Abstract
Background and objectives: Natriuretic peptides have been suggested to be of value in risk stratification in dialysis patients. Data in patients on peritoneal dialysis remain limited.
Design, setting, participants, & measurements: Patients of the ADEMEX trial (ADEquacy of peritoneal dialysis in MEXico) were randomized to a control group [standard 4 × 2L continuous ambulatory peritoneal dialysis (CAPD); n = 484] and an intervention group (CAPD with a target creatinine clearance ≥60L/wk/1.73 m2; n = 481). Natriuretic peptides were measured at baseline and correlated with other parameters as well as evaluated for effects on patient outcomes.
Results: Control group and intervention group were comparable at baseline with respect to all measured parameters. Baseline values of natriuretic peptides were elevated and correlated significantly with levels of residual renal function but not with body size or diabetes. Baseline values of N-terminal fragment of B-type natriuretic peptide (NT-proBNP) but not proANP(1–30), proANP(31–67), or proANP(1–98) were independently highly predictive of overall survival and cardiovascular mortality. Volume removal was also significantly correlated with patient survival.
Conclusions. NT-proBNP have a significant predictive value for survival of CAPD patients and may be of value in guiding risk stratification and potentially targeted therapeutic interventions.
Plasma levels of cardiac natriuretic peptides are elevated in patients with chronic kidney disease, owing to impairment of renal function, hypertension, hypervolemia, and/or concomitant heart disease (1–7). Atrial natriuretic peptide (ANP) and particularly brain natriuretic peptide (BNP) levels are linked independently to left ventricular mass (3–5,8–16) and function (3,6–17) and predict total and cardiovascular mortality (1,3,8,10,12,18) as well as cardiac events (12,19). ANP and BNP decrease significantly during hemodialysis treatment but increase again during the interdialytic interval (1,2,4,6,7,14,17,20–23). Levels in patients on peritoneal dialysis (PD) have been found to be lower than in patients on hemodialysis (11,24–26), but the correlations with left ventricular function and structure are maintained in both types of dialysis modalities (11,15,27,28).
The high mortality of patients on peritoneal dialysis and the failure of dialytic interventions to alter this mortality (29,30) necessitate renewed attention into novel methods of stratification and identification of patients at highest risk to be targeted for specific interventions. Cardiac natriuretic peptides are increasingly considered to fulfill this role in nonrenal patients. Evaluations of cardiac natriuretic peptides in patients on PD have been limited by small numbers (3,9,11,12,15,24–26) and only one study examined correlations between natriuretic peptide levels and outcomes (12). The PD population enrolled in the ADEMEX trial offered us the opportunity to evaluate cardiac natriuretic peptides and their value in predicting outcomes in the largest clinical trial ever performed on PD (29,30). It is hoped that such an evaluation would identify patients at risk even in the absence of overt clinical disease and hence facilitate or encourage interventions with salutary outcomes.
Concise Methods
We conducted a prospective randomized controlled clinical trial called ADEMEX, which examined the effect of increasing PD small solute clearances on select patient outcomes in individuals with ESRD treated with CAPD (29,30). The local clinical research committees of all participating centers approved the study protocol, and all study subjects gave written informed consent. Patients were recruited from 24 dialysis centers in 14 Mexican cities. The study enrolled 965 patients on CAPD between June 1998 and May 1999. Patients were randomized into either a control group (n = 484 patients receiving a standard dose of 4 × 2L CAPD) or an intervention group (n = 481 patients receiving a modified CAPD prescription aimed at achieving a creatinine clearance ≥60L/wk/1.73 m2). By design, the study was terminated in May 2001 when the last enrolled patient completed 2 yr of follow-up. A complete description of the study design, patient characteristics, etc. can be found in our previous publications (29,30). Although the ADEMEX trial was not specifically designed to evaluate the prognostic significance of cardiac natriuretic peptides, the availability of never-thawed frozen plasma samples offered us the opportunity to undertake such an evaluation. Similar approaches have been successfully implemented in other large clinical trials (31).
Determinations of cardiac natriuretic peptide concentrations were performed on baseline samples obtained from patients subsequently randomized to the control and intervention groups. We elected to measure the levels of inactive longer NH2-terminal fragments (i.e. NT-proANP and NT-proBNP) rather than the biologically active shorter COOH-terminal peptides (i.e. ANP or BNP) because of the longer half-lives and consequently higher plasma concentrations of NT peptides. It has been suggested that these inactive peptides better fit the definition of a disease marker than do circulating concentrations of ANP or BNP (32). Furthermore, because multiple assays for proANP exist with variable sensitivities that may affect clinical relevance (32), we simultaneously examined the concentrations of the full 98 amino acid NT peptide proANP(1–98) as well as smaller fragments [proANP(1–30) and proANP(31–67)] (32). There is no universal consensus on which assays of proANP are best for risk stratification, hence our decision to examine multiple peptides.
Plasma concentrations of proANP(1–30), proANP(31–67), and proANP(1–98) were determined by competitive enzyme immunoassays (Biomedica, Vienna, Austria). The N-terminal fragment of NT-proBNP was determined by immunoassay (ECLIA, Roche, Mannheim, Germany). Plasma albumin was measured by the bromocresol purple method. Plasma concentration of interleukin-6 (IL-6) was measured by an immunoassay (R&D Systems, Wiesbaden, Germany) and N-high sensitive C-reactive protein (hsCRP) was determined by an immunonephelometric assay (Dade-Behring, Vienna, Austria).
