beneficiaries with longer potential follow-up might have a higher frequency of outcomes, outcomes were assessed over a 12-mo period from the date of the first test used to determine the entry eGFR, but no later than the end of the study date, because it was assumed the stage of CKD during this first test would determine further medical treatment. For stage 3 CKD, the target frequency of testing was annual, and was quarterly for stage 4 CKD. Laboratory results extracted included: serum intact parathyroid hormone (PTH), serum calcium, serum phosphorus, serum hemoglobin, serum ferritin, serum iron saturation, serum albumin, serum LDL cholesterol, serum 25-hydroxyvitamin D (reported as all such determinations ordered, although KDOQI recommendations are dependent on the level of PTH), urine protein, and urine microalbumin (the latter also analyzed restricted to patients with diabetes). Prescriptions extracted included: angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARB), erythropoiesis-stimulating agents (ESAs), 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), and nonsteroidal anti-inflammatory drugs (NSAID). Because targets for anemia management (iron panel, ferritin, ESA use) are contingent on the level of hemoglobin, these measures are presented dependent on minimum target levels of hemoglobin ≤11 g/dl. BP was not obtained electronically in the electronic health record during the time of the study and was not reported in this study.
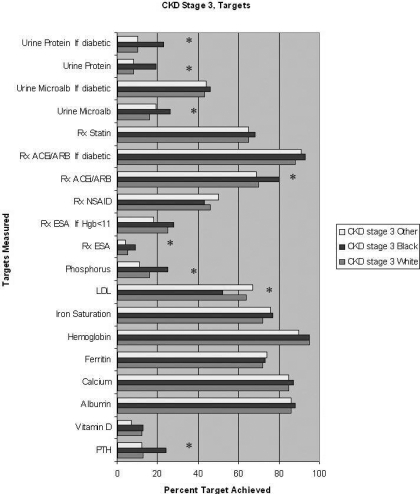
Figure 1.
Among stage 3 chronic kidney disease (CKD) patients, univariate analysis of percentage compliance with Kidney Disease Outcomes Quality Initiative (KDOQI)-recommended monitoring of laboratory results and recommended medication use of each race (* represents P value <0.05 for Black race compared with White race). Data shown for ferritin and iron saturation are limited to patients with hemoglobin ≤11 g/dl. ESA, erythropoiesis stimulating agent; PTH, parathyroid hormone; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blockers; NSAID, nonsteroidal anti-inflammatory drugs
Follow-Up
Available data did not include information on patient vital status unless they died while hospitalized. To ensure that beneficiaries identified in the study were available to providers, and that noncompliance was not due to beneficiary death or loss to follow-up, we included beneficiaries in the study only if they had evidence of continued appointments or some other form of medical activity (reporting of laboratory or radiology results, filled medication prescriptions, kept appointments) in the system for 6 mo after the official end of the study date of April 30, 2006. This essentially collapsed beneficiaries who died and beneficiaries who were lost to follow-up, which our data systems could not distinguish, into one category.
Dependent Variables
Variables included age (continuous), race (Black versus White versus other), sex, eGFR as a continuous variable, CKD stage (3 versus 4), and comorbid conditions to include coronary artery disease, congestive heart failure, diabetes mellitus, and hypertension. Comorbid conditions were defined as presence of two or more ICD-9 diagnoses during the study period.
Statistical Analyses
SPSS version 12.0 was used for statistical analysis. In univariate analysis, χ2 testing was used for categorical variables and t test was used for continuous variables with a normal distribution. Alpha values were set at 0.05 (two-tailed). Alternate tests were used for special circumstances (Fisher exact test for categorical variables with violations of Cochran's assumptions, the Wilcoxon rank sum test or Mann–Whitney test as alternatives for the t test for continuous variables without Gaussian distributions). Multiple logistic regression analysis was performed separately to assess factors independently associated with lab draws and prescriptions. Variables that were significant with a P value of <0.05 after multivariate analysis were considered to be independently associated with lab draws and prescriptions. The predictive accuracy of the model was assessed using the concordance c index, which is equivalent to the area under the receiver operator curve for the model (1.0 indicating perfect agreement, 0.5 indicating random agreement). Model fit was assessed using Hosmer–Lemeshow diagnostics.
