Abstract
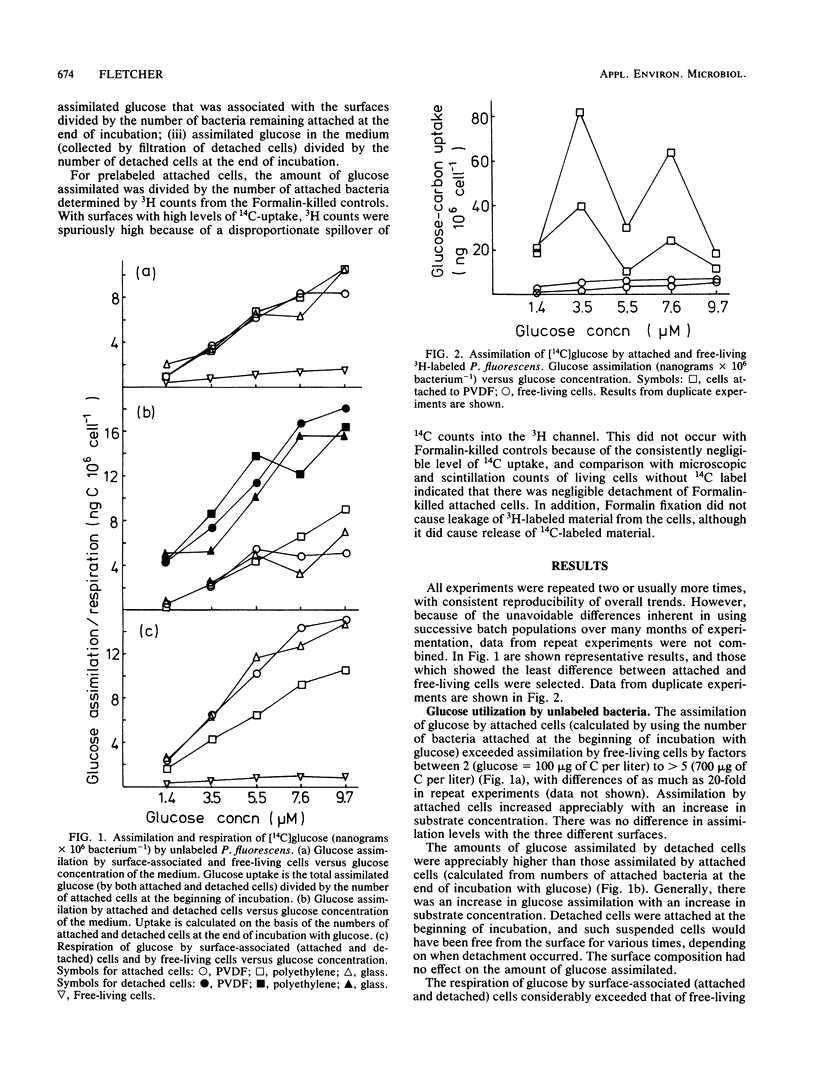
The assimilation and respiration of glucose by attached and free-living Pseudomonas fluorescens were compared. The attachment surfaces were polyvinylidene fluoride, polyethylene, and glass. Specific uptake of [14C]glucose was determined after bacterial biomass was measured by (i) microscopic counts or (ii) prelabeling of cells by providing [3H]leucine as substrate, followed by dual-labeling scintillation counting. The glucose concentration was 1.4, 3.5, 5.5, 7.6, or 9.7 μM. Glucose assimilation by cells which became detached from the surfaces during incubation with glucose was also measured after the detached cells were collected by filtration. The composition of the substratum had no effect on the amount of glucose assimilated by attached cells. Glucose assimilation by attached cells exceeded that by free-living cells by a factor of between 2 and 5 or more, and respiration of glucose by surface-associated cells was greater than that by free-living bacteria. Glucose assimilation by detached cells was greater than that by attached bacteria. Measurements of biomass by microscopic counts gave more consistent results that those obtained with dual-labeling, but in general, results obtained by both methods were corroborative.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bright J. J., Fletcher M. Amino Acid assimilation and electron transport system activity in attached and free-living marine bacteria. Appl Environ Microbiol. 1983 Mar;45(3):818–825. doi: 10.1128/aem.45.3.818-825.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Gerchakov S. M., Millero F. J. Effects of inorganic particles on metabolism by a periphytic marine bacterium. Appl Environ Microbiol. 1983 Feb;45(2):411–417. doi: 10.1128/aem.45.2.411-417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey B., Kjelleberg S., Marshall K. C. Responses of marine bacteria under starvation conditions at a solid-water interface. Appl Environ Microbiol. 1983 Jan;45(1):43–47. doi: 10.1128/aem.45.1.43-47.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Mitchell R. Contribution of particle-bound bacteria to total microheterotrophic activity in five ponds and two marshes. Appl Environ Microbiol. 1982 Jan;43(1):200–209. doi: 10.1128/aem.43.1.200-209.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Humphrey B. A., Marshall K. C. Effect of interfaces on small, starved marine bacteria. Appl Environ Microbiol. 1982 May;43(5):1166–1172. doi: 10.1128/aem.43.5.1166-1172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Humphrey B. A., Marshall K. C. Initial phases of starvation and activity of bacteria at surfaces. Appl Environ Microbiol. 1983 Nov;46(5):978–984. doi: 10.1128/aem.46.5.978-984.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J. H., Fletcher M. Influence of substratum wettability on attachment of freshwater bacteria to solid surfaces. Appl Environ Microbiol. 1983 Mar;45(3):811–817. doi: 10.1128/aem.45.3.811-817.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotzky G. Influence of clay minerals on microorganisms. 3. Effect of particle size, cation exchange capacity, and surface area on bacteria. Can J Microbiol. 1966 Dec;12(6):1235–1246. doi: 10.1139/m66-165. [DOI] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]