Pearson's χ2 test and Fisher's exact test were used to compare discrete baseline patient characteristics (e.g. gender, diabetes, comorbidity) whereas the t test and Wilcoxon's rank-sum test were used to compare continuous variables at baseline (e.g. age, serum chemistries, etc.). For those continuous variables having a skewed distribution (e.g. NT-proBNP), comparisons were also made based on log-transformed values of the original variable. Results of these different statistical tests were consistent for each of the continuous variables. Therefore, because the t test is robust against departures from normality provided the variance is similar between the groups being compared, all reported P values for comparing continuous variables are those based on the t test. Life table techniques in combination with a log-rank test as well as Cox proportional hazards regression were used to evaluate patient survival (33,34).
Results
Patient Characteristics and Baseline Findings
The original design of the trial of two separate groups (control and intervention) was respected in the analysis examining the effects of natriuretic peptides. Patients in the two groups were similar at baseline with respect to various demographic measurements, comorbidities, dialysis parameters at baseline, select serum chemistries, inflammatory markers, and cardiac natriuretic peptides (Tables 1 and 2). By study design, patients in the intervention group had significantly greater peritoneal clearances compared with those in the control group. Specifically, over the course of the study, mean peritoneal creatinine clearances (mean ± 1 SEM) averaged 46 ± 0.45 L/wk/1.73 m2 for the control group and 57 ± 0.48 L/wk/1.73 m2 for the intervention group (P < 0.001). Likewise, the mean peritoneal Kt/V values averaged 1.62 ± 0.02 for control patients and 2.13 ± 0.02 for intervention patients (P < 0.001). There was no significant difference in the time averaged GFR between the two groups (intervention-control mean difference of −0.20 ± 0.11 ml/min, P = NS), nor were there differences in normalized protein nitrogen appearance (nPNA), and serum albumin values over the course of the study.
Table 1.
Baseline clinical characteristics of the two study groups
| Parameter | Control Group | Intervention Group | P value |
|---|---|---|---|
| n | 484 | 481 | |
| Age (yr) | 47.9 ± 14.10 | 46.6 ± 13.7 | NS |
| Gender men:women (%) | 292:192 (60:40) | 271:210 (56:44) | NS |
| Diabetes | 217 (45%) | 201 (42%) | NS |
| Hypertension | 322 (66%) | 311 (65%) | NS |
| Systolic BP (mmHg) | 152.5 ± 26.8 | 151.7 ± 24.9 | NS |
| Diastolic BP (mmHg) | 89.6 ± 13.8 | 90.5 ± 13.9 | NS |
| Pulse pressure (mmHg) | 62.7 ± 20.8 | 61.2 ± 19.3 | NS |
| Heart rate (beats per min) | 80.3 ± 10.4 | 80.0 ± 10.8 | NS |
| Incident: prevalent (%) | 197:287 (41%:59%) | 205:276 (43%:57%) | NS |
| Anephric (GFR <1 ml/min) | 270 (56%) | 257 (54%) | NS |
Values are mean ± SD. Incident: prevalent refers to the vintage of patients on dialysis. Incident patients by definition had been on dialysis for less than 3 mo when enrolled in the study and prevalent patients for more than 3 mo.
Table 2.
Baseline laboratory measurements of the two study groups
| Parameter | Control Group | Intervention Group | P value |
|---|---|---|---|
| n | 484 | 481 | |
| GFR (ml/min) | 1.66 ± 2.45 | 1.54 ± 2.0 | NS |
| Urine volume (L/day) | 0.43 ± 0.54 | 0.44 ± 0.52 | NS |
| Peritoneal ultrafiltration (L/24 h) | 0.56 ± 0.68 | 0.61 ± 0.70 | NS |
| Total fluid removed (L/24 h) | 0.99 ± 0.71 | 1.06 ± 0.70 | NS |
| Albumin (g/dl) | 2.87 ± 0.64 | 2.95 ± 0.64 | NS |
| NPNA (g/kg/day) | 0.81 ± 0.22 | 0.80 ± 0.21 | NS |
| NT-proBNP (pg/ml) | 13,547 ± 13,761 | 13,123 ± 13,902 | NS |
| proANP(1–30) (nmol/L) | 2.54 ± 2.20 | 2.36 ± 2.13 | NS |
| proANP(1–98) (nmol/L) | 0.63 ± 0.98 | 0.60 ± 0.98 | NS |
| proANP(31–67) (nmol/L) | 3.46 ± 1.86 | 3.69 ± 1.88 | NS |
| IL-6 (pg/ml) | 8.96 ± 11.06 | 7.73 ± 10.10 | NS |
| HsCRP (mg/dl) | 1.06 ± 2.25 | 0.78 ± 1.37 | NS |
Values are mean ± SD; nPNA, normalized protein nitrogen appearance; NT-proBNP, N-terminal fragment of B-type natriuretic peptide; proANP, pro-atrial natriuretic peptide, IL-6, interleukin-6, HsCRP, highly sensitive C-reactive protein
Correlates of Baseline Natriuretic Peptides
Anephric patients (defined by a GFR <1 ml/min) had significantly higher plasma levels of NT-proBNP, proANP(1–30), and proANP(31–67) as compared with the nonanephric patients (defined by a GFR >1 ml/min) (Table 3). ProANP(1–98) did not differ between the anephric group and the nonanephric group. When patients were subdivided into those with diabetes and those without diabetes, no difference was observed between diabetic and nondiabetic patients for any of the natriuretic peptides irrespective of the level of renal function. The pattern described for the effects of anephric status on natriuretic peptides, however, continued to be observed independent of diabetic status: diabetic and nondiabetic anephric patients had significantly higher plasma levels of NT-proBNP, proANP(1–30), and proANP(31–67) as compared with diabetic and nondiabetic nonanephric patients. Again, proANP(1–98) did not differ between anephric and nonanephric patients (Table 3).