Results
Among the 8318 total beneficiaries who were evaluated; 5849 (70.3%) were White, 1344 (16.2%) were Black, and 1125 (13.5%) were of other/unknown race. Of 7729 stage 3 CKD beneficiaries, 16% had at least 1 visit to nephrology. Of 589 stage 4 CKD beneficiaries, 64% had at least 1 visit to nephrology. Black beneficiaries were significantly more likely than White beneficiaries to be seen by nephrology, 28 versus 14% for stage 3 beneficiaries, respectively, and 76 versus 62% for stage 4 beneficiaries. Black beneficiaries represented 15.6% of the stage 3 CKD cohort and 23.6% of the stage 4 CKD cohort. Beneficiaries seen by nephrology were significantly more likely to be male, younger, and have a lower eGFR. Stage 3 CKD beneficiaries seen by nephrology were significantly more likely to have an ICD-9 diagnosis of coronary artery disease, congestive heart failure, diabetes mellitus, and hypertension.
Table 1: In unadjusted analysis, compared with White beneficiaries, Blacks were significantly more likely to be male, younger than 65 yr-old, seen by nephrology, and have stage 4 CKD. Black beneficiaries were significantly less likely to have coronary artery disease but more likely to have congestive heart failure, diabetes mellitus, and hypertension.
Table 1.
Demographic data by race
| Variable | Race
|
P value | ||
|---|---|---|---|---|
| White | Black | Other | ||
| Female gender | 2823 (51.7%) | 675 (49.8%) | 477 (57.6%) | <0.001 |
| Age over 65 | 4524 (77.3%) | 898 (66.8%) | 705 (62.7%) | <0.001 |
| Seen by nephrology | 999 (17.1)% | 448 (33.3%) | 177 (15.7%) | <0.001 |
| CKD stage 3 | 5459 (93.3%) | 1205 (89.7%) | 1065 (94.7%) | <0.001 |
| CKD stage 4 | 390 (6.7%) | 139 (10.3%) | 60 (5.3%) | <0.001 |
| Dx CAD | 1621 (27.7%) | 323 (24.0%) | 176 (15.6%) | <0.001 |
| Dx CHF | 869 (14.9%) | 231 (17.2%) | 64 (5.7%) | <0.001 |
| Dx DM | 1843 (31.5%) | 679 (51.9%) | 427 (38.0%) | <0.001 |
| Dx HTN | 4880 (83.4%) | 1226 (91.2%) | 927 (82.4%) | <0.001 |
Dx, diagnosis; CKD, chronic kidney disease; CAD, coronary artery disease, CHF, congestive heart failure; DM, diabetes mellitus; HTN, hypertension
Figure 1: In unadjusted analysis of CKD stage 3 patients, there was no difference between Blacks and Whites for monitoring of serum calcium and albumin levels. Blacks were significantly more likely than Whites to have most other levels monitored with the exception of serum hemoglobin. The only laboratory target achieved significantly less frequently in Black, compared with White, beneficiaries was LDL cholesterol. Blacks were significantly more likely than Whites to be prescribed ACE inhibitors/ARB, and statins, and appropriately less likely to be prescribed NSAID, with no difference in ESA use.
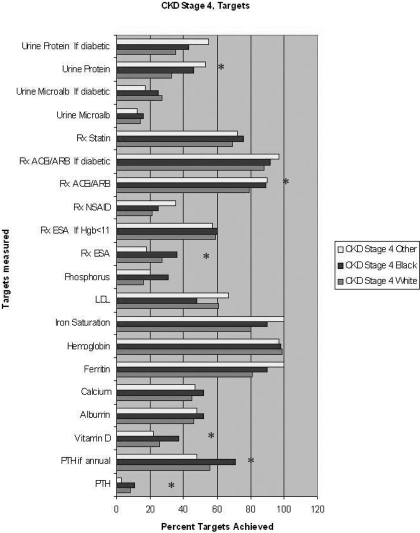
Figure 2 shows results for CKD stage 4 patients. Results were similar to that for stage 3 CKD patients. LDL levels were ordered less frequently in blacks than whites but this difference was not statistically significant.
