Abstract
Background and objectives: Kidney transplantation is the most desired and cost-effective modality of renal replacement therapy for patients with irreversible chronic kidney failure (end-stage renal disease, stage 5 chronic kidney disease). Despite emerging evidence that the best outcomes accrue to patients who receive a transplant early in the course of renal replacement therapy, only 2.5% of incident patients with end-stage renal disease undergo transplantation as their initial modality of treatment, a figure largely unchanged for at least a decade.
Design, setting, participants, & measurements: The National Kidney Foundation convened a Kidney Disease Outcomes Quality Initiative (KDOQI) conference in Washington, DC, March 19 through 20, 2007, to examine the issue. Fifty-two participants representing transplant centers, dialysis providers, and payers were divided into three work groups to address the impact of early transplantation on the chronic kidney disease paradigm, educational needs of patients and professionals, and finances of renal replacement therapy.
Results: Participants explored the benefits of early transplantation on costs and outcomes, identified current barriers (at multiple levels) that impede access to early transplantation, and recommended specific interventions to overcome those barriers.
Conclusions: With implementation of early education, referral to a transplant center coincident with creation of vascular access, timely transplant evaluation, and identification of potential living donors, early transplantation can be an option for substantially more patients with chronic kidney disease.
Transplantation was the first successful modality of renal replacement therapy (RRT) for irreversible chronic kidney disease (CKD; stage 5); however, its broad applicability has been limited by immunologic rejection, adverse effects of immunosuppressant agents, and a relative shortage of available organs. After implementation of Medicare funding for RRT in 1972, long-term dialysis rapidly evolved as first-line treatment. In 1978, Rennie (1) summarized the prevailing situation: “Even although it offers a much better quality of life while it works, a transplant in most cases (of kidney failure) can be considered only a temporary respite from the basic form of treatment, which is dialysis.” Despite many remarkable advances during the past three decades, with transplantation now viewed unequivocally as offering the best survival and quality of life for candidates across all demographic groups, current practice remains that described by Rennie (2). Notwithstanding strong evidence that transplantation is most successful when implemented before onset of long-term dialysis, only 2.5% of patients with end-stage renal disease undergo transplantation as initial RRT (3–5).
This persistent finding has been subject to numerous explanations, often subjective and speculative, and thus far not amenable to remedy. In response to this conundrum, the National Kidney Foundation (NKF) convened a conference to address the issue of early transplantation within its Kidney Disease Outcomes Quality Initiative (KDOQI) framework, held in Washington, DC, March 19 through 20, 2007. Fifty-two participants representing transplant centers, dialysis providers, and payers were divided into three working groups. The first (work group 1) addressed the issue of how optimally to position kidney transplantation within the current CKD staging and treatment paradigms (6). Work group 2’s task was to formulate recommendations regarding educational and training implications required to promote early transplantation. Finally, given the critical importance of fiscal issues in RRT, work group 3 evaluated how finances might impede access to transplantation for patients with CKD and was charged with formulating potential remedies. This article is a summary of the deliberations, findings, and recommendations of these three work groups.
The first challenge for the conference was to determine the focus of deliberations: Was preemptive (before the onset of dialysis) or early (performed within the first 6 to 12 mo after initiation of dialysis) transplantation to be the primary concern? It was noted that both terms (preemptive and early) are adjectives that refer to the timing of transplantation and impart urgency to the process. Current data indicate recipient and allograft survival benefits for patients who receive a transplant within the first year of RRT; with each additional year of dialysis therapy, survival is compromised (7). Whether there are additional advantages associated with true preemptive transplantation, after correction for multiple interrelated risk factors, is less certain (8,9). Even so, it seems that patients and payers benefit from preemptive transplantation by avoiding medical complications and costs associated with initiation of dialysis, vascular access, and loss of employment; therefore, the participants chose to emphasize preemptive transplantation as the ideal, with the understanding that the unpredictability of advanced CKD and the shortage of organs from deceased donors necessitates that the next best option for many candidates will be transplantation as early in the course of RRT as possible.