Table 3.
Cardiac natriuretic peptides by renal function status and diabetes
| Parameter | n | Anephric (GFR < 1 ml/min) | n | Nonanephric (GFR > 1 ml/min) | P value |
|---|---|---|---|---|---|
| All patients | |||||
| NT-proBNP (pg/ml) | 429 | 17,396 ± 14,067 | 356 | 8364 ± 11,729 | <0.0001 |
| proANP(1–30) (nmol/L) | 277 | 2.95 ± 2.25 | 245 | 1.89 ± 1.92 | <0.0001 |
| proANP(1–98) (nmol/L) | 277 | 0.67 ± 1.06 | 245 | 0.55 ± 0.89 | NS |
| proANP(31–67) (nmol/L) | 276 | 3.91 ± 1.90 | 245 | 3.20 ± 1.77 | <0.0001 |
| Diabetic patients | |||||
| NT-proBNP (pg/ml) | 156 | 18,443 ± 13,865 | 185 | 10,314 ± 12,369 | <0.0001 |
| proANP(1–30) (nmol/L) | 101 | 2.81 ± 2.23 | 132 | 1.97 ± 1.92 | <0.01 |
| proANP(1–98) (nmol/L) | 101 | 0.59 ± 0.99 | 132 | 0.51 ± 0.83 | NS |
| proANP(31–67) (nmol/L) | 100 | 3.96 ± 1.88 | 132 | 3.33 ± 1.77 | <0.01 |
| Nondiabetic patients | |||||
| NT-proBNP (pg/ml) | 273 | 16,798 ± 14,172 | 171 | 6254 ± 10,632 | <0.0001 |
| proANP(1–30) (nmol/L) | 176 | 3.03 ± 2.26 | 113 | 1.80 ± 1.93 | <0.0001 |
| proANP(1–98) (nmol/L) | 176 | 0.72 ± 1.09 | 113 | 0.59 ± 0.95 | NS |
| proANP(31–67) (nmol/L) | 176 | 3.89 ± 1.92 | 113 | 3.04 ± 1.76 | <0.001 |
Values are mean ± SD. There was no difference between diabetic or nondiabetic patients for any of the peptides in either the anephric or the nonanephric groups.
Baseline levels of natriuretic peptides correlated with a variety of parameters and among themselves (Table 4). NT-proBNP was found to correlate with the other natriuretic peptides, mainly with pro-ANP(1–30) (Table 4). There was a direct correlation between NT-proBNP and systolic BP and an inverse correlation between NT-proBNP and both urine volume and GFR. The correlation between NT-proBNP and GFR was significantly reduced (to r = −0.09) when controlled for urine volume. The other natriuretic peptides also correlated with systolic BP, urine volume and GFR, but these correlations were greatly reduced when corrected for NT-proBNP. None of the natriuretic peptides was correlated with age, gender, or any anthropomorphic measures.
Table 4.
Bivariate correlations of natriuretic peptides
| NT-proBNP | proANP (1–98) | proANP (1–30) | proANP (31–67) | |
|---|---|---|---|---|
| NT-proBNP | 1 | 0.165a | 0.610a | 0.285a |
| proANP(1–98) | 0.165a | 1 | 0.208a | 0.013 |
| proANP(1–30) | 0.610a | 0.208a | 1 | 0.386a |
| proANP(31–67) | 0.285a | 0.013 | 0.386a | 1 |
| Systolic BP | 0.256a | -0.010 | 0.161a | 0.133a |
| Urine volume | −0.318a | −0.086b | −0.250a | −0.189a |
| GFR | −0.305a | −0.057 | −0.240a | −0.202a |
| Albumin | −0.255a | −0.009 | −0.158a | −0.049 |
| IL-6 | 0.269a | 0.089** | 0.198a | 0.089b |
| HsCRP | 0.144a | 0.009 | 0.074 | −0.012 |
*Correlation is significant at the 0.01 level.
Correlation is significant at the 0.05 level
NT-proBNP correlated with inflammatory markers (inversely with albumin and directly with IL-6 and hsCRP). The correlation between NT-proBNP and albumin was reduced after correction for IL-6 but remained significant (r = −0.175, P < 0.001). The correlation between NT-proBNP and hsCRP lost its significance after controlling for IL-6. Controlling for urine volume did not eliminate the significant correlations between NT-proBNP and albumin (r = −0.2524, P < 0.001) or NT-proBNP and IL-6 (r = 0.2552, P < 0.001).