Figure 2.
Among stage 4 CKD patients, univariate analysis of percentage compliance with KDOQI recommended monitoring of laboratory results and recommended medication use of each race (* represents P value <0.05 for Black race compared with White race). For monitoring of calcium, phosphorous, albumin, and PTH, target was considered achieved if at least four tests were ordered during a 12-mo period, as per the Methods section. Other targets were set at annual intervals (for labs) or active prescription (for medications). Data shown for ferritin and iron saturation are limited to patients with hemoglobin ≤11 g/dl.
Table 2: Logistic regression analysis of demographic factors including eGFR that were associated with Black race showed many significant differences. Blacks were significantly younger, had significantly lower eGFR, had significantly higher prevalence of diabetes and hypertension, but significantly lower prevalence of coronary artery disease.
Table 2.
Logistic regression analysis of Black versus White race, demographics
| Variable | Black (versus White race)
|
P value | |
|---|---|---|---|
| Adjusted Odds Ratio | 95% CI | ||
| Age (per year) | 0.97 | 0.96 to 0.97 | <0.001 |
| Male | 1.11 | 0.98 to 1.26 | 0.09 |
| Diabetes | 1.93 | 1.71 to 2.19 | <0.001 |
| CAD | 0.77 | 0.66 to 0.90 | 0.001 |
| CHF | 1.44 | 1.20 to 1.72 | <0.001 |
| HTN | 2.09 | 1.69 to 2.58 | <0.001 |
| CKD stage (4 vs. 3) | 1.44 | 1.16 to 1.79 | <0.001 |
Variables in model included mean first eGFR (to define CKD stage later), gender, diagnoses of diabetes, CAD, heart failure, or hypertension, and age.
Table 3: In this table showing the results of multiple different logistic regression analyses for the association of race (Black and other, using White race as the reference) with study goals, adjusted for baseline demographic factors and stage of CKD, Blacks were significantly more likely than Whites to be referred to nephrology, have monitoring goals for PTH and phosphorous (among CKD stage 3 patients). Because current KDOQI guidelines do not distinguish targets for LDL, urine protein/microalbumin monitoring, and medication use by stage of CKD, results are shown both in aggregate and stratified by stage. In aggregate and among stage 3 CKD, Blacks were significantly more likely to have urine protein ordered, have ACE or ARB prescribed, and appropriately significantly less likely to have NSAID prescribed. They were significantly less likely to have LDL monitored regardless of CKD stage. No significant differences were noted between Blacks and Whites among CKD stage 4 patients, although the number of patients in this category was much smaller than in stage 3. Other race was significantly associated with less frequent monitoring of phosphorus, hemoglobin, or vitamin D.
Table 3.