Advantages of Preemptive Transplantation: Smoothing Peaks and Valleys
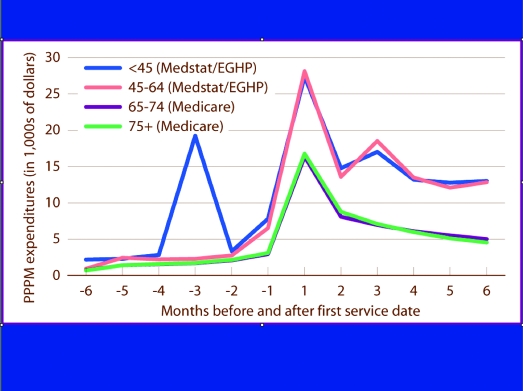
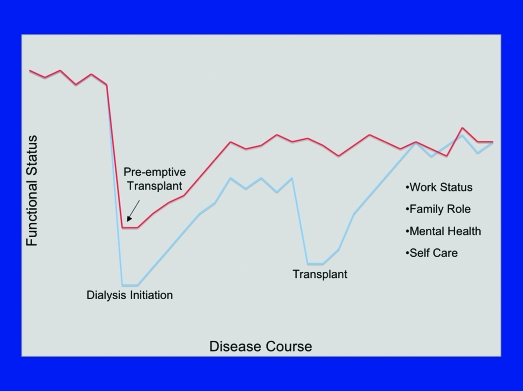
It is now well established that early kidney transplantation is associated with optimal outcomes in terms of patient and graft survival (7–10). Not as widely appreciated is the potential salutary impact of preemptive transplantation on peaks (in cost, morbidity, and mortality) and valleys (in employability and quality of life) that occur with transitions in CKD care (5) (Figures 1 and 2). Whereas mortality within the first year of initiation of RRT has steadily declined for patients who are on peritoneal dialysis and those who receive transplants, early mortality on hemodialysis remains high and relatively unchanged since the mid-1990s (5). These data indicate the importance of effective transitioning of patients between CKD and ESRD care and have provided impetus for the “Fistula First” initiative of the Center for Medicare and Medicaid Services (CMS) (11). When a patient begins RRT, or transitions from one modality of care to another, there is a dramatic decline in quality-of-life measures (12,13). Furthermore, of patients who were on dialysis for >1 yr, only 24% returned to work after transplantation, compared with at least one half of those who received a transplant preemptively (14). It is also clear that duration of disability before transplantation influences return-to-work rates and preservation of family dynamics (15). A key benefit of preemptive transplantation may therefore reside in avoiding these coincident positive and negative peaks in mortality and quality of life, respectively, by smoothing the transition to RRT: For an appropriate candidate, “Transplant First” should always be the goal.
Figure 1.
Expenditures associated with institution of long-term dialysis for patients transitioning from chronic kidney disease (CKD) care to renal replacement therapy (RRT) in 2003, by age. Per-person per-month expenditures for the transition to ESRD Medicare, incident patients with Medicare as primary provider; Medstat/employee group health plan [EGHP], patients enrolled for full year in both 2003 and 2004 (5).
Figure 2.
Decline in functional status associated with institution of dialysis, recovery, then a secondary decline associated with transplantation. Preemptive transplantation, by reducing transitions from two to one, has the potential to decrease substantially the adverse impact of RRT on quality-of-life measures (Rebecca Hays, NKF/KDOQI Conference on Early Kidney Transplantation, Washington, DC, 2007).
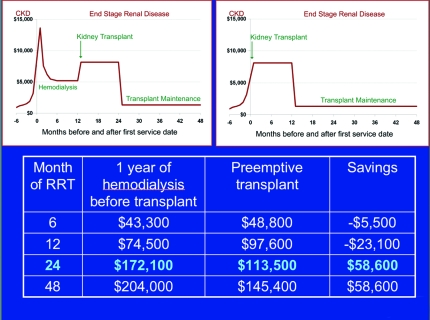
CKD and RRT consume an increasing portion of health care expenditures in the United States. Recent CMS data indicate that, in 2003, patients with CKD consumed almost 25% of the Medicare budget (up from 14% in 1993), with 7.1% solely to support the Medicare ESRD program (5). Although the incidence of ESRD seems to have stabilized for the first time in two decades, patients are surviving longer on RRT, prevalent cases are increasing, and expenditures continue to escalate. Overall, costs attributable to maintenance of a kidney transplant are less than one third those that are associated with long-term dialysis. It is now clear that transplants performed preemptively reduce the frequency of costly complications such as delayed graft function, acute rejection, and allograft failure (10,16). Although available estimates remain inexact, it is likely that by also avoiding the initiation of dialysis with its attendant complications, preemptive transplantation imparts substantial cost savings to the Medicare ESRD program. Estimates performed by Eugene Schweitzer for this conference (Figure 3) indicated that the lengthier the period of dialysis avoided, the greater the cost savings to be realized.
Figure 3.
By estimating per-month expenditures for patients aged 45 to 64 as 85% of those documented in a 67-yr-old, it is possible to approximate the financial impact of preemptive transplantation versus transplantation that occurs after 12 mo of hemodialysis. At 2 yr after onset of RRT, expenditures for the patient who undergoes preemptive transplantation are 34% less than in a comparable patient who undergoes 12 mo of hemodialysis before transplantation. In general, the longer a patient spends on dialysis before transplantation, the greater the cost savings that might accrue with preemptive transplantation (Eugene Schweitzer, NKF/KDOQI Conference on Early Kidney Transplantation, Washington, DC, 2007).
Demographics of Preemptive Transplantation
More than 60% of incident patients with ESRD have been followed by a nephrologist for at least 6 mo before institution of RRT (5); however, only 5.7% of incident patients with ESRD are placed on the waiting list before beginning RRT, whereas another 0.8% undergo preemptive living-donor (LD) transplantation without being placed on the waiting list. Of the roughly 7% of incident patients who had ESRD and were evaluated before beginning dialysis and found to be suitable candidates, fully 39% received a transplant preemptively, almost one third of whom received deceased-donor kidneys. Overall, although these figures document transplantation to be the initial modality of therapy for only 2.5% of incident patients with ESRD, among minorities, only 1% undergo preemptive transplants, numbers that have remained substantially unchanged for at least a decade. The US Scientific Registry of Transplant Recipients noted that approximately 26% of LD transplants were performed preemptively, with another one quarter occurring within the first year of RRT. Somewhat surprising, 11% of transplants from deceased donors occur before onset of dialysis, with 25% of listed patients receiving a transplant within 1 yr after beginning RRT. Many of these patients are recipients of zero HLA antigen-mismatched grafts, a category likely to decrease under anticipated revisions of the current deceased-donor kidney allocation system in the United States (17). Going forward, it is likely that preemptive transplantation will be increasingly linked to the availability of LD.
