Abstract
Background and Objectives: Aminoglycoside antibiotics are commonly used in chronic kidney disease stage 5 patients. The purpose of this study was to characterize gentamicin pharmacokinetics, dialytic clearance, and removal by hemodialysis and to develop appropriate dosing strategies.
Design Setting, Participants, and Measurements: Eight subjects receiving chronic, thrice-weekly hemodialysis with no measurable residual renal function received gentamicin after a hemodialysis session. Blood samples were collected serially, and serum concentrations of gentamicin were determined.
Results: Median (range) systemic clearance, volume of distribution at steady state, and terminal elimination half-life were 3.89 ml/min (2.69–4.81 ml/min), 13.5 L (8.7–17.9 L), and 39.4 h (32.0–53.6 h), respectively. Median (range) dialytic clearance, estimated amount removed, and percent maximum rebound were 103.5 ml/min (87.2–132.7 ml/min), 39.6 mg (19.7–43.9 mg), and 38.7% (0%–71.8%), respectively. Gentamicin dialytic clearance was statistically significantly related to creatinine dialytic clearance (r2 = 0.52, P = 0.04), although this relationship is not likely to be strong enough to serve as a surrogate for gentamicin monitoring. The pharmacokinetic model was used to simulate gentamicin serum concentrations over a one-wk period.
Conclusions: In clinical situations where gentamicin is used as the primary therapy in a patient receiving hemodialysis with a CAHP hemodialyzer, conventional doses after each dialysis session are not as efficient at achieving treatment targets as predialysis dosing with larger doses.
According to the U.S. Renal Data System, infections are a major cause of morbidity and mortality in hemodialysis patients (1). Several studies have been performed evaluating gentamicin pharmacokinetics in patients with chronic kidney disease (2–4), and numerous others have evaluated gentamicin removal by “traditional” hemodialysis, (2,3,5–11) newer dialytic techniques (12), and alternative dosing strategies (13–16). The removal of gentamicin by the dialyzers studied is highly variable across these studies and is due in part to the different types of dialyzers used, length of dialysis sessions, hemodialysis operating characteristics, patient characteristics, delivered Kt/V, etc.
Current dosing guidelines for gentamicin use in patients with renal failure (17) receiving hemodialysis suggest that one half of the “full dose” should be given after hemodialysis. The widely used guideline did not make reference to the type of dialyzer being used or any other hemodialysis treatment characteristics, even though these characteristics greatly influence gentamicin dialytic clearance (7). Because gentamicin and other aminoglycosides have a narrow therapeutic index, optimization of therapy while minimizing the risk of toxicity to residual renal function or vestibular toxicity is important for these patients with renal failure who have prolonged exposure to the drug. In addition, because the peak gentamicin serum concentration to minimum inhibitory concentration (MIC) ratio and area under the curve (AUC)/MIC ratio are the most important predictors of efficacy for aminoglycosides, opportunities to maximize therapeutic goals while minimizing risk of toxicity are important (18). Efforts to maximize these pharmacodynamic parameters have been successful in other populations (19,20), but not in patients receiving hemodialysis. This is due to the difficulty in achieving high peak/MIC ratios while minimizing “trough” concentrations with conventional dosing regimens. A novel way to administer aminoglycosides to patients receiving hemodialysis, such that pharmacodynamics may be maximized, was described a decade ago (13) and more recently evaluated (14,15). These studies evaluated the potential for administering aminoglycosides immediately before hemodialysis, rather than after hemodialysis. Administration in this fashion more closely mimics extended interval aminoglycoside dosing, maximizing peak concentrations while minimizing extended exposure.
The purpose of this study was several-fold: to characterize gentamicin pharmacokinetics in otherwise healthy patients with chronic kidney disease requiring chronic hemodialysis, to determine the dialytic clearance and removal of gentamicin by hemodialysis with a CAHP-210 hemodialyzer, and to investigate the relationship between gentamicin removal and urea and creatinine removal during hemodialysis. Finally, our study was performed to investigate novel gentamicin dosing strategies through pharmacokinetic/pharmacodynamic simulation techniques to explore regimens that may maximize pharmacokinetic/pharmacodynamic relationships as compared with conventional gentamicin regimens used in patients with chronic kidney disease stage 5 receiving hemodialysis.
Concise Methods
Subjects and Study Protocol
Eight subjects from the outpatient hemodialysis unit at Indiana University Hospital (Indianapolis, IN) were enrolled in the study. Individuals were considered for enrollment if they were at least 18 yr of age, received maintenance hemodialysis three times weekly, had no acute concurrent illness, and their postdialysis body weight was within 30% of their ideal body weight. Subjects were excluded from the study if they had a history of gentamicin allergy or had received gentamicin within 3 wk of study initiation. The study was approved by the Indiana University-Purdue University Indianapolis Institutional Review Board. All subjects gave written informed consent before participating in the study.