Logistic regression analysis of Black race as a correlate of study goals by race, using White race as reference
| Variable | Black (versus White)
|
Other (versus White)
|
||||
|---|---|---|---|---|---|---|
| Adjusted Odds Ratio | 95% CI | P value | Adjusted Odds Ratio | 95% CI | P value | |
| Stage 3 CKD only | ||||||
| race as a correlate of compliance with monitoring for: | ||||||
| referral to nephrology (versus no visits) | 2.04 | 1.75 to 2.38 | <0.001 | 0.89 | 0.74 to 1.09 | 0.28 |
| PTH | 2.24 | 1.79 to 2.80 | <0.001 | 0.80 | 0.57 to 1.11 | 0.18 |
| Vitamin D | 1.07 | 0.89 to 1.31 | 0.45 | 0.58 | 0.45 to 0.74 | <0.001 |
| calcium | 1.05 | 0.86 to 1.27 | 0.65 | 0.97 | 0.80 to 1.17 | 0.75 |
| phosphorus | 1.60 | 1.37 to 1.87 | <0.001 | 0.63 | 0.51 to 0.78 | <0.001 |
| hemoglobin | 1.20 | 0.89 to 1.61 | 0.22 | 0.57 | 0.47 to 0.75 | <0.001 |
| ferritina | 0.98 | 0.65 to 1.47 | 0.92 | 1.16 | 0.65 to 2.08 | 0.62 |
| iron saturationa | 1.16 | 0.76 to 1.76 | 0.49 | 1.16 | 0.79 to 1.70 | 0.63 |
| albumin | 1.01 | 0.83 to 1.21 | 0.91 | 0.94 | 0.77 to 1.14 | 0.52 |
| LDL | 0.48 | 0.42 to 0.55 | <0.001 | 0.95 | 0.82 to 1.10 | 0.51 |
| urine microalbumin | 1.17 | 0.98 to 1.39 | 0.08 | 0.96 | 0.78 to 1.17 | 0.66 |
| limited to diabetics | 1.11 | 0.91 to 1.33 | 0.30 | 0.91 | 0.73 to 1.14 | 0.42 |
| urine protein | 1.73 | 1.39 to 2.15 | <0.001 | 0.93 | 0.72 to 1.20 | 0.58 |
| race as a correlate of compliance with prescription for: | ||||||
| ACEi or ARB | 1.25 | 1.06 to 1.49 | 0.01 | 1.01 | 0.86 to 1.19 | 0.85 |
| ESAa | 1.15 | 0.76 to 1.75 | 0.51 | 0.65 | 0.33 to 1.27 | 0.21 |
| NSAID | 0.97 | 0.85 to 1.10 | 0.61 | 1.02 | 0.89 to 1.16 | 0.83 |
| statin | 0.94 | 0.81 to 1.09 | 0.41 | 1.07 | 0.92 to 1.25 | 0.35 |
| Stage 4 CKD Only | ||||||
| race as a correlate of compliance with monitoring for: | ||||||
| referral to nephrology (versus no visits) | 1.33 | 0.83 to 2.13 | 0.24 | 0.69 | 0.39 to 1.23 | 0.21 |
| PTH | 1.00 | 0.50 to 2.03 | 0.99 | 0.32 | 0.07 to 1.43 | 0.14 |
| Vitamin D | 1.59 | 1.02 to 2.48 | 0.04 | 0.76 | 0.39 to 1.47 | 0.41 |
| calcium | 0.98 | 0.64 to 1.49 | 0.91 | 1.04 | 0.59 to 1.84 | 0.90 |
| phosphorus | 1.52 | 0.92 to 2.50 | 0.10 | 1.11 | 0.54 to 2.30 | 0.78 |
| hemoglobin | 0.38 | 0.08 to 1.80 | 0.22 | 0.34 | 0.06 to 1.83 | 0.21 |
| ferritin* | 1.70 | 0.50 to 5.79 | 0.39 | NA | ||
| iron saturationa | 1.43 | 0.41 to 5.05 | 0.58 | NA | ||
| albumin | 0.93 | 0.61 to 1.41 | 0.73 | 1.05 | 0.59 to 1.85 | 0.88 |
| LDL | 0.40 | 0.26 to 0.62 | <0.001 | 1.26 | 0.83 to 1.91 | 0.28 |
| urine microalbumin | 0.77 | 0.42 to 1.40 | 0.76 | 0.77 | 0.31 to 1.89 | 0.77 |
| limited to diabetics | 0.86 | 0.46 to 1.64 | 0.66 | 0.61 | 0.21 to 1.76 | 0.36 |
| urine protein | 1.26 | 0.80 to 1.97 | 0.31 | 2.93 | 1.49 to 5.73 | 0.002 |
| race as a correlate of compliance with prescription for: | ||||||
| ACEi or ARB | 1.42 | 0.75 to 2.72 | 0.28 | 2.52 | 0.99 to 6.37 | 0.05 |
| ESAa | 1.03 | 0.47 to 2.29 | 0.93 | 1.41 | 0.39 to 5.11 | 0.60 |
| NSAID | 1.16 | 0.71 to 1.91 | 0.55 | 2.01 | 1.10 to 3.69 | 0.02 |
| statin | 1.08 | 0.65 to 1.79 | 0.76 | 1.18 | 0.61 to 2.30 | 0.61 |
| Targets not distinguished by stage | ||||||
| race as a correlate of compliance with monitoring for: | ||||||