Older patients and those with diabetes are less likely than younger patients (<39 yr of age) and patients without diabetes to undergo preemptive referral, placement on the waiting list, and transplantation (3). As implied previously, minority patients (black, Native American, and Hispanic) are also substantially less likely than white patients to undergo early referral and transplantation. Although these nonmodifiable risk factors are of undeniable importance, insurance status and geographic location also exert substantial influence on access to early transplantation. For instance, patients with employer-sponsored health benefits are four times more likely to be placed on the waiting list before transplantation than those with Medicare, Medicaid, or both (an effect at least partially dependent on the age of the insured populations); however, insurance status does not seem to influence likelihood that a candidate, once listed, will undergo preemptive transplantation.
Before the Washington conference, a Web-based survey was distributed to 5900 nephrologists in the United States under the auspices of the NKF and ASN (Pradel FG, Jain R, Mullins CD, Vassalotti J, Bartlett ST, unpublished observations). Despite a response rate of just under 10%, several trends were evident. Most respondents maintained a positive attitude toward preemptive transplantation but would welcome additional education and guidelines regarding its benefits and the provision of appropriate posttransplantation care. Although the majority also agreed that early referral could threaten the financial health of dialysis centers, quality assessment of providers should include transplant referral rates and educational efforts regarding transplantation. The majority of respondents opined that currently available commercial RRT educational tools do not adequately address preemptive transplantation.
Preemptive Transplantation and the CKD Paradigm
Dissemination of the KDOQI staging and treatment guidelines for CKD in 2002 has already exerted a profound impact on the practice of medicine in the United States (6). Designed to promote early recognition and intervention in patients with what might be termed subclinical kidney disease, it spells out new metrics for monitoring kidney function (including estimated GFR [eGFR]) and guidelines for therapy. The KDOQI guidelines recommend that patients with eGFR <30 ml/min/1.73 m2 be prepared for dialysis and transplantation but do not describe the preemptive transplant option in detail.
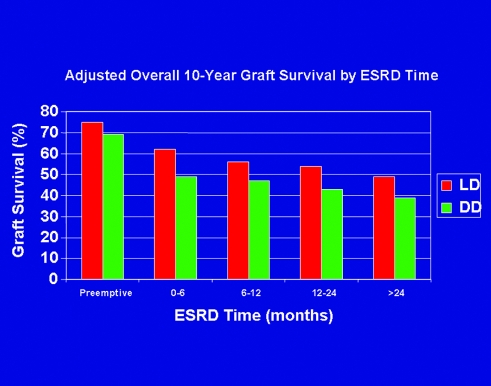
For appropriate candidates, kidney transplantation from a LD or deceased donor provides the best outcomes among available modalities of RRT; time spent on dialysis awaiting referral for transplantation increases mortality and compromises outcomes after transplantation (7). (Figure 4) There are no data to indicate that any defined subgroup of patients with CKD benefits from dialysis before transplantation. Thus, for the two thirds of patients who have ESRD and are seen by a nephrologist at least 6 mo before beginning RRT, referral for transplantation before or at the same time as creation of vascular access should be the standard. Indeed, published guidelines from the Renal Physicians Association already stipulate such action (18). Consistent with the recently adopted Medicare “final rule,” each transplant center should define and promulgate criteria for transplant candidacy; patients who have CKD and meet those criteria should be referred to the transplant center for evaluation in a timely manner (19).
Figure 4.
Impact of duration of time undergoing dialysis on allograft survival at 10 yr after transplantation for recipients of kidneys from living (LD) and deceased (DD) donors (7).
Such a policy will require recognition and acknowledgment of progressive CKD by practitioners and incorporation of early transplantation among options in educational efforts regarding modalities of RRT. In most nephrology practices, such education is instituted in CKD stage 3 (eGFR 30 to <60 ml/min/1.73 m2) or early stage 4 (eGFR 15 to <30 ml/min/1.73 m2); work group 1 agreed that this timing seemed appropriate and should be accompanied by referral for transplant evaluation in early stage 4 CKD. By the time eGFR declines to ≤20 ml/min/1.73 m2, appropriate candidates should have been identified and placed on the waiting list, consistent with current national policy within the United States. These referral and listing recommendations are also applicable to patients with failing allografts (20). The patient whose first contact with a nephrologist occurs later in the course of CKD (stage 5 or at initiation of dialysis) should be referred promptly for transplant evaluation.
Less clear than the timing of referral is the optimal timing for the transplant itself to occur. The outcome benefits of preemptive transplantation do not seem related to native kidney GFR at the time of surgery (21). Recent data indicate that mean eGFR for patients who undergo preemptive transplantation is 9.9 ml/min (21) and that residual GFR at the time of transplantation exerts little influence on eGFR of the allograft 6 mo later. Thus, appropriate timing of preemptive transplantation should be individualized, based on patient variables including rate of progression of CKD, symptoms attributable to CKD, management of comorbidities that affect candidacy, and (in some cases) donor and candidate convenience. For most patients, appropriate timing will be late in stage 4 or early in stage 5 CKD (eGFR ≤20 ml/min/1.73 m2). There is no evidence that transplantation even earlier in the course of CKD produces additional benefit or that measures to preserve native kidney function maximally should not be implemented.