After completion of a scheduled hemodialysis session, each subject received gentamicin 1.5 mg/kg as a one-hour intravenous infusion, through the venous hemodialysis access needle that was kept in the patient for this purpose. Venous blood samples were obtained immediately before and 0.5, 1, 1.5, 2, 3, and 5 h after the infusion was started from the predialyzer port of the subject's hemodialysis access that was kept in the patient for this purpose. Subjects returned to the hemodialysis center the following day, and the 24 h blood sample was obtained by direct venipuncture. Subjects returned to the hemodialysis center the following day for their next hemodialysis session and at approximately 44 h after the initiation of gentamicin administration (just before their hemodialysis session), a blood sample was obtained from the predialyzer port of the subject's hemodialysis access.
At the midpoint of the hemodialysis session, simultaneous blood samples were obtained from the predialyzer and postdialyzer access ports of the hemodialyzer circuit. Additional venous blood samples were collected at the end of that hemodialysis period, and 0.5, 1, 2, and 4 h after the completion of hemodialysis.
All blood samples described above were collected into nonheparinized evacuated blood collection test tubes and allowed to clot at room temperature for at least 30 min. The samples were then centrifuged and the serum harvested and stored in the −70°C freezer until assayed.
All subjects were dialyzed with a new, nonreused cellulose acetate high performance (CAHP-210) hemodialyzer with a surface area of 2.1 m2, and ultrafiltration coefficient of 13.2 ml/h per mmHg. The hemodialysis treatment was 3.5 to 4 h long. The hemodialysis operating characteristics were the same as used for their regularly scheduled hemodialysis treatments.
Analytical Methods
Serum concentrations of gentamicin were determined by the enzyme multiplied immunoassay technique. Linear calibration curves were obtained for gentamicin over the concentration range of 0.5 to 10 μg/ml. Samples with concentrations above this range were diluted and reassayed. The within-day and between-day coefficients of variation for replicate control samples were less than 12% at 1 μg/ml and 8 μg/ml. Urea nitrogen and creatinine concentrations were determined by colorimetric methods. This assay had an intra- and interassay coefficient of variation of less than 5%, for both creatinine and urea nitrogen.
Pharmacokinetic Analysis
Differential equations describing a two-compartment, open-pharmacokinetic model were fit to each subject's gentamicin serum concentration-time data using the Bayesian estimator in the Adapt II (21) software package.
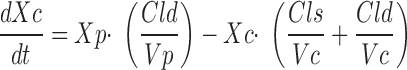 |
(1) |
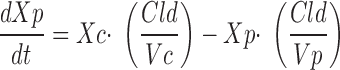 |
(2) |
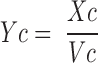 |
(3) |
where Xc and Xp are the amounts of gentamicin in the central and peripheral compartments, respectively, Vc and Vp are the apparent volume of distribution in the central and peripheral compartments, respectively, Cld is the distribution clearance between the central and peripheral compartment, Cls is systemic clearance, and Yc is the ADAPT output equation describing the estimated gentamicin serum concentrations. Volumes of distribution were assumed to be constant during the entire study period. Systemic clearance of gentamicin was assumed to occur from the central compartment only. Based upon the fitted pharmacokinetic parameters obtained by this model (Vc, Vp, Cld, and Cls), apparent volume of distribution at steady state (Vss) was calculated as the sum of Vc and Vp, and interdialytic elimination rate constant and interdialytic elimination half-life (t1/2) were calculated by standard equations.
Dialytic clearance (Cldial) was estimated by equation 4, the Fick Equation,
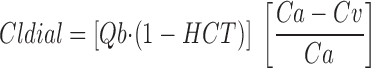 |
(4) |
where HCT is hematocrit, Qb is blood flow rate, Ca is the observed concentration of solute (gentamicin, creatinine, urea) in the arterial port of the dialyzer circuit, and Cv is the observed concentration of solute (gentamicin, creatinine, urea) in the venous port of the dialyzer circuit.
Rebound was calculated using equation 5:
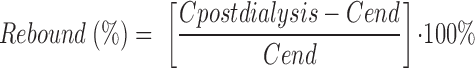 |
(5) |
where Cpostdialysis is the observed concentration of solute obtained from the venous port of the dialyzer circuit after hemodialysis was completed and Cend is the maximum observed concentration of solute after hemodialysis was completed.