| LDL | 0.43 | 0.38 to 0.49 | <0.001 | 0.95 | 0.82 to 1.09 | 0.45 |
| urine microalbumin | 1.14 | 0.97 to 1.35 | 0.11 | 0.98 | 0.81 to 1.19 | 0.86 |
| limited to diabetics | 1.09 | 0.91 to 1.33 | 0.33 | 0.95 | 0.76 to 1.19 | 0.68 |
| urine protein | 1.89 | 1.59 to 2.26 | <0.001 | 1.07 | 0.85 to 1.33 | 0.59 |
| race as a correlate of compliance with prescription for: | ||||||
| ACEi or ARB | 1.29 | 1.09 to 1.52 | 0.003 | 1.05 | 0.89 to 1.23 | 0.55 |
| ESAa | 1.19 | 0.79 to 1.79 | 0.40 | 0.62 | 0.32 to 1.20 | 0.16 |
| NSAID | 0.90 | 0.79 to 1.02 | 0.011 | 1.06 | 0.93 to 1.21 | 0.36 |
| statin | 0.91 | 0.79 to 1.05 | 0.20 | 1.09 | 0.94 to 1.27 | 0.23 |
95% CI, 95% confidence interval; ESA, erythropoiesis stimulating agent; PTH, parathyroid hormone; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blockers; NSAID, nonsteroidal anti-inflammatory drugs.
Each item in the far left column was an outcome (independent variable). Results shown are the adjusted odds ratio of Black race and other race (compared to White race as reference) as a predictor of goals. All models also run with variables of gender, age, diagnoses of diabetes, CAD, heart failure, or hypertension.
Analysis limited to beneficiaries with hemoglobin ≤11 g/dl.
Patients classified as Other race were significantly less likely than Whites to have Vitamin D, phosphorous, or hemoglobin ordered among stage 3 CKD. Among stage 4 CKD, however, they were significantly more likely to have urine protein ordered, have ACE or ARB used, and also significantly more likely to have NSAID used.
Discussion
In this cohort of DOD beneficiaries with prevalent CKD stage 3 and 4, the only target that was performed significantly less frequently in Blacks than in Whites was ordering of LDL cholesterol. However, this was the lone exception; for all other targets, the frequency of compliance with recommended targets was not statistically different for Blacks compared with Whites, and in many cases significantly higher, even in adjusted analysis. After adjusting for other factors, we found that Black beneficiaries were referred to nephrology more frequently than comparable White beneficiaries. This must be interpreted in light of the automated eGFR reporting during the study period that did not automatically correct for Black race, but suggested multiplying the reported eGFR by 1.18. Provider compliance with this extra step was not assessed. In addition, many guidelines at the time, including the Veterans Administration (VA)-DOD CKD guidelines (10), were based solely on serum creatinine levels, which might have been expected to lead to underestimation of actual eGFR and, in a truly race-neutral environment, led to over-referral of Blacks when compared with Whites. These findings suggest unintended consequences for adjusting (or failing to adjust) for Black race in the standard eGFR equations. Not inconsistent with this observation, a recent study of the VA health system did not indicate that universal use of automated reporting of eGFR resulted in improved delivery of quality care (11); however, a specific assessment of racial disparities was not performed. Nevertheless, this is one of the few reported examples in US nephrology where access to care is comparable for Blacks and Whites.