Given the current imbalance between supply and demand of kidneys for transplantation and proposed modifications in national kidney allocation policy of organs from deceased donors that are likely to favor those who are already on dialysis (17), it must be recognized that the future of preemptive transplantation is tightly intertwined with availability of LD. This inescapable corollary implies that early education of patients and families (whether occurring in nephrology practices, dialysis facilities, or transplant centers) must incorporate a timely discussion of issues surrounding LD transplantation. This requirement underscores recent emphases on donor education, health, and autonomy already initiated in the transplant community (22,23).
These recommendations may require additional refinement for specific groups of patients. Among children, for whom consequences of long-term dialysis are widely recognized, preemptive transplantation is already much more common than in adults, and current allocation algorithms make early transplantation from deceased donors more accessible (3). In addition, contemplation of decades of RRT may affect choices when there are multiple donor options, especially regarding siblings and parents. Among patients with type 1 diabetes, benefits of preemptive transplantation are well defined, but availability of pancreas transplantation may influence the choice of approaches. Indeed, recent data indicate that outcomes with simultaneous kidney-pancreas transplantation from a deceased donor may rival those associated with preemptive transplantation of a kidney alone from a LD (24,25). Finally, although elderly patients make up the fastest growing component of incident patients with ESRD and clearly benefit from preemptive transplantation, an appropriate LD may be less readily identifiable, again necessitating individualization of approaches (26).
Making Preemptive Transplantation Normative: Overcoming Barriers with Training and Education
Although benefits of preemptive transplantation have been documented in the literature for almost a decade, its continued rarity in clinical practice indicates the existence of substantial barriers to implementation. These include impediments at each step in the transplantation process, many of which have been previously identified but not yet remedied (27).
At the patient level, documentation of progressive CKD is often a laboratory finding dissociated from physical symptoms and relatively easy to deny or overlook until very advanced. Someone who has an eGFR of 30 ml/min/1.73 m2 and basically feels well and continues to work may be averse to evaluating modalities of RRT. Responses may range from overt denial to depression-related inertia that prevents timely decision-making. Substantial information deficits regarding transplantation as an option have been well documented, particularly among minority patients (28,29). The relative inability of nephrologists to predict an accurate timeline for intervention adds to the problem (20). Finally, navigating the financial hurdles associated with transplantation, often beyond the expertise of practicing nephrologists and off the radar of many dialysis providers, may be staggering for individual patients (especially those who rely on government resources). Availability of accurate information regarding transplantation (including the role of LD) as an integral part of pre-RRT education is essential. Candidates should be made aware that after referral, the transplant center can provide counseling to help deal with the intricacies of transplant finances and the burden of the transplant evaluation process.
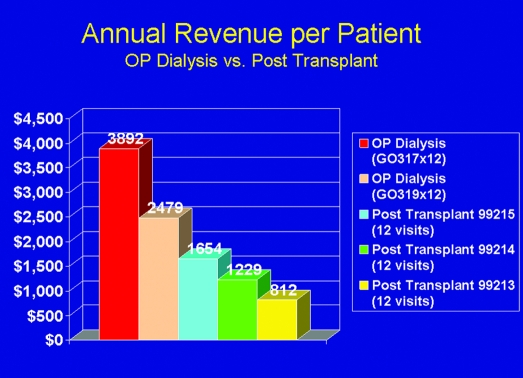
At the physician level, generalists must become better informed regarding recognition of CKD and appropriate timing of referral to a nephrologist. Nephrology fellowship training must include adequate exposure to transplantation issues, with emphasis on defining candidacy and helping patients prepare for transplantation. Continuing education for nephrologists (e.g., NephSAP, American Society of Nephrology, NKF) must include state-of-the-art transplant teaching. Having an adequate number of nephrologists available to see and care for patients early in the CKD pipeline will likely require more manpower in the field. Financial structures within practices must allow adequate time and staffing for transplant teaching and posttransplantation care (currently substantially underfunded relative to dialysis-related care; Figure 5).
Figure 5.
Comparison of Medicare reimbursement to a nephrology practice on an annual per-patient basis for care of a patient on dialysis (at two different frequencies of visits) versus posttransplantation office visits (assuming one visit per month) at three different levels of care. In most practices, transplant recipients are seen much less often than on a monthly basis (Andrew Howard, NKF/KDOQI Conference on Early Kidney Transplantation, Washington, DC, 2007).
The recent development of CKD clinics devoted to preparation of patients for RRT might ultimately improve early patient education and referral for transplantation. Unfortunately, little standardization exists regarding practice in these clinics, which are often primarily funded by the dialysis industry; not surprising, emphasis is on preparation for long-term dialysis. Referral and transplant rates (including preemptive) for nephrologists and dialysis providers should be included as a quality parameter made available to the public and should be incorporated into reimbursement policy (bundled payments, pay for performance).