Equilibrated Kt/Vurea was calculated using an “equilibrated” single pool model that used a predialysis and 30-min postdialysis BUN using the method of Daugirdas (22). Urea volume of distribution (Vurea) was calculated by the Watson method (23). The 30-min equilibrated urea reduction ratios was defined as:
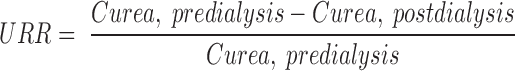 |
(6) |
Statistical Analysis
Linear regression analysis was used to investigate the relationship between measures of urea and creatinine removal and measures of gentamicin removal. Statistical analysis was performed using JMP for Windows (SAS Institute, Cary, NC). Parameters were considered statistically significant at P < 0.05.
Simulations
Population simulations with output noise were performed using the ADAPT II computer program (21) to evaluate the ability of several gentamicin dosing regimens to obtain effective peak concentrations while maintaining trough concentrations that are likely to avoid toxicity. Serum concentrations were simulated following 5 different gentamicin dosing regimens. The five gentamicin regimens were as follows: Regimen A, 2 mg/kg loading dose, followed by 1 mg/kg administered immediately after each dialysis session; Regimen B, 3.5 mg/kg loading dose, followed by 3.5 mg/kg administered 1 h before each dialysis session; Regimen C, 4 mg/kg loading dose, followed by 3 mg/kg administered 1 h before each dialysis session; Regimen D, 3.1 mg/kg loading dose, followed by 2.75 mg/kg administered immediately 1 h before each dialysis session; and Regimen E, 3 mg/kg loading dose, followed by 3 mg/kg administered immediately 1 h before each dialysis session. The dialysis regimen studied was a 2 d-2 d-3 d interdialytic period.
Each population simulation consisted of 500 simulated patients with the output error derived from our pharmacokinetic model using the mean ± SD pharmacokinetic parameters (Vc, Vp, Cls, Cld, and Cldial) and variance parameters in this study. The serum concentrations were simulated for one wk after the first gentamicin dose and included 3 doses of gentamicin and 3 dialysis sessions. At each peak (30 min after the end of the 30 min infusion), the percent of simulated patients who achieved a target of ≥8 μg/ml, predose or predialysis or predose concentration <2 μg/ml, AUC ≥140 μg/h per ml and AUC ≤240 μg/h per ml 48 h postdose (14,15). The final predialysis or predose concentration was obtained 164 h after the initial dose or 72 h after the third dose because of the nature of the hemodialysis scheduling.
Results
Six males and two females completed the study. The median (range) ages and weights were: 51.5 years (39–71 years) and 76 kg (37–84 kg), respectively. The median (range) dose of gentamicin was: 1.5 mg/kg (1.2 to 1.7 mg/kg). All subjects had been undergoing thrice-weekly hemodialysis for at least one year before participation.
Individual and group median gentamicin pharmacokinetic parameters are illustrated in Table 1. Overall, there was good agreement between observed and model-derived serum concentrations. In all subjects taken together, 82% of the fitted data points were within 5% of the observed data points and 100% of the fitted data points were within 10.1% of the observed data points.
Table 1.
Individual and median gentamicin pharmacokinetic parameter estimates and body weight in study subjects
| Subject No. | Cls (ml/min) | Cld (ml/min) | Vc (L) | Vp (L) | Vss (L) | t1/2β (h) | Weight (kg) |
|---|---|---|---|---|---|---|---|
| 1 | 4.07 | 188 | 5.30 | 9.06 | 14.4 | 41.1 | 75 |
| 2 | 3.84 | 199 | 5.62 | 11.3 | 16.9 | 51.4 | 80 |
| 3 | 2.69 | 95.9 | 3.32 | 5.36 | 8.67 | 37.6 | 37 |
| 4 | 3.94 | 150 | 5.24 | 7.43 | 12.7 | 37.5 | 81 |
| 5 | 3.27 | 195 | 2.69 | 6.31 | 8.99 | 32.0 | 61 |
| 6 | 4.46 | 142 | 4.31 | 8.34 | 12.7 | 33.2 | 84 |
| 7 | 4.81 | 175 | 7.47 | 10.4 | 17.9 | 43.5 | 77 |
| 8 | 3.48 | 240 | 6.61 | 9.47 | 16.1 | 53.6 | 65 |
| Median | 3.89 | 182 | 5.27 | 8.70 | 13.5 | 39.4 | 76 |
Cls, systemic clearance; Cld, distribution clearance between the central and peripheral compartment; Vc, apparent volume of distribution in the central compartment; Vp, apparent volume of distribution in the peripheral compartment; Vss, apparent volume of distribution at steady-state; and t1/2β, terminal elimination half-life (i.e., interdialytic half-life).