Predictive Value of Baseline Natriuretic Peptides
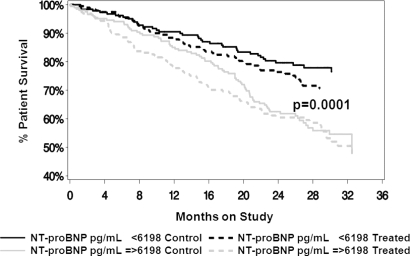
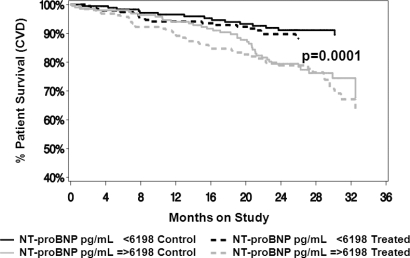
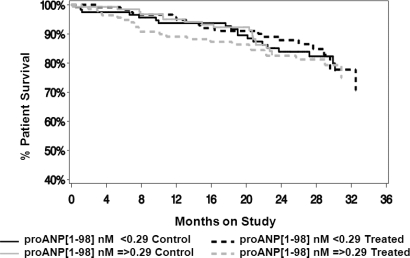
The value of baseline natriuretic peptides in predicting outcomes was explored by defining the population on the basis of median values of the respective natriuretic peptides (above or below the median value for the parameter of interest). NT-proBNP had a significant effect on overall survival (all cause mortality; P < 0.001) that was independent of whether patients were on the control prescription (control) or the enhanced treatment (treatment) (Figure 1). The same was true of the effects of NT-proBNP on cardiovascular mortality independent of the treatment assignment (P < 0.001) (Figure 2). Cardiovascular mortality was defined as death due to any cardiac or cerebrovascular disease or event. In contrast, proANP(1–98) had no effect on overall survival (all cause mortality) in either treatment assignment group (Figure 3). ProANP(1–98) also had no effect on cardiovascular survival in either treatment assignment group (data not shown). Similarly, neither proANP(1–30) nor proANP(31–67) had any effect on patient overall survival or patient cardiovascular mortality (data not shown).
Figure 1.
Patient overall survival (all cause mortality) by N-terminal pro-brain natriuretic peptide (NT-proBNP) and treatment assignment. The control (control) and the intervention (Treatment) groups were divided based on the median value of NT-proBNP. NT-proBNP had a significant effect on survival (P < 0.001) that was independent of the treatment assignment.
Figure 2.
Patient cardiovascular survival (cardiovascular mortality) by N-terminal pro-brain natriuretic peptide (NT-proBNP) and treatment assignment. The control (control) and the intervention (Treatment) groups were divided based on the median value of NT-proBNP. NT-proBNP had a significant effect on survival (P < 0.001) that was independent of the treatment assignment.
Figure 3.
Patient overall survival (all cause mortality) by pro-atrial natriuretic peptide proANP(1-98) and treatment assignment. The control (control) and the intervention (Treatment) groups were divided based on the median value of proANP(1-98). ProANP(1-98) had no effect on survival in either treatment assignment groups.
To explore whether the observed effect of NT-proBNP on overall and cardiovascular survival was due to its association with another parameter, we performed a Cox stepwise regression for overall mortality risk (Table 5) including a variety of other parameters that influence survival in the model. In this analysis, we subdivided the values of IL-6, hsCRP, and NT-proBNP into quintiles to further refine the stratification of patients at risk. In this model, hsCRP was not associated with survival. The lower quintile of IL-6 exhibited better survival. Each of the lower two quintiles of NT-proBNP was significantly associated with overall survival with the other quintiles as the collective controls. The survival benefit was similar when the two lower quintiles were combined by the choice of a single cutoff value for the analysis (<3465 pg/ml).
Table 5.
Cox stepwise regression for overall mortality risk
| Variable | Relative Risk | 95% Confidence interval | P value |
|---|---|---|---|
| Age (per 10 yr increase) | 1.18 | 1.052 to 1.324 | 0.0046 |
| Diabetes | 1.92 | 1.454 to 2.543 | <0.0001 |
| Peripheral vascular disease | 3.33 | 1.052 to 10.576 | 0.0408 |
| Baseline albumin (g/dl) | 0.61 | 0.498 to 0.738 | <0.0001 |
| Baseline nPNA (g/kg/day) | 0.49 | 0.262 to 0.919 | 0.0262 |
| Peritoneal ultrafiltration <0.4 L/day | 1.53 | 1.175 to 1.995 | 0.0016 |
| Urinary volume (per 0.1 L) | 0.93 | 0.890 to 0.969 | 0.0007 |
| IL-6 < 2.965 (pg/ml) | 0.58 | 0.362 to 0.940 | 0.0267 |
| NT-proBNP < 889 (pg/ml) | 0.63 | 0.406 to 0.967 | 0.0345 |
| 889 ≤ NT-proBNP <3465 (pg/ml) | 0.64 | 0.446 to 0.920 | 0.0160 |
The values for peritoneal ultrafiltration represent the lowest quintile of the time-dependent analysis. Time-dependent analysis was also used for urine volume. The value for IL-6 represents the lowest quintile of the baseline value. For NT-proBNP, the two lowest quintiles are those identified in the table.
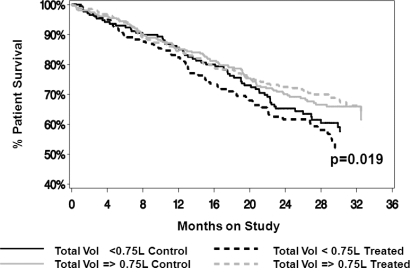
In the aforementioned analysis, we included time-dependent measures for peritoneal ultrafiltration and urine volume because of the expected relevance of volume homeostasis components to natriuretic peptides. Because the issue of volume control is increasingly relevant in defining treatment guidelines in PD, we have further explored the effect of baseline fluid removal on prediction of outcomes. Groups were divided based on a value of fluid removal identified in the European Automated Peritoneal Dialysis Study (EAPOS) as correlated with survival (35). Lower values of total fluid removal were significantly associated with an increase in all cause mortality (P < 0.01) independent of the treatment assignment (Figure 4). Likewise, lower values of total fluid removal were significantly associated with increased cardiovascular mortality (P < 0.05) independent of the treatment assignment. Because fluid removal may vary over time, we explored the effect of this parameter on survival in a time-dependent analysis. Adjusted relative risks associated with time-dependent values of peritoneal ultrafiltration divided by quintiles were calculated. Relative risk calculations were adjusted for treatment group, gender, age, diabetes, cardiovascular disease, peripheral vascular disease, hypertension, prior time on dialysis, baseline values of GFR, total body water, albumin, nPNA, and time-dependent values of urinary volume. Patients in the lowest quintile had significantly higher relative risk of death.