Despite the relative equity of care for Blacks compared with Whites, overall compliance with targets was relatively low, with less than 50% of targets achieved and a substantial portion were at 20% or less of compliance (Figure 1 and 2). Many of the targets that were achieved significantly more frequently in Blacks versus Whites were in the latter category. Thus, success is “relative.” Given differences in study populations and definitions of targets, available studies for comparison are few. However, among stage 3 CKD patients, the use of ACE/ARB was higher for both diabetics and nondiabetic patients than as reported in the VA system by Wyatt et al. (11). Their study assessed ordering of urinalysis, not specifically urine protein or microalbumin, and thus comparison is not possible. Comparison of our stage 4 CKD targets is more difficult because the two available studies, by Lenz et al. and Patwardhan et al., studied stage 4 and 5 CKD patients not yet on dialysis; Lenz et al. stratified by stage 4 CKD but also stratified by length of time the patients had seen a nephrologist, which was not our primary study objective. However, comparing our stage 4 achieved targets in Figure 2 with these studies, our quarterly assessment of PTH (at least for Black and White patients) exceeded the 9% reported for patients who had seen a nephrologist for less than 6 mo, but below the 20% for those who had seen a nephrologist longer. Compared with other measures reported by Lenz, our achievement of targets for calcium, phosphorous, and albumin were lower but our achievement of targets for hemoglobin was higher. Lenz et al. did not report use of medications. While Patwardhan aggregated both stage 4 and stage 5 CKD, our achievement of targets was higher for hemoglobin, anemia evaluation, and monitoring of PTH (annual, as Patwardhan reported, not quarterly as KDOQI recommends). Monitoring for LDL was comparable.
To our knowledge, the specific issue of racial disparities in provision of CKD care has not been investigated in detail. Most recent reports of CKD care have either not explicitly assessed racial disparities (8), or have been among atypical patient populations (chronic allograft failure) in predominantly White populations (12). One of the few reports that assessed the role of race in the provision of CKD care (stage 4 to 5 CKD) in an American-dedicated CKD clinic serving an urban, socioeconomically disadvantaged minority population found that providers achieved significantly poorer performance of KDOQI goals in Black beneficiaries than Whites (13). A recent study of CKD stage 3 to 4 care in the VA health system did not specifically assess care delivery by race (11).
Efforts aimed at reducing racial disparities in the traditional US health system have focused on reducing barriers to insurance coverage and other financial inequalities. The DOD system may represent a reasonable scenario for what might be achieved through such efforts. Alternatively, in the DOD setting (and perhaps others), reliance primarily on serum creatinine level might result in a relative advantage for Blacks, both in comparison to Whites and to other races, especially patients whose serum creatinine levels were relatively low compared with their true GFR. However, such an advantage was not noted in an urban, socioeconomically disadvantaged US population (13). Despite these caveats, the demographics and burden of disease shown for our cohort in Table 1 are remarkably similar to other reported CKD cohorts.
Reported advantages for Blacks in US medical care are few, but do exist. Most noteworthy is the consistent finding that Blacks on long-term dialysis have better survival compared with their White counterparts, as noted in the US Renal Data System annual reports. No explanation for this situation has been widely accepted; however, Black dialysis patients are generally younger and have less coronary artery disease than their White counterparts, just as in this cohort. Black dialysis patients also have lower rates of kidney transplantation than Whites, with the net result that healthier Blacks remain on dialysis whereas healthier Whites are removed from the dialysis population, possibly skewing these results. No such differential in follow-up is plausible for the DOD population.
Because improved compliance with CKD care among patients who have seen nephrologists, compared with non-nephrologists, has been widely reported previously (8,13,14), we felt it was critical to account for nephrology referral in reporting these outcomes, although assessing the effect of nephrology referral was not a primary objective in this study. It is important to distinguish that our methods of analysis did not permit us to determine which clinic actually ordered the tests, and we primarily used nephrology referral as an adjustment variable.
Because all other targets were achieved at least as often if not more frequently among Blacks than Whites, the one exception, monitoring of LDL levels, is puzzling. According to recent KDOQI guidelines on management of dyslipidemias, all adults with CKD (eGFR <60 cc/min/1.73 m2) should be evaluated for dyslipidemia. However, this guideline was not released before the timeframe of the study. Previous management should have depended on National Centers for Environmental Prediction guidelines, but even adjusted for the lower prevalence of coronary artery disease and younger age, the lower rate of ordering of LDL labs in Blacks persisted. Disparity of this sort was not noted in the VA health system (15) or in civilian medical systems (16). Ordering of LDL levels may also be particularly driven by socioeconomic status, and in many physicians' personal experience, patients are often much more concerned about their “cholesterol” level than other aspects of their health that may be much more pressing, perhaps in a manner similar to advertisement-driven requests for medication prescriptions (the “ask your doctor” phenomenon). Our hypothesis would be that White patients, compared with Blacks, would be more likely to request lipid testing, and thus influence provider monitoring, adjusted for other factors, in the DOD system. This finding certainly warrants further investigation into our own health targets, which may or may not have implications for other health systems.