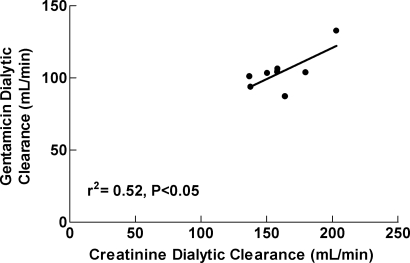
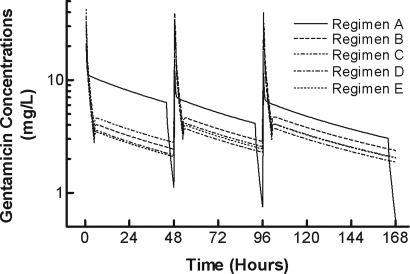
Individual and median estimates of gentamicin and urea dialytic clearance, amount of gentamicin removed, and gentamicin rebound characteristics are shown in Table 2. The median (range) blood flow rates, dialysate flow rates, duration of dialysis, and hematocrits were: 400 ml/min (400–450 ml/min), 600 ml/min (600–800 ml/min), 4.0 h (3.5–4.0 h), and 35.4% (31.4%–47.1%), respectively. All subjects had been undergoing thrice-weekly hemodialysis for at least one year before participation. As shown in Figure 1, gentamicin dialytic clearance was statistically significantly related to creatinine dialytic clearance (r2 = 0.52, P = 0.04) but not urea dialytic clearance (r2 = 0.40, P = 0.09), urea reduction ratio (r2 = 0.21, P = 0.16), or urea equilibrated Kt/V (r2 = 0.40 P = 0.09) (data not shown). Mean ± SD concentration-time profiles for the 5 doses are depicted in Figure 2. The simulated data are depicted in Tables 3 and 4.
Table 2.
Individual and median gentamicin dialytic clearance, gentamicin removal estimates, urea removal estimates, and rebound characteristics
| Cldialurea (ml/min) | CldialCr (ml/min) | Cldialgent (ml/min) | eKt/Vurea | Tmax,rebound (h) | Maximum Rebound (%) | |
|---|---|---|---|---|---|---|
| 1 | 211 | 150 | 103 | 1.38 | 2 | 35.6 |
| 2 | 157 | 137 | 101 | 1.26 | 2 | 60.4 |
| 3 | 210 | 158 | 106 | 1.75 | 4 | 62.2 |
| 4 | 177 | 158 | 104 | 1.44 | 4 | 25.9 |
| 5 | 223 | 180 | 104 | 1.33 | 2 | 71.8 |
| 6 | 192 | 138 | 94.0 | 1.20 | 4 | 41.8 |
| 7 | 193 | 164 | 87.2 | 1.44 | 4 | 29.9 |
| 8 | 243 | 203 | 133 | 1.58 | 1 | 0 |
| Median | 201 | 158 | 104 | 1.41 | 3 | 38.7 |
Cldial, dialytic clearance; Vurea, estimated urea volume of distribution; eKT/Vurea, 30-min equilibrated KT/Vurea.
Figure 1.
Relationship between gentamicin dialytic clearance and creatinine dialytic clearance. The relationship was significant (r2 = 0.52, P < 0.05). The closed circles (•) represent the individual data points and the solid line represents the best-fit line to the data.
Figure 2.
Gentamicin mean concentration-time profiles for simulated dosing regimens A (solid line), B, C, D, and E (hatched lines). The profiles are shown for the intradialyitic and interdialytic periods for one week after the administration of the first drug dose. The times for the dialysis session were 44 to 48, 92 to 96, and 164 to 168 h.
Table 3.