Figure 4.
Patient overall survival (all cause mortality) by total fluid removal (urinary and peritoneal) and treatment assignment. Groups were divided based on a value of fluid removal identified in the European Automated Peritoneal Dialysis Study (EAPOS) as correlated with survival (35). Total fluid removal (Vol) had a significant effect on survival (P < 0.01) that was independent of the treatment assignment.
Discussion
The findings of this study can be summarized as follows: plasma levels of cardiac natriuretic peptides are elevated in patients on PD; the levels observed correlate with the level of residual renal function and systolic BP; NT-proBNP [but neither proANP(1–30) nor proANP(31–67) or proANP(1–98)] is a powerful predictor of total as well as cardiovascular mortality.
Levels of cardiac natriuretic peptides have been known to reflect the severity of chronic kidney disease and to rise with diminishing GFR (36,37). Levels have been found to be elevated in patients on dialysis and to respond to manipulations in extracellular volume status (1,2,4,6,7,14,17,20–23). In the study presented here, we observed high levels of cardiac natriuretic peptides in patients on PD. These levels were higher in anephric patients than in patients with some degree of residual renal function. In concordance with other studies (38–40), we found no difference in natriuretic peptides levels between diabetic and nondiabetic patients. Others, however, had identified diabetes and obesity as associated with low plasma natriuretic peptide levels in the general population, and determined the negative effects of obesity and diabetes on natriuretic peptide levels to be additive (41). In patients on PD in this study, neither diabetes nor anthropomorphic parameters had any effects on natriuretic peptides levels.
ANP and particularly BNP levels are linked independently to left ventricular mass (3–5,8–16) and function (3,6–17) and predict total and cardiovascular mortality (1,3,8,10,12,18,40). This study, however, demonstrates that only NT-proBNP and not proANP(1–30), proANP(31–67), or proANP(1–98) is of predictive value in estimating all-cause and cardiovascular mortality in PD patients. ANP and BNP are synthesized and secreted mainly by cardiomyocytes. It is believed that ANP is preferentially produced in the atria, whereas BNP is preferentially synthesized in the ventricles, particularly in patients with chronic cardiac diseases (42). Chronic cardiac dysfunction induces the secretion of a greater amount of BNP than ANP (42). This may explain why in our study as well as in others [reviewed in (42)] NT-proBNP is a better diagnostic/prognostic index compared with proANP. Furthermore, previous studies had utilized only one representative assay for proANP peptides. Because differing assays may have different sensitivities, which may affect clinical relevance, we thought it important to simultaneously evaluate multiple assays. Our results suggest that the poor predictive value of proANP peptides is not because of a selection of a low sensitivity assay.
NT-proBNP is an inactive cleavage product with much slower clearance than the biologically active BNP (43,44). Its plasma concentration, therefore, principally reflects myocardial secretion over a prolonged time. Prior investigations have identified plasma BNP and NT-proBNP levels as independent predictors of mortality or cardiovascular events in populations with chronic heart failure (45,46), acute coronary syndromes (47,48), prior myocardial infarction (49,50), established vascular disease or elevated coronary risk (51,52), stable coronary heart disease (53), and in community-based samples (54,55). Evaluations in patients on PD have been limited by small numbers (3,9,11,12,15,24–26) and only one examined correlation between natriuretic peptide levels and outcomes (12).
Wang et al. showed that NT-proBNP is an important risk predictor of cardiovascular congestion, mortality, and adverse cardiovascular outcomes in chronic PD patients and adds important prognostic information beyond that contributed by left ventricular hypertrophy, systolic dysfunction, and other conventional risk factors (12). In the study presented here, we found that NT-proBNP was an independent predictor of all-cause mortality as well as cardiovascular mortality. The predictive value of NT-proBNP persisted even after correction for other factors known to influence outcomes in patients on PD including age, diabetes, presence of peripheral vascular disease, baseline albumin and nPNA, urine volume, peritoneal ultrafiltration, and measures of chronic inflammatory status (IL-6, hsCRP) (Table 5). Concordant with other studies (56), we found that urine volume was an independent predictor of outcome, and although levels of NT-proBNP correlated with levels of residual renal function (Tables 3 and 4), the effect of NT-proBNP on patient outcome persisted even after correction for the effect of urine volume (Table 5).
We and others (35) have found a relationship between peritoneal ultrafiltration and patient outcome. In the EAPOS trial, baseline peritoneal ultrafiltration was predictive of outcome, but not time-dependent peritoneal ultrafiltration (35). In the study presented here, we have reproduced the findings of EAPOS pertaining to baseline peritoneal ultrafiltration and have shown that when examined by quintiles, time-dependent peritoneal ultrafiltration is also predictive of outcomes (only for the lowest quintile used in Table 5). This analysis was performed because of the expected importance of controlling for factors affecting volume homeostasis when exploring the role of cardiac natriuretic peptides. Care should be taken, however, in the interpretation of these findings out of this particular context. The relevance of fluid removal volume in the absence of concomitant information about salt/fluid intake and volume status is questionable.