A few other targets in the study warrant specific comment. Although relative rates of ordering of PTH and vitamin D levels favored Blacks in this study, overall rates of ordering these tests was low. This is not surprising because the dates of the study came immediately after the release of the first KDOQI clinical practice guidelines for bone and mineral metabolism, which set the standard for these tests. Many practitioners had previously based their ordering of PTH on serum calcium or phosphorous levels, and ordering of Vitamin D levels was unusual. However, we think this provides useful baseline data, and indicates that Black beneficiaries did not receive significantly less attention to these goals even before national guidelines were disseminated. Our clinic made the monitoring of PTH and Vitamin D levels its main performance improvement project for the 2006 academic year partly on the basis of these data.
In contrast to the results of our primary comparison of Black versus White race, the frequency of reaching targets for Other races was significantly lower for three measures (phosphorous, hemoglobin, and Vitamin D) and in general much more likely to be lower than Whites, the reverse of the trend observed for Blacks. During the time period, DOD racial categories did not have an indicator for Hispanic race, and the number of Asians was too small to assess separately. The use of the MDRD equation for estimating GFR does not make allowance for race other than Black, and to date the consensus from available literature does not suggest this is necessary. Furthermore, we did not have information on height or weight to calculate patient body surface area or ideal body weight. There are known reported limitations of the widely used MDRD equation, particularly for Asians (17), which are only partly explained by differences in body size. Because our information on this category is by definition quite limited, our conclusions must also be quite limited. It does suggest, however, that the use of the current eGFR equations to guide provision of CKD care may have limitations to which providers should be alerted, and that using the current eGFR equation may not solve certain disparities in providing CKD care. This may be related to the increased use of NSAID among this group. The increased ordering of urine protein and ACE or ARB was unexpected but might be due to small sample size and coincidental higher prevalence of certain glomerular diseases (such as IgA nephropathy) in this group, which we did not specifically assess in the study.
This study has several limitations that may influence interpretation of its results. As with all administrative databases, race was self-reported in the DOD. The validity of racial categories is being called into question, and in the military environment, racial combinations (especially Black and Asian, as well as Black Hispanic) are likely more common than in the general population, which may increase the possibility of misclassification. Lack of information on Hispanic race in particular could have resulted in misclassification bias if Hispanics are more likely to refer to themselves as “White” than “Black.” Reporting of race generally remains mutually exclusive and is often a matter of patient/family preference and/or controversial. However, outcomes associated with race are an institutionalized aspect of American culture, and therefore medical care (e.g., reporting of US Renal Data System, originally intended to inform Congress, routinely includes race). In addition, we did our best to avoid including beneficiaries who had acute kidney injury or dialysis treatments, but in the absence of individual chart audit, which was not possible with this protocol, we cannot absolutely exclude misclassification bias. We cannot exclude misclassification or under-ascertainment of comorbid conditions, although the methods we used have been generally reported in the literature. The provision of targets over a 12-mo period is arbitrary but consistent with national recommendations. As a retrospective cohort study, we cannot exclude observation bias in which sicker patients would be more likely to have laboratory results ordered than those who remain healthy, independent of compliance with targets. However, our outcomes did not assess the total number of laboratory tests or medications ordered but focused on meeting national consensus recommendations. Also, as a retrospective cohort study, we could not exclude residual confounding. As shown in the tables, there were many demographic differences between Black and White beneficiaries. By definition we could not adjust for unmeasured confounders.