AUC, Cmax, and Cmin following the first, second, and third doses of gentamicin given before (Regimen A) or after (Regimens B-E) hemodialysis
| Regimen | First Dose
|
Second Dose
|
Third Dose
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC (μg · h per ml) | Cmax (μg/ml) | Cmin (μg/ml) | AUC (μg · h per ml) | Cmax (μg/ml) | Cmin (μg/ml) | AUC (μg · h per ml) | Cmax (μg/ml) | Cmin (μg/ml) | |
| A | 383 ± 102 | 13.1 ± 2.06 | 6.31 ± 2.76 | 245 ± 74.5 | 7.94 ± 1.21 | 4.12 ± 1.99 | 236 ± 71.3 | 7.48 ± 1.11 | 3.04 ± 1.87 |
| B | 192 ± 46.4 | 22.9 ± 3.60 | 2.44 ± 1.21 | 220 ± 65.3 | 25.3 ± 3.72 | 2.85 ± 1.57 | 225 ± 70.7 | 25.7 ± 3.78 | 2.36 ± 1.66 |
| C | 219 ± 53.0 | 26.1 ± 4.11 | 2.79 ± 1.38 | 197 ± 61.6 | 22.4 ± 3.30 | 2.57 ± 1.45 | 195 ± 62.2 | 22.1 ± 3.26 | 2.05 ± 1.45 |
| D | 170 ± 41.0 | 20.3 ± 3.19 | 2.16 ± 1.07 | 176 ± 53.3 | 20.1 ± 2.96 | 2.29 ± 1.27 | 178 ± 56.1 | 20.2 ± 2.98 | 1.86 ± 1.31 |
| E | 165 ± 39.7 | 19.6 ± 3.09 | 2.09 ± 1.04 | 189 ± 56.0 | 21.7 ± 3.19 | 2.45 ± 1.34 | 193 ± 60.6 | 22.0 ± 3.24 | 2.03 ± 1.42 |
Values are mean ± SD. Regimens and targets are described in the text.
Table 4.
Percent of simulated patients at AUC, Cmax, and Cmin following the first, second, and third doses of gentamicin given before (Regimen A) or after (Regimens B-E) hemodialysis
| Regimen | First Dose
|
Second Dose
|
Third Dose
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (μg · h per ml)
|
Cmax (μg/ml) ≥8 | Cmin (μg/ml) <2 | AUC (μg · h per ml)
|
Cmax (μg/ml) ≥8 | Cmin (μg/ml) <2 | AUC (μg · h per ml)
|
Cmax (μg/ml) ≥8 | Cmin (μg/ml) <2 | |||||||
| ≤240 | ≥140 | 140–240 | ≤240 | ≥140 | 140–240 | ≤240 | ≥140 | 140–240 | |||||||
| A | 8.0 | 99.4 | 7.4 | 99.8 | 7.4 | 46.4 | 90.4 | 36.8 | 45.6 | 16.8 | 51 | 90.2 | 41.2 | 31.8 | 33.6 |
| B | 84.4 | 85.2 | 69.6 | 100 | 38 | 64.2 | 88.2 | 52.4 | 100 | 32.6 | 61 | 88.2 | 49.2 | 100 | 47.4 |
| C | 64.8 | 93.4 | 58.2 | 100 | 30 | 75.6 | 79.8 | 55.4 | 100 | 39 | 77 | 78.8 | 55.8 | 100 | 53.8 |
| D | 95.8 | 74.6 | 70.4 | 100 | 44.2 | 87.6 | 73.2 | 60.8 | 100 | 44 | 86.4 | 73.2 | 59.6 | 100 | 58.8 |
| E | 96.8 | 71.8 | 68.6 | 100 | 47.4 | 81.8 | 78.2 | 60 | 100 | 39.8 | 78.4 | 78.8 | 57.2 | 100 | 54.6 |
Targets represents those with AUC less than or equal to 240 μg/h per ml, greater than or equal to 140 μg/h per ml, and 140 to 240 μg/h per ml; peak concentrations more than the target of 8 μg/ml or those with minimum concentrations less than 2 μg/ml.
Discussion
The removal of gentamicin by hemodialysis is highly variable, depending on the dialyzers used, length of dialysis sessions, hemodialysis operating, and patient characteristics, etc. Gentamicin and other aminoglycosides have a narrow therapeutic index, and as such, optimization of therapy while minimizing the risk of toxicity is especially important for patients with chronic kidney disease stage 5 requiring hemodialysis who have prolonged exposure to the drug. In addition, because the peak to MIC ratio or AUC/MIC ratio are the most important predictors of efficacy for aminoglycosides, opportunities to maximize therapeutic goals while minimizing risk of toxicity are essential (18).
Dosing guidelines (17) for gentamicin use in patients with chronic kidney disease receiving hemodialysis simply suggest that one half of the “full dose” should be given after hemodialysis. In this study, we determined the pharmacokinetics of gentamicin and its removal by a contemporary hemodialyzer in a group of otherwise healthy subjects with chronic kidney disease. We then performed simulations from the data with the ultimate goal of testing commonly used dosing regimens of gentamicin to develop a rational dosing method for this drug.