In a previous report (29), we had described the observation of a very small (100 ml/d) albeit statistically significant difference in time-averaged daily peritoneal ultrafiltration between the control and the intervention groups. The two groups, however, did not differ in terms of survival (29). It is difficult to assign clinical significance to this minimal difference. As pertains to natriuretic peptides, however, the study presented here demonstrated that the predictive value of baseline NT-proBNP was independent of treatment assignment.
Along with myocardial stretch, a variety of other factors have been found to stimulate or enhance secretion of BNP in vitro, including myocardial ischemia and paracrine or endocrine agents (57). This may explain why the predictive value of plasma NT-proBNP concentration was not eliminated by correction for other indices of volume balance. It may also imply that levels of NT-proBNP may be dependent on local and circulating factors that drive inflammation, fibrosis, and hypertrophy throughout the cardiovascular system (57). Indeed, we did find significant correlations between levels of NT-proBNP and inflammatory markers such as IL-6, hsCRP, and albumin. It is well known that cytokines (particularly TNF-α, IL-1, and IL-6) have a direct stimulating effect on cardiac natriuretic peptide secretion independent of other stimuli (42).
In the Cox regression model (Table 5), the various biomarkers had significant independent predictive value for all-cause mortality (Table 5). The persistence of the effect of NT-proBNP in the presence of other factors such as overt clinical disease, fluid removal, and inflammatory mediators suggests that it is an important indicator relevant for risk stratification. It has been suggested that combined use of biomarkers may enhance their predictive value (58). Scoring methods for such biomarker combinations, however, have not been systematized or validated and may be confounded by intermarker correlations.
In conclusion, our results suggest that cardiac natriuretic peptides such as NT-proBNP are independently predictive of all-cause mortality and cardiovascular mortality. Elevated levels of NT-proBNP may identify CAPD patients at risk for higher cardiovascular mortality. These observations invite serious consideration of these biomarkers as potential triggers for targeted therapeutic intervention. We recognize that correlation of a biomarker with an outcome is insufficient on its own to trigger a therapeutic intervention or even define the nature of the intervention. However, several lines of evidence suggest the need for evaluative studies. First, cardiovascular mortality remains exceedingly high in ESRD, and NT-proBNP is linked prognostically as well as diagnostically to cardiovascular disease burden. Second, levels of NT-proBNP are responsive to therapeutic interventions. Third, NT-proBNP guided treatment of patients with congestive heart failure has resulted in improved outcomes (59).
Disclosures
None.
Acknowledgments
This work was sponsored by Concejo Nacional de Ciencia y Tecnología, México (Grant CONACYT. U42396-M, 2002). The authors are grateful to all of the investigators and institutions that participated in the ADEMEX trial that are listed in previous publications (29,30). The authors appreciate the excellent technical assistance of Dr. Ch. Bieglmayer (Central Laboratory of the Medical University of Vienna) and Anna-Maria Raffetseder.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Horl WH: Natriuretic peptides in acute and chronic kidney disease and during renal replacement therapy. J Investig Med 53: 366–370, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Suresh M, Farrington K: Natriuretic peptides and the dialysis patient. Semin Dial 18: 409–419, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Zoccali C, Mallamaci F, Benedetto FA, Tripepi G, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Cottini E, Malatino LS: Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol 12: 1508–1515, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Ishizaka Y, Yamamoto Y, Fukunaga T, Yokota N, Kida O, Kitamura K, Kangawa K, Minamino N, Matsuo H, Eto T: Plasma concentration of human brain natriuretic peptide in patients on hemodialysis. Am J Kidney Dis 24: 461–472, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Fagugli RM, Palumbo B, Ricciardi D, Pasini P, Santirosi P, Vecchi L, Pasticci F, Palumbo R: Association between brain natriuretic peptide and extracellular water in hemodialysis patients. Nephron Clin Pract 95: c60–c66, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Racek J, Kralova H, Trefil L, Rajdl D, Eiselt J: Brain natriuretic peptide and N-terminal proBNP in chronic haemodialysis patients. Nephron Clin Pract 103: c162–c172, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Sheen V, Bhalla V, Tulua-Tata A, Bhalla MA, Weiss D, Chiu A, Abdeen O, Mullaney S and Maisel A: The use of B-type natriuretic peptide to assess volume status in patients with end-stage renal disease. Am Heart J 153: 244 e1–e5, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cataliotti A, Malatino LS, Jougasaki M, Zoccali C, Castellino P, Giacone G, Bellanuova I, Tripepi R, Seminara G, Parlongo S, Stancanelli B, Bonanno G, Fatuzzo P, Rapisarda F, Belluardo P, Signorelli SS, Heublein DM, Lainchbury JG, Leskinen HK, Bailey KR, Redfield MM, Burnett JC Jr: Circulating natriuretic peptide concentrations in patients with end-stage renal disease: Role of brain natriuretic peptide as a biomarker for ventricular remodeling. Mayo Clin Proc 76: 1111–1119, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Mallamaci F, Zoccali C, Tripepi G, Benedetto FA, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Stancanelli B, Malatino LS: Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int 59: 1559–1566, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Naganuma T, Sugimura K, Wada S, Yasumoto R, Sugimura T, Masuda C, Uchida J, Nakatani T: The prognostic role of brain natriuretic peptides in hemodialysis patients. Am J Nephrol 22: 437–444, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Nakatani T, Naganuma T, Masuda C, Uchida J, Sugimura T, Sugimura K: Significance of brain natriuretic peptides in patients on continuous ambulatory peritoneal dialysis. Intl J Mol Med 10: 457–461, 2002 [PubMed] [Google Scholar]