Transplant centers must also assume greater responsibility for education and training, as well as facilitate the process to preemptive transplantation. The greatest reservoir of knowledge regarding state-of-the-art practices resides within transplant centers; appropriate information must be readily accessible to patients and dialysis providers. The referral and evaluation processes must be transparent and readily accessible. It is the responsibility of the transplant center to ensure that evaluation occurs in a timely manner and that decisions regarding candidacy are communicated to patient and referring nephrologist, as mandated by recent CMS guidelines (19). Transplant centers must provide staffing that is adequate to implement these recommendations; rates of preemptive transplantation should be available in center-specific reports.
Finally, considering the central role of the LD in preemptive transplantation, identification and education of potential donors must be given greater emphasis. There remains substantial disparity from center to center and patient to patient (3). Promotion of preemptive transplantation requires efficiency, and donors must be made aware of the importance of timeliness. Criteria defining LD acceptability should be standardized and promulgated to practicing nephrologists, along with information regarding the emerging options of paired donation and desensitization (30,31). Going forward, the evolution of these criteria depends on gleaning accurate and comprehensive data regarding long-term LD risk. Evaluation processes must likewise become more standardized. The desire to donate a kidney cannot be allowed to go unfulfilled by financial disincentives; the burden of our stated dependence on LD should not continue to be borne exclusively by donors themselves (32). Our ongoing interest in LD kidney transplantation, however, must always be tempered by a commitment to serve first the best interests of the potential donor, with respect for donor autonomy, as outlined in 2000 and now codified in federal regulations (19,22).
Finances of Preemptive Transplantation
The Social Security Act of 1972 made RRT an entitlement for most Americans, guaranteeing payment for transplantation or long-term dialysis services. CMS policy mandates timely access to transplant evaluation for Medicare beneficiaries; however, the complex regulations that now govern its implementation are daunting to patients and providers alike. Virtually all expenses that are associated with pretransplantation referral, evaluation, and placement on the waiting list of candidates are potentially reimbursable to transplant centers as organ acquisition costs (OAC). The problem, however, involves the complex interplay among private and government payers and the negotiation of these hurdles in a manner timely enough to allow preemptive transplantation to occur.
Because Medicare is the primary payer for kidney transplantation in the United States, its policies are critical in promoting preemptive transplantation. A patient must be eligible, entitled, and enrolled in the Medicare program (Table 1). Medicare enrollment at the time of transplantation is essential for long-term care: Those not enrolled, even if adequately covered by a private insurer (employee group health plan [EGHP]), will be ineligible for Medicare assistance with costs of immunosuppressants later in life. Coordination of benefits defines the relationship between ESRD Medicare and EGHP. The standard average kidney OAC is calculated from the total pretransplantation costs divided by the number of kidney transplants performed at that center. Ultimately, the OAC may end up as two to three times the cost of the transplant itself. Typically, for patients with private insurance, the OAC is included in a “global fee” paid at the time of transplantation. For patients with Medicare, it is paid to the center by CMS. In some states, however, Medicaid does not pay OAC, which ultimately limits access to the transplantation process.
Table 1.
Definitions of important terms in Medicare financing of kidney transplantationa
| Eligible | Patient paid into Social Security/Medicare for a required number of work quarters (or is the dependent of someone who has) and eligible based on age (>65 yr), disability, or ESRD |
| Entitled | ESRD entitlement begins |
| first day of the month in which beneficiary begins dialysis self-care program | |
| first day of the third full month of dialysis (in-center hemodialysis) | |
| two months (from first of month) before transplantation (if at a Medicare-approved facility) | |
| Enrolled | Beneficiary must file completed application for Part A and/or Part B; Part A no premium, Part B requires a monthly premium ($93.50/mo) |
| Enrollment can occur | |
| after Medical Evidence report (form 2728) is filed with CMS | |
| once transplant surgery takes place; for example, surgery is March 19, 2007; enrollment can occur for 2 mo before March 1 (January 1, 2007); Part A: application must be filed; Part B: must apply and pay premiums for January to March at $93.50/mo | |
| COB | For a patient with private insurance, 30 mo after the Medicare effective date (initiation of dialysis or transplantation), Medicare becomes primary (if patient is eligible, entitled, and enrolled) and private insurer becomes secondary |
| Medicare coverage remains active for only 36 mo after transplantation unless the patient remains Medicare eligible for other reasons (age, disability); in the case of preemptive transplantation in an otherwise healthy recipient <65 yr of age, Medicare would be primary for only 6 mo | |
| OAC | These include but are not limited to |
| costs incurred by the transplant center in the identification and evaluation of all potential recipients and LD | |
| costs incurred in LD nephrectomy (Part A; not including physician/surgeon fees that must be claimed under Part B) | |
| cost of procuring organs from deceased donors, including fee to organ procurement organization | |
| costs incurred in the maintenance of waiting list |
COB, coordination of benefits; LD, living donor; OAC, organ acquisition costs.