The pharmacokinetics of gentamicin in patients with chronic kidney disease have been reported numerous times in the literature and summarized (24). The pharmacokinetic parameter estimates obtained in the current study are similar with those earlier papers. As expected, the systemic clearance is lower and terminal elimination half-life is longer than that reported in normal individuals. Although it is difficult to compare dialytic clearance values from study to study because of the differences noted above, the median dialytic clearance values of 103.5 ml/min are at least consistent with previous data using other contemporary hemodialyzers of similar permeability (5,11). In addition, in vitro dialytic clearances of gentamicin with the CAHP-210 hemodialyzer in our laboratory are comparable with these clearance values (25).
Hemodialysis is generally dosed based on urea removal. Authors of Kidney Dialysis Outcomes Quality Initiative recommend a selected minimum Kt/Vurea for all patients. Because of the variability that exists in gentamicin dialytic removal, knowledge of the relationship between gentamicin removal and urea removal is important to predict gentamicin removal. Because Kt/Vurea is the most commonly used measure of dialysis adequacy, we sought to investigate the relationship between gentamicin dialytic removal and urea dialytic removal. Alternatively, because creatinine is another commonly measured laboratory value to assess renal disease and dialytic clearance, it may also be a useful marker. As illustrated in Figure 1, gentamicin dialytic clearance was statistically significantly predicted by creatinine dialytic clearance, but not the more clinically used Kt/Vurea or urea reduction ratios. In addition, even though there was a statistically significant relationship between gentamicin clearance and both urea and creatinine clearance, it is not clear if either would be useful in predicting the first dose or subsequent doses of gentamicin. Studies investigating the relationship between measures of dialysis adequacy and gentamicin removal during per-dialysis dosing of gentamicin are necessary.
As has been reported in previous studies (2,5,26), we observed substantial rebound of gentamicin concentrations after the discontinuation of hemodialysis. The median maximal percent increase in gentamicin concentrations was 38.7% and occurred a median of 3 hours after the completion of hemodialysis. Consistent with previous data (2,5,26), the degree of rebound was extremely variable ranging from 0% to 70%. The rebound phenomenon is the result of the slower transfer of drug from the peripheral tissues to plasma than dialytic removal of the drug. The time to maximum rebound observed in this study differed from what has been reported in the literature. This difference is partially due to the change in the time of collecting gentamicin samples after hemodialysis. In this study, we collected data for 4 hours after dialysis, whereas others have collected blood samples for shorter time periods. Even with the extended sampling time to 4 hours after dialysis, 3 of the 8 subjects in this study had a time to maximum rebound of 4 hours, suggesting that a longer time period of collecting data after hemodialysis may be necessary to accurately assess the amount of gentamicin removed by hemodialysis. Our data are in contrast to a recent study (11), which suggests that a single sample 1 h following dialysis incorporated into a Bayesian computer model allows adequate characterization of gentamicin rebound following hemodialysis.
Based on the pharmacokinetics of gentamicin and its removal by hemodialysis observed in this study, we simulated one commonly used gentamicin dosing strategy and 4 novel dosing regimens for patients receiving hemodialysis. Two of these regimens have been previously explored in an earlier publication (14,15), and one was found to be the most desirable for this type of dosing. We performed simulations, assuming a hemodialysis treatment scheme of 2 d-2 d-3 d intradialytic periods. Serum concentrations over the simulated one-wk period are illustrated in Figure 2 and Table 3.
Aminoglycoside antibiotics are concentration-dependent bacteriocidal drugs and, as such, are most efficacious when high peak/MIC or AUC/MIC ratios are achieved (18). Peak concentrations that exceed the MIC of susceptible organisms by 8- to 10-fold are associated with clinical response rates of ≥90% and can reduce the rates of emergence of aminoglycoside resistant mutants (18). Extended interval or “once daily” dosing schemes are frequently used for aminoglycosides to maximize the pharmacokinetic/pharmacodynamic relationships. These dosing schemes also allow for serum concentrations of aminoglycosides to fall well below toxic concentrations for large portions of the dosing interval and minimize exposure in the kidney and ear. Uptake of aminoglycosides into those tissues is more efficient at low sustained concentrations (i.e., troughs) rather than high intermittent concentrations (i.e., peaks) (18). Because of the relatively long postantiobiotic effect that exists for aminoglycosides, in theory extended interval dosing schemes maximize efficacy and minimize toxicity.