- 12.Wang AY, Lam CW, Yu CM, Wang M, Chan IH, Zhang Y, Lui SF, Sanderson JE: N-terminal pro-brain natriuretic peptide: An independent risk predictor of cardiovascular congestion, mortality, and adverse cardiovascular outcomes in chronic peritoneal dialysis patients. J Am Soc Nephrol 18: 321–330, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Yoshihara F, Horio T, Nakamura S, Yoshii M, Ogata C, Nakahama H, Inenaga T, Kangawa K, Kawano Y: Adrenomedullin reflects cardiac dysfunction, excessive blood volume, and inflammation in hemodialysis patients. Kidney Int 68: 1355–1363, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Nishikimi T, Futoo Y, Tamano K, Takahashi M, Suzuki T, Minami J, Honda T, Uetake S, Asakawa H, Kobayashi N, Horinaka S, Ishimitsu T, Matsuoka H: Plasma brain natriuretic peptide levels in chronic hemodialysis patients: Influence of coronary artery disease. Am J Kidney Dis 37: 1201–1208, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Lee JA, Kim DH, Yoo SJ, Oh DJ, Yu SH, Kang ET: Association between serum N-terminal pro-brain natriuretic peptide concentration and left ventricular dysfunction and extracellular water in continuous ambulatory peritoneal dialysis patients. Perit Dial Int 26: 360–365, 2006 [PubMed] [Google Scholar]
- 16.Nitta K, Kawashima A, Yumura W, Naruse M, Oba T, Kabaya T, Nihei H: Plasma concentration of brain natriuretic peptide as an indicator of cardiac ventricular function in patients on hemodialysis. Am J Nephrol 18: 411–415, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Osajima A, Okazaki M, Kato H, Anai H, Tsuda Y, Segawa K, Tanaka H, Tamura M, Takasugi M, Nakashima Y: Clinical significance of natriuretic peptides and cyclic GMP in hemodialysis patients with coronary artery disease. Am J Nephrol 21: 112–119, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Odar-Cederlof I, Ericsson F, Theodorsson E, Kjellstrand CM: Neuropeptide-Y and atrial natriuretic peptide as prognostic markers in patients on hemodialysis. ASAIO J 49: 74–80, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Goto T, Takase H, Toriyama T, Sugiura T, Kurita Y, Tsuru N, Masuda H, Hayashi K, Ueda R, Dohi Y: Increased circulating levels of natriuretic peptides predict future cardiac event in patients with chronic hemodialysis. Nephron 92: 610–615, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Ito S, Murai S, Takada N, Ozasa A, Hanada M, Sugiyama M, Suzuki K, Nagae Y, Inagaki T, Takeda Y, Fukutomi T, Joh T: Relationship between Doppler transmitral flow velocity pattern and plasma atrial and brain natriuretic peptide concentrations in anuric patients on maintenance hemodialysis. Int Heart J 47: 401–408, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Kohse KP, Feifel K, Mayer-Wehrstein R: Differential regulation of brain and atrial natriuretic peptides in hemodialysis patients. Clin Nephrol 40: 83–90, 1993 [PubMed] [Google Scholar]
- 22.Takagi T, Nishikawa M, Inada M: Changes in plasma atrial natriuretic peptide during hemodialysis: Mechanism of elevated levels in patients with chronic renal failure. Endocr J 40: 257–261, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Haug C, Metzele A, Steffgen J, Grunert A: Changes in brain natriuretic peptide and atrial natriuretic peptide plasma concentrations during hemodialysis in patients with chronic renal failure. Horm Metab Res 26: 246–249, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Taskapan MC, Ulutas O, Aksoy Y, Senel S, Sahin I, Kosar F, Taskapan H: Brain natriuretic peptide and its relationship to left ventricular hypertrophy in patients on peritoneal dialysis or hemodialysis less than 3 years. Ren Fail 28: 133–139, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Ando R, Matsuda O, Miyake S, Yoshiyama N: Plasma levels of human atrial natriuretic factor in patients treated by hemodialysis and continuous ambulatory peritoneal dialysis. Nephron 50: 225–228, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Plum J, Schoenicke G, Kleophas W, Kulas W, Steffens F, Azem A, Grabensee B: Comparison of body fluid distribution between chronic haemodialysis and peritoneal dialysis patients as assessed by biophysical and biochemical methods. Nephrol Dial Transplant 16: 2378–2385, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Khan IA, Fink J, Nass C, Chen H, Christenson R, deFilippi CR: N-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide for identifying coronary artery disease and left ventricular hypertrophy in ambulatory chronic kidney disease patients. Am J Cardiol 97: 1530–1534, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Zeng C, Wei T, Jin L, Wang L: Value of B-type natriuretic peptide in diagnosing left ventricular dysfunction in dialysis-dependent patients. Intern Med J 36: 552–557, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S: Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 13: 1307–1320, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Paniagua R, Amato D, Vonesh E, Guo A, Mujais S: Health-related quality of life predicts outcomes but is not affected by peritoneal clearance: The ADEMEX trial. Kidney Int 67: 1093–1104, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Masson S, Latini R, Anand IS, Vago T, Angelici L, Barlera S, Missov ED, Clerico A, Tognoni G, Cohn JN: Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the Valsartan Heart Failure (Val-HeFT) data. Clin Chem 52: 