Under these complex definitions, there are several scenarios that are clear financial disincentives to transplant centers’ performing preemptive transplantation:
Patients who are not Medicare eligible (Medicaid only)
Patients with EGHP (not enrolled in Medicare) with a limit on donor benefits
Patients enrolled in Medicare Part A only (no physician fees recoverable because patient not enrolled or does not pay premiums in Part B)
Indeed, there are identifiable financial incentives and disincentives for all participants in the process (Table 2). As is apparent from Table 2, financial disincentives are primarily grouped among dialysis providers. Even sponsors of EGHP, thought to have the most to lose by paying for a transplant during their 30 mo at risk, were acknowledged to recoup substantial savings from transplants that were performed on patients who were on dialysis for <20 to 24 mo (one executive noted that costs of the transplant are offset by a breakeven point of 233 days of long-term dialysis).
Table 2.
Financial incentives and disincentives for various participants in the process of preemptive kidney transplantationa
| Participant | Incentive | Disincentive |
|---|---|---|
| Recipient | Less disruptive to earning potential, employment | Difficult for patients with only Medicare coverage because of timing and out-of-pocket requirements |
| Maintain EGHP | ||
| Less impact on lifetime maximum (avoiding dialysis expenditures) | ||
| Living donor | Lost wages, travel expenses, and other associated costs (as with all LD scenarios) | |
| The urgency of timing may add difficulty in dealing with lost wages, travel expenses, and other associated costs | ||
| Hospital/transplant center | Less costly (higher margin); less DGF | Administrative burden (EGHP) and potential risk (Medicare) |
| Decreased OAC; reduces waiting list expense | Recovering OAC for Medicaid-only patients | |
| Better outcomes attract patients and contracts | ||
| Transplant surgeon/physician | Greater percentage of transplants paid by EGHP increases reimbursement | Limited reimbursement from Medicare-only patients, particularly if no Part B coverage |
| Nontransplant physicians | None | Lack of financial support for educating patient with CKD regarding transplantation |
| Loss of dialysis revenue for patients who may not have had any coverage before initiation of RRT | ||
| Poor reimbursement for posttransplantation care relative to dialysis (50 to 80% less per patient per year) | ||
| Complexity of case overwhelms reimbursement benefit for PCP | ||
| EGHP | Avoids costs of dialysis and its complications before transplant (greatest financial benefit accrues with least time in COB period spent on dialysis) | Potential high "churn" reduces the savings opportunity (a "mythical" disincentive) |
| Lower costs of preemptive transplant | Fear of transplanting prematurely, given member churn | |
| Fulfills obligation and social responsibility by affirming optimal patient care | ||
| Preserves other types of insurance (disability and reinsurance claims). | ||
| Medicare | Cost savings (particularly with LD transplants) | Premature transplantation |
| Medicaid | Cost savings | Premature transplantation |
CKD, chronic kidney disease; DGF, delayed graft function; EGHP, employee group health plan; PCP, primary care provider; RRT, renal replacement therapy\
Surmounting these financial hurdles is most difficult for patients who rely (or are destined to rely) on Medicare coverage for RRT. Support of a social worker or financial counselor during CKD management is essential. As the principal payer for RRT, it must be clearly understood that Medicare has the most to gain from implementation of new policies to promote preemptive transplantation.
Recommendations and Summary
In short, recent trends in kidney transplantation have documented the primacy of a functional allograft as the optimal treatment for most patients with ESRD, with the best outcomes among those who receive a transplant early in the course of RRT. These findings have yet to be translated into changes in clinical practice, as only 2.5% of patients with ESRD underwent preemptive transplantation in 2004. Implementation of a number of cost-effective changes in clinical practice and reimbursement structure (as identified by participants in this conference and summarized in Table 3) has the potential to remedy the situation, resulting in progressive improvement in projected outcomes for patients with ESRD in the United States. Mange and Weir (33), in 2003, raised the central issue highlighted in the conference: Why not preemptive transplantation? Why not, indeed!
Table 3.
Recommendations of the NKF/KDOQI Conference on Early Transplantationa
| Clinical Recommendations | Financial Recommendations |
|---|---|
| Increase access to preemptive transplantation by promoting early patient education (CKD stage 3) regarding transplantation as an RRT option; promoting early referral (CKD stage 4) to a transplant center; promoting knowledge regarding LD kidney transplantation among patients with CKD and providers | Modify eligibility for Medicare ESRD to begin at late stage 4 or early stage 5 CKD (eGFR ≤15 to 20 ml/min) |
Improve funding for support services in CKD clinics:
| |
| Improve efficiency of evaluation at transplant centers and of communication between transplant centers and referring physicians: staffing adequate to make 6 wk from referral to listing as the standard | Support Part B premium reimbursement by third parties (as with COBRA) |
| Increase percentage of LD transplants performed preemptively from 26 to 50% | Promote measures to increase availability of kidneys for transplantation: provide adequate funding for the Organ Donation Recovery and Improvement Act; a national program to protect LD from financial disincentives and health risks associated with donor nephrectomy |
| Create benchmarks to measure performance:preemptive referral and transplantation rates for nephrologists and dialysis providers;evaluation time and preemptive transplant rates for transplant centers | Increase resource availability for:
|
| Standardization of Medicaid coverage for kidney transplantation,including reimbursement of OAC | |
| Higher reimbursement rates for dialysis units with higher case mix–adjusted transplant rates (cost neutral if lower rates for dialysis units with lower case mix–adjusted transplant rates) |
eGFR, estimated GFR; NKF/KDOQI, National Kidney Foundation Kidney Disease Outcomes Quality Initiative.