Patients with chronic kidney disease receiving hemodialysis present dosing challenges. Extended interval aminoglycosides are not used routinely in this patient population because of the lack of intrinsic renal function and resultant reduced aminoglycoside clearance. The reduced renal clearance result in extended periods of elevated “trough” concentrations. Gentamicin dosing regimens in hemodialysis patients typically require dosing after each hemodialysis session. Failure to redose after each hemodialysis session results in prolonged periods of time when serum concentrations are just above or near the MIC of the organism resulting in poor bacteriocidal activity and potential development of resistance. Traditional gentamicin regimens used in patients with normal renal function typically are aimed at achieving peak concentrations ≥8 μg/ml, depending on the site and severity of the infection, and trough concentrations that are less than 2 μg/ml. We used these arbitrary cutoffs as our targets for peak and trough concentrations because no data exist in dialysis patients defining appropriate peak and “trough” concentrations.
Recent data in hemodialysis patients receiving aminoglycosides suggest that extended interval aminoglycosides may be a reasonable dosing method if the drugs are administered just before hemodialysis (14,16). This allows high peak concentrations followed by removal by hemodialysis, minimizing sustained elevated serum concentrations. Traditional gentamicin regimens used in patients with normal renal function typically were aimed at 24-h AUC between 70 and 120 μg/h per ml (14,15), depending on the site and severity of the infection. Because no such data exist in dialysis patients, we used 48-h AUC values that were twice the 24-h AUC values for target values. This approach has been used previously (14,15). Tables 3 and 4 show the data from the simulations and indicate that target concentrations are achievable in patients with chronic kidney disease, receiving hemodialysis with the CAHP hemodialyzer. These results clearly suggest that the predialysis dosing of aminoglycosides is likely to be more effective and possibly less toxic than the postdialysis dosing. After the first dose, all regimens were effective in achieving peak concentrations greater than or equal to 8 μg/ml (at least 99.8% ≥ target). Subsequent doses were equally effective in the simulated patients receiving predialysis doses, at achieving the target but not in the conventional postdialysis regimen, where less than 50% of simulated patients achieved the target peak concentrations. Even with the higher doses administered in the predialysis group, all of the regimens and doses had substantially higher percentages of patients with minimum concentrations less than 2 μg/ml as compared with the conventional dosing. A maximum 48-h AUC of 240 and a minimum AUC of 140 was proposed previously as reasonable targets in dialysis patients receiving gentamicin (14,15). As evidenced by the data in Tables 3 and 4, all average AUC data fall within the range of desirable AUCs. As expected, based on Cmax and Cmin observations, substantially more simulated patients receiving the predialysis dosing had AUCs within the target range than simulated patients receiving conventional dosing after hemodialysis. Those simulated patients were primarily outside the range because of excessive exposure rather than insufficient exposure.
Conclusion
Hemodialysis with the CAHP-210 hemodialyzer is highly effective in removing gentamicin. However, gentamicin clearance does not relate to urea clearance, Kt/Vurea, or urea reduction ratio; consequently, the predictability of gentamicin dialytic clearance cannot be garnered from the urea removal indices. While creatinine dialytic clearance did significantly relate to gentamicin dialytic clearance, this relationship was not strong enough to serve as a surrogate for gentamicin monitoring. Gentamicin serum concentrations should be monitored to assure adequate care. Finally, in clinical situations where gentamicin is used as the primary therapy in a patient receiving hemodialysis with a CAHP hemodialyzer, loading doses of 2 mg/kg followed by maintenance doses of 1 mg/kg after each dialysis session are not as efficient at achieving pharmacokinetic/pharmacodynamic targets as predialysis dosing with larger doses, regardless of the regimen. Future studies should evaluate the use of prehemodialysis dosing of aminoglycosides in hemodialysis patients as this may maximize pharmacodynamic endpoints while minimizing exposure to aminoglycosides.
Disclosure
Supported in part by a grant from the National Kidney Foundation of Indiana, Inc., Indianapolis, Indiana. The authors report no conflicts of interest.
Published online ahead of print. Publication date available at www.cjasn.org.