In summary, in a cohort of stage 3 and 4 CKD patients receiving their care in the DOD health system, compliance with recommended targets was not significantly lower for Black, compared with White, beneficiaries with the exception of less frequent monitoring of LDL cholesterol levels. In a health system that provides medical benefits to its beneficiaries equitably and without cost, disparities between care provided to Black versus White beneficiaries documented in other aspects of US medical care were largely not observed. The results observed for beneficiaries categorized as Other race are preliminary and suggest the need for further study.
Disclosures
None.
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Racial Disparities in Chronic Kidney Disease: Tragedy, Opportunity, or Both?,” on pages 314–316.
S.W.G.'s current affiliation is Nephrology Service, Naval Medical Center, Portsmouth, Virginia.
Disclaimer: The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy or Army, Department of Defense, nor the US Government.
References
- 1.Popescu I, Vaughan-Sarrazin MS, Rosenthal GE: Differences in mortality and use of revascularization in Black and White beneficiaries with acute MI admitted to hospitals with and without revascularization services. JAMA 297: 2489–2495, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Satcher D, Fryer GE, McCann J, Troutman A, Woolf SH, Rust G: What if we were equal? A comparison of the Black-White mortality gap in 1960 and 2000. Health Affairs 24: 459–465, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Eckhoff DE, Young CJ, Gaston RS, Fineman SW, Deierhoi MH, Foushee MT, Brown RN, Diethelm AG: Racial disparities in renal allograft survival: A public health issue? J Am Coll Surg 204: 894–902, 2007 [DOI] [PubMed] [Google Scholar]
- 4.McClellan W, Warnock DG, McClure L, Campbell RC, Newsome BB, Howard V, Cushman M, Howard G: Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol 17: 1710–1715, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Garg PP, Diener-West M, Powe NR: Reducing racial disparities in transplant activation: Whom should we target? Am J Kidney Dis 37: 921–931, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Shone LP, Dick AW, Klein JD, Zwanziger J, Szilagyi PG: Reduction in racial and ethnic disparities after enrollment in the State Children's Health Insurance Program. Pediatrics 115: e697–705, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Hyman JJ, Reid BC, Mongeau SW, York AK: The military oral health care system as a model for eliminating disparities in oral health. J Am Dent Assoc 137: 372–378, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Patwardhan MB, Samsa GP, Matchar DB, Haley WB: Advanced chronic kidney disease patterns among nephrologists and non-nephrologists: A database analysis. Clin J Am Soc Nephrol 2: 277–283, 2007 [DOI] [PubMed] [Google Scholar]
- 9.http://www.kidney.org/professionals/KDOQI/guidelines_ckd/p5_lab_g4.htm, Table 47, Equation Number 3, accessed August 16, 2007
- 10.http://www.oqp.med.va.gov/cpg/ESRD/ESRD_Base.htm, accessed August 20, 2007
- 11.Wyatt C, Konduri V, Eng J, Rohatgi R: Reporting of estimated GFR in the primary care clinic. Am J Kidney Dis 49: 634–641, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Ansell D, Udayaraj UP, Steenkamp R, Dudley CR: Chronic renal failure in kidney transplant recipients. Do they receive optimum care?: Data from the UK renal registry. Am J Transplant 7: 1167–1176, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Lenz O, Mekala DP, Patel DV, Fornoni A, Metz D, Roth D: Barriers to successful care for chronic kidney disease. BMC Nephrol 6: 11, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, Powe NR: The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 137: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Woodard LD, Kressin NR, Petersen LA: Is lipid-lowering therapy underused by African Americans at high risk of coronary heart disease within the VA health care system? Am J Public Health 94: 2112–2117, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuart-Shor EM, Nannini A, Ostrem M, Russell GE, Mittleman MA: The prevalence of blood pressure and cholesterol monitoring in Boston among non-Hispanic Blacks, Hispanics, and non-Hispanic Whites Ethn Dis 16: 375–383, 2006 [PubMed] [Google Scholar]
- 17.Jafar TH, Schmid CH, Levey AS: Serum creatinine as marker of kidney function in South Asians: A study of reduced GFR in adults in Pakistan. J Am Soc Nephrol 16: 1413–1419, 2005 [DOI] [PubMed] [Google Scholar]