Disclosures
Francoise Pradel received a grant from the National Kidney Foundation to conduct a Web-based survey about nephrologists’ attitudes toward preemptive transplantation.
Acknowledgments
We acknowledge the conference co-chairs Robert S. Gaston, MD, and Stephen T. Bartlett, MD, and participants by workgroup: Workgroup 1: Transplant and the CKD Paradigm Chairs: Alan Leichtman, MD, University of Michigan; Robert M. Merion, MD, University of Michigan Health System; Ruben L. Velez, MD, Dallas Nephrology Associates.
Participants: Valarie B. Ashby, MA, Kidney Epidemiology and Cost Center, University of Michigan; David A. Axelrod, MD, MBA, Dartmouth-Hitchcock Medical Center; Bryan N. Becker, MD, University of Wisconsin-Madison; Margaret Bia, MD, Yale School of Medicine; David J. Cohen, MD, Columbia University Medical Center; Gabriel M. Danovitch, MD, University of California, Los Angeles (UCLA); Sandy Feng, MD, PhD, University of California San Francisco; Suzanne Gagne, RN, CNN, Fresenius Medical Care Education Department; Bertram L. Kasiske, MD, Hennepin County Medical Center; Douglas S. Keith, MD, McGill University Health Center, Royal Victoria Hospital; Michael V. Rocco, MD, MSCE, Wake Forest University School of Medicine; Victor V. Rozas, MD, Great Lakes Renal Network; Charlie Thomas, LCSW, ACSW, Banner Good Samaritan Medical Center–Transplant Services; Debra Washington; Winfred W. Williams, MD, General Hospital, Harvard Medical School, Richard B. Simches Research Center. Workgroup 2: Training/Education
Chairs: Connie Davis, MD, University of Washington; Dale A. Distant, MD, SUNY Downstate; John J. Friedewald, MD, Northwestern University Feinberg School of Medicine; Andrew Howard, MD.
Participants: Allan J. Collins, MD, University of Minnesota and Chronic Disease Research Group; Rebecca Hays, MSW, APSW, University of Wisconsin Hospital and Clinics, Transplant Clinic; Jenny Kitsen, ESRD Network 1—Network of New England; David K. Klassen, MD, University of Maryland; Elizabeth S. Ommen, MD, Mount Sinai School of Medicine; Francoise Pradel, PhD, University of Maryland School of Pharmacy; Trent Tipple, MD, Columbus Children’s Hospital/Ohio State University College of Medicine; Joseph Vassalotti, MD, National Kidney Foundation; Pedro Vergne-Marini, MD, Methodist Hospitals of Dallas. Workgroup 3: Financial Model/Removing Disincentives
Chairs: Michael Abecassis, MD, Northwestern Memorial Hospital; Francis Delmonico, MD, Massachusetts General Hospital; Edward R. Jones, MD, Delaware Valley Nephrology, Germantown Hospital & Medical Center; Robert A. Metzger, MD, TransLife at Florida Hospital.
Participants: James Burdick, MD, HHS/HRSA/HSB/Division of Transplantation; Mary Beth Callahan, ACSW/LCSW, Dallas Transplant Institute; Dolph Chianchiano, JD, MPA, National Kidney Foundation; James Coates, MD, Aetna; Peter Crooks, MD, Kaiser Permanente Renal Program; Derrick Latos, MD, ESRD Network 5; Deb McGrew, MHA, University of Alabama at Birmingham; Richard J. Migliori, MD, Health Solutions Group; Catherine Paykin, MSSW, National Kidney Foundation; Eugene J. Schweitzer, MD, University of Maryland Baltimore; Wadi N. Suki, MD, The Kidney Institute; Paul E. Turer, MD, MBA, Mid-Atlantic Nephrology Associates; Richard A. White, Guardian Life Insurance Company; James J. Wynn, MD, Medical College of Georgia.