S. J. M. is currently affiliated with Clarian Health Partners, Inc., Indianapolis, IN.
M. K. S. is currently affiliated with Eli Lilly and Company, Indianapolis, IN.
References
- 1.U.S. Renal Data System: 2006 Annual Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, U.S. Renal Data, 2006
- 2.Matzke GR, Halstenson CE, Keane WF: Hemodialysis elimination rates and clearance of gentamicin and tobramycin. Antimicrob Agents Chemother 25: 128–130, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letourneau-Saheb L, Lapierre L, Daigneault R, Prud'Homme M, St-Louis G, Serois G: Gentamicin pharmacokinetics during hemodialysis in patients suffering from chronic renal failure. Int J Clin Pharmacol Biopharm 15: 116–120, 1977 [PubMed] [Google Scholar]
- 4.Dager WE, King JH: Aminoglycosides in intermittent hemodialysis: pharmacokinetics with individual dosing. Ann Pharmacother 40: 9–14, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Amin NB, Padhi ID, Touchette MA, Patel RV, Dunfee TP, Anandan JV: Characterization of gentamicin pharmacokinetics in patients hemodialyzed with high-flux polysulfone membranes. Am J Kidney Dis 34: 222–227, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R, Toto RD: Gentamicin clearance during hemodialysis: a comparison of high-efficiency cuprammonium rayon and conventional cellulose ester hemodialyzers. Am J Kidney Dis 22: 296–299, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R, Cronin RE: Heterogeneity in gentamicin clearance between high-efficiency hemodialyzers. Am J Kidney Dis 23: 47–51, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Danish M, Schultz R, Jusko WJ: Pharmacokinetics of gentamicin and kanamycin during hemodialysis. Antimicrob Agents Chemother 6: 841–847, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halpren BA, Axline SG, Coplon NS, Brown DM: Clearance of gentamicin during hemodialysis: comparison of four artificial kidneys. J Infect Dis 133: 627–636, 1976 [DOI] [PubMed] [Google Scholar]
- 10.Christopher TG, Korn D, Blair AD, Forrey AW, O'Neill MA, Cutler RE: Gentamicin pharmacokinetics during hemodialysis. Kidney Int 6: 38–44, 1974 [DOI] [PubMed] [Google Scholar]
- 11.Vercaigne LM, Ariano RE, Zacharias JM: Bayesian pharmacokinetics of gentamicin in a haemodialysis population. Clin Pharmacokinet 43: 205–210, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Manley HJ, Bailie GR, McClaran ML, Bender WL: Gentamicin pharmacokinetics during slow daily home hemodialysis. Kidney Int 63: 1072–1078, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Matsuo H, Hayashi J, Ono K, Andoh K, Andoh Y, Sano Y, Saruki K, Tanaka J, Yamashita M, Nakamura K, Kubo K: Administration of aminoglycosides to hemodialysis patients immediately before dialysis: a new dosing modality. Antimicrob Agents Chemother 41: 2597–2601, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teigen MM, Duffull S, Dang L, Johnson DW: Dosing of gentamicin in patients with end-stage renal disease receiving hemodialysis. J Clin Pharmacol 46: 1259–1267, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Dang L, Duffull S: Development of a semimechanistic model to describe the pharmacokinetics of gentamicin in patients receiving hemodialysis. J Clin Pharmacol 46: 662–673, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kamel Mohamed OH, Wahba IM, Watnick S, Earle SB, Bennett WM, Ayres JW, Munar MY: Administration of tobramycin in the beginning of the hemodialysis session: a novel intradialytic dosing regimen. Clin J Am Soc Nephrol 2: 694–699, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Aronoff GR, Bennett WM, Berns JS, Brier ME, Kasbekar N, Mueller BA, Pasko DA, Smoyer WE: Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children, 5th ed., Philadelphia, American College of Physicians, 2007
- 18.Craig WA: Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26: 1–10; quiz 11–12, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Begg EJ, Barclay ML, Duffull SB: A suggested approach to once-daily aminoglycoside dosing. Br J Clin Pharmacol 39: 605–609, 1995 [PMC free article] [PubMed] [Google Scholar]
- 20.Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R: Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 39: 650–655, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Argenio DZ, Schumitzky A: ADAPT II Release 4, User's Guide, Los Angeles, Biomedical Simulations Resource, University of Southern California, 1997
- 22.Daugirdas JT: Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol 4: 1205–1213, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Watson PE, Watson ID, Batt RD: Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 33: 27–39, 1980 [DOI] [PubMed] [Google Scholar]
- 24.Zaske DE: Aminoglycosides. In: Applied Pharmacokinetics Principles of Therapeutic Drug Monitoring, 3rd ed., edited by Evans WE, Schentag JJ, Jusko WJ, Vancouver, Applied Therapeutics, 2006
- 25.Sowinski KM, Magner S, Lucksiri A, Hamburger RJ, Mueller BA, Scott MK: Solute removal characteristics of the CAHP-210 hemodialyzer: in vitro and in vivo performance [Abstract]. Pharmacotherapy 21: 1288–1289, 2001 [Google Scholar]
- 26.Halstenson CE, Berkseth RO, Mann HJ, Matzke GR: Aminoglycoside redistribution phenomenon after hemodialysis: netilmicin and tobramycin. Int J Clin Pharmacol Ther Toxicol 25: 50–55, 1987 [PubMed] [Google Scholar]