1528–1538, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Clerico A, Emdin M: Diagnostic accuracy and prognostic relevance of the measurement of cardiac natriuretic peptides: A review. Clin Chem 50: 33–50, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Brown EA, Davies SJ, Rutherford P, Meeus F, Borras M, Riegel W, Divino Filho JC, Vonesh E, van Bree M: Survival of functionally anuric patients on automated peritoneal dialysis: The European APD Outcome Study. J Am Soc Nephrol 14: 2948–2957, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Mark PB, Stewart GA, Gansevoort RT, Petrie CJ, McDonagh TA, Dargie HJ, Rodger RS, Jardine AG: Diagnostic potential of circulating natriuretic peptides in chronic kidney disease. Nephrol Dial Transplant 21: 402–410, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Takami Y, Horio T, Iwashima Y, Takiuchi S, Kamide K, Yoshihara F, Nakamura S, Nakahama H, Inenaga T, Kangawa K, Kawano Y: Diagnostic and prognostic value of plasma brain natriuretic peptide in non-dialysis-dependent CRF. Am J Kidney Dis 44: 420–428, 2004 [PubMed] [Google Scholar]
- 38.Ortega O, Gallar P, Munoz M, Rodriguez I, Carreno A, Ortiz M, Molina A, Oliet A, Lozano L, Vigil A: Association between C-reactive protein levels and N-terminal pro-B-type natriuretic peptide in pre-dialysis patients. Nephron Clin Pract 97: c125–c130, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Schleiffer T, Nagel D, Franz H, Falk M, Valentiner I, Wildburg G, Stark M, Brass H: Endothelin and atrial natriuretic peptide in non-insulin-dependent diabetic versus nondiabetic patients on chronic hemodialysis. Ren Fail 16: 747–758, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Sommerer C, Beimler J, Schwenger V, Heckele N, Katus HA, Giannitsis E, Zeier M: Cardiac biomarkers and survival in haemodialysis patients. Eur J Clin Invest 37: 350–356, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS: Impact of obesity on plasma natriuretic peptide levels. Circulation 109: 594–600, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Clerico A, Recchia FA, Passino C, Emdin M: Cardiac endocrine function is an essential component of the homeostatic regulation network: Physiological and clinical implications. Am J Physiol Heart Circ Physiol 290: H17–H29, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Pemberton CJ, Johnson ML, Yandle TG, Espiner EA: Deconvolution analysis of cardiac natriuretic peptides during acute volume overload. Hypertension 36: 355–359, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Kragelund C, Omland T: B-type natriuretic peptide (BNP) or N-terminal-proBNP for the diagnosis of heart failure: Which peptide is the better choice? Scand J Clin Lab Invest 65: 629–632, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R: B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 105: 2392–2397, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Tsutamoto T, Wada A, Maeda K, Hisanaga T, Maeda Y, Fukai D, Ohnishi M, Sugimoto Y, Kinoshita M: Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: Prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 96: 509–516, 1997 [DOI] [PubMed] [Google Scholar]
- 47.de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E: The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 345: 1014–1021, 2001 [DOI] [PubMed] [Google Scholar]
- 48.James SK, Lindahl B, Siegbahn A, Stridsberg M, Venge P, Armstrong P, Barnathan ES, Califf R, Topol EJ, Simoons ML, Wallentin L: N-terminal pro-brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: A Global Utilization of Strategies To Open occluded arteries (GUSTO)-IV substudy. Circulation 108: 275–281, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Luchner A, Hengstenberg C, Lowel H, Buchner S, Schunkert H, Riegger GA, Holmer S: NT-ProBNP in outpatients after myocardial infarction: Interaction between symptoms and left ventricular function and optimized cut-points. J Card Fail 11: S21–S27, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, Frampton C, Turner J, Crozier IG, Yandle TG: B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation 107: 2786–2792, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Blankenberg S, McQueen MJ, Smieja M, Pogue J, Balion C, Lonn E, Rupprecht HJ, Bickel C, Tiret L, Cambien F, Gerstein H, Munzel T, Yusuf S: Comparative impact of multiple biomarkers and N-terminal pro-brain natriuretic peptide in the context of conventional risk factors for the prediction of recurrent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) study. Circulation 114: 201–208, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R: N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med 352: 666–675, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA: N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA 297: 169–176, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS: Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 350: 655–663, 2004 [DOI] [PubMed] [Google Scholar]
- 55.McKie PM, Rodeheffer RJ, Cataliotti A, Martin FL, Urban LH, Mahoney DW, Jacobsen SJ, Redfield MM, Burnett JC Jr: Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide: Biomarkers for mortality in a large community-based cohort free of heart failure. Hypertension 47: 874–880, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bargman JM, Thorpe KE, Churchill DN: Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Konstam MA: Natriuretic peptides and cardiovascular events: More than a stretch. JAMA 297: 212–214, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Mallamaci F, Tripepi G, Cutrupi S, Malatino LS, Zoccali C: Prognostic value of combined use of biomarkers of inflammation, endothelial dysfunction, and myocardiopathy in patients with ESRD. Kidney Intl 67: 2330–2337, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM: Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 355: 1126–1130, 2000 [DOI] [PubMed] [Google Scholar]