KDOQI is a trademark of the National Kidney Foundation, Inc.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Rennie D: Home dialysis and the costs of uremia. N Engl J Med 298: 399–400, 1978 [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB: Geographic variability in access to primary kidney transplantation in the United States, 1996–2005. Am J Transplant 7[Suppl 1]: 1412–1423, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Meier-Kriesche HU, Port FK, Ojo AO, Rudich SM, Hanson JA, Cibrik DM, Leichtman AB, Kaplan B: Effect of waiting time on renal transplant outcome. Kidney Int 58: 1311–1317, 2000 [DOI] [PubMed] [Google Scholar]
- 5.US Renal Data System: USRDS 2006 Annual Data Report. Available at: http://www.usrds.org/adr_2006.htm. Accessed June 19, 2007
- 6.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 7.Meier-Kriesche HU, Kaplan B: Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: A paired donor kidney analysis. Transplantation 74: 1377–1381, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Gill JS, Tonelli M, Johnson N, Pereira BJG: Why do preemptive kidney transplant recipients have an allograft survival advantage? Transplantation 78: 873–879, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Mange KC, Joffe MM, Feldman HI: Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 344: 726–731, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Innocenti GR, Wadei HM, Prieto M, Dean PG, Ramos EJ, Textor S, Khamash H, Larson TS, Cosio F, Kosberg K, Fix L, Bauer C, Stegall MD: Preemptive living donor kidney transplantation: Do the benefits extend to all recipients? Transplantation 83: 144–149, 2007 [DOI] [PubMed] [Google Scholar]
- 11.National Vascular Access Improvement Initiative. Fistula First. Available at: htt://www.cms.hhs.gov/ESRDQualityImproveInit/04_FistulaFirstBreakthrough.asp. Accessed May 29, 2007 [PubMed]
- 12.Kimmel PL: Psychosocial factors in dialysis patients. Kidney Int 59: 1599–1613, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Watnick S, Kirwin P, Mahnensmith R, Concato J: The prevalence and treatment of depression among patients starting dialysis. Am J Kidney Dis 41: 105–110, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Gaston RS, Thomas C: Psychiatric and psychosocial issues in kidney transplantation. In: Medical Management of Kidney Transplantation, edited by Weir MR, Philadelphia, Lippincott Williams & Wilkins, 2005, pp 231–237
- 15.Muerher RJ, Becker BN: Life after transplantation: New transitions in quality of life and psychological distress. Semin Dial 18: 124–131, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT: Preemptive kidney transplantation: The advantage and the advantaged. J Am Soc Nephrol 13: 1358–1364, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Stegall MD: The development of kidney allocation policy. Am J Kidney Dis 46: 974–975, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Bolton WK: Renal Physicians Association Clinical Practice Guideline: Appropriate patient preparation for renal replacement therapy. J Am Soc Nephrol 14: 1406–1410, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Medicare and Medicaid Services (CMS). Final Rule: Hospital Conditions of Participation: Requirements for Approval and Re-approval of Transplant Centers to Perform Organ Transplants. Available at: http://www.cms.hhs.gov/CFCsandCoPs/downloads/trancenterreg2007.pdf Accessed May 29, 2007 [PubMed]
- 20.Djamali A, Kendziorski C, Brazy PC, Becker BN: Disease progression and outcomes in chronic kidney disease and renal transplantation. Kidney Int 64: 1800–1807, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Ishani A, Ibrahim HN, Gilbertson D, Collins AJ: The impact of residual renal function on graft and patient survival rates in recipients of preemptive renal transplants. Am J Kidney Dis 42: 1275–1282, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Abecassis M, Adams M, Adams P, Arnold RM, Atkins CR, Barr ML, Bennett WM, Bia M, Briscoe DM, Burdick J, Corry RJ, Davis J, Delmonico FL, Gaston RS, Harmon W, Jacobs CL, Kahn J, Leichtman A, Miller C, Moss D, Newmann JM, Rosen LS, Siminoff L, Spital A, Starnes VA, Thomas C, Tyler LS, Williams L, Wright FH, Youngner S, Live Organ Donor Consensus Group: Consensus statement on the live organ donor. JAMA 284: 2919–2926, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Delmonico F, Council of the Transplantation Society: A report of the Amsterdam Forum on the Care of the Live Kidney Donor: Data and medical guidelines. Transplantation 79[Suppl]: S53–S66, 2005 [PubMed] [Google Scholar]
- 24.Becker BN, Rush SH, Dykstra DM, Becker YT, Port FK: Preemptive transplantation for patients with diabetes-related kidney disease. Arch Intern Med 166: 44–48, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Ojo AO, Meier-Kriesche H-U, Hanson JA, Leichtman A, Magee JC, Cibrik D, Wolfe RA, Port FK, Agodoa L, Kaufman DB, Kaplan B: The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation 71: 82–90, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Danovitch G, Savransky E: Challenges in the counseling and management of older kidney transplant candidates. Am J Kidney Dis 47[Suppl 2]: S86–S97, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Gaston RS, Danovitch GM, Adams PL, Wynn JJ, Merion RM, Deierhoi MH, Metzger RA, Cecka JM, Harmon WE, Leichtman AB, Spital A, Blumberg E, Herzog CA, Wolfe RA, Tyan DB, Roberts J, Rohrer R, Port FK, Delmonico FL.: The report of a national conference on the wait list for kidney transplantation. Am J Transplant 3: 775–785, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Ayanian JZ, Cleary PD, Keogh JH, Noonan SJ, David-Kasdan JA, Epstein AM: Physicians’ beliefs about racial differences in referral for renal transplantation. Am J Kidney Dis 43: 350–357, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM: The effect of patient’s preferences on racial differences in access to renal transplantation. N Engl J Med 341: 1661–1669, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Gloor JM, DeGoey SR, Pineda AA, Moore SB, Prieto M, Nyberg SL, Larson TS, Griffin MD, Textor SC, Velosa JA, Schwab TR, Fix LA, Stegall MD: Overcoming a positive crossmatch in living-donor kidney transplantation. Am J Transplant 3: 1017–1023, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Park K, Moon JI, Kim SI, Kim YS: Exchange donor program in kidney transplantation. Transplantation 67: 336–338, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Gaston RS, Danovitch GM, Epstein RA, Kahn JP, Matas AJ, Schnitzler MA: Limiting financial disincentives in live organ donation: A rational solution to the kidney shortage. Am J Transplant 6: 2548–2555, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Mange KC, Weir MR: Preemptive renal transplantation: Why not? Am J Transplant 3: 1336–1340, 2003 [DOI] [PubMed] [Google Scholar]