Abstract
Background and objectives: The level of glomerular filtration rate at which hemoglobin declines in chronic kidney disease is poorly described in the pediatric population.
Design, setting, participants, & measurements: This cross-sectional study of North American children with chronic kidney disease examined the association of glomerular filtration rate, determined by the plasma disappearance of iohexol, and hemoglobin concentration.
Results: Of the 340 patients studied, the mean age was 11 ± 4 yr, the mean glomerular filtration rate was 42 ± 14 ml/min per 1.73 m2, and the mean hemoglobin was 12.5 ± 1.5. Below a glomerular filtration rate of 43, the hemoglobin declined by 0.3 g/dl (95% confidence interval −0.2 to −0.5) for every 5-ml/min per 1.73 m2 decrease in glomerular filtration rate. Above a glomerular filtration rate of 43 ml/min per 1.73 m2, the hemoglobin showed a nonsignificant decline of 0.1 g/dl for every 5-ml/min per 1.73 m2 decrease in glomerular filtration rate.
Conclusions: In pediatric patients with chronic kidney disease, hemoglobin declines as an iohexol-determined glomerular filtration rate decreases below 43 ml/min per 1.73 m2. Because serum creatinine–based estimated glomerular filtration rates may overestimate measured glomerular filtration rate in this population, clinicians need to be mindful of the potential for hemoglobin decline and anemia even at early stages of chronic kidney disease, as determined by current Schwartz formula estimates. Future longitudinal analyses will further characterize the relationship between glomerular filtration rate and hemoglobin, including elucidation of reasons for the heterogeneity of this association among individuals.
The adverse health effects of anemia in adult and pediatric patients with chronic kidney disease (CKD) are both common and profound. Anemia has been associated with increased mortality, limitations in physical activity, and adverse effects on quality of life. Among children in the 2005 End Stage Renal Disease Clinical Performance Measures Project, 95% of 1598 prevalent pediatric patients with ESRD were anemic; 95% of patients who were receiving hemodialysis and 94% of patients who were receiving peritoneal dialysis were prescribed erythropoiesis-stimulating agents (ESA) (1). A lower prevalence of anemia is observed at earlier stages of CKD in pediatric patients. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) stages CKD as follows: Stage 1, evidence of kidney damage with normal or increased GFR; stage 2, GFR 60 to 89 ml/min per 1.73 m2; stage 3, GFR 30 to 59 ml/min per 1.73 m2; stage 4, GFR 15 to 29 ml/min per 1.73 m2; stage 5, GFR <15 ml/min per 1.73 m2 or on dialysis (2). In a recent single-center, cross-sectional study of 366 children and adolescents with CKD, approximately 30% of patients with stages 1 and 2 CKD reported prevalent anemia, defined as hemoglobin <12 mg/dl or medical treatment for anemia, whereas 66% of patients with stage 3 and 93% of patients with stages 4 and 5 CKD were anemic. GFR was estimated from serum cystatin C in this study (3).
The lower prevalence of anemia in patients with earlier stages of CKD suggests an association between hemoglobin and GFR. In adults, hemoglobin has been reported to decline below a GFR threshold of 40 to 60 ml/min per 1.73 m2 as measured by the Modification of Diet in Renal Disease (MDRD) or Cockcroft-Gault equation (4,5). This association, including the existence of a “GFR threshold,” has not been clearly described in children. This study aimed to define more clearly the relationship between hemoglobin and GFR in the pediatric CKD population. To accomplish this, we used the relatively large sample size and precise measurement of GFR offered by the Chronic Kidney Disease in Children Prospective Cohort Study (CKiD).
Concise Methods
Study Design and Population
The CKiD Study, a multicenter, prospective cohort sponsored by the National Institutes of Health, aims to enroll 540 children with mild to moderate CKD. Details of the CKiD Study design have been previously published (6). Briefly, eligible children are aged 1 to 16 yr and have a Schwartz-estimated GFR between 30 and 90 ml/min per 1.73 m2 (7,8). Exclusion criteria are renal, other solid-organ, bone marrow, or stem cell transplantation; dialysis treatment within the past 3 mo; cancer/leukemia diagnosis or HIV diagnosis/treatment within past 12 mo; current pregnancy or pregnancy within the past 12 mo; history of structural heart disease; genetic syndromes involving the central nervous system; and history of severe to profound mental retardation. This study is a cross-sectional analysis of the first 340 children enrolled in CKiD by April 2007 with available hemoglobin and GFR measurements.
Measurements
GFR was determined by plasma iohexol disappearance curves with four time points at 10, 30, 120, and 300 min after infusion of 5 ml of iohexol; details of the GFR assessment methods have been published previously (9). Blood samples for hemoglobin measurement were drawn from each patient on the same day the GFR was measured. Hemoglobin measurements were performed at the local laboratories of the participating CKiD clinical sites. These clinical laboratories are required to perform regular quality assurance evaluations and careful calibration of laboratory instruments to ensure accuracy and reproducibility. Proficiency testing surveys recently published by the College of American Pathologists demonstrate coefficients of variation of approximately 1 to 2% for the various instruments performing hemoglobin measurements (10).
Demographic and clinical information collected at the baseline study visit of interest for this analysis included age, gender, race/ethnicity, height, weight, underlying CKD diagnosis, and use of ESA and iron supplements during the past 30 d. Race was categorized as white, black, and other. Anemia was defined as having a hemoglobin value below the fifth percentile for age and gender or use of an ESA during the past 30 d (11). Three categories of primary CKD diagnosis were used: (1) Glomerular (includes chronic glomerulonephritis, congenital nephrotic syndrome, Denys-Drash syndrome, diabetic nephropathy, familial nephritis, FSGS, hemolytic uremic syndrome, Henoch-Schönlein nephritis, idiopathic crescentic glomerulonephritis, IgA nephropathy, membranoproliferative glomerulonephritis types 1 and 2, membranous nephropathy, sickle cell nephropathy, and systemic immunologic disease including systemic lupus erythematosus); (2) genitourinary, cystic, hereditary (includes aplastic/hypoplastic/dysplastic kidneys, medullary cystic disease/juvenile nephronophthisis, obstructive uropathy, polycystic kidney disease [both autosomal dominant and recessive], pyelonephritis/interstitial nephritis, reflux nephropathy, and syndrome of agenesis of abdominal musculature); and (3) other nonglomerular or unknown.
Statistical Analyses
Study population characteristics are described in Table 1. Means and SD are used to describe continuous variables; percentages and frequencies are used to describe categorical variables.
Table 1.
Characteristics of 340 pediatric patients with CKDa
| Characteristic | Value |
|---|---|
| Age (yr; mean ± SD) | 11 ± 4 |
| Male (% [n]) | 61 (207) |
| Race (% [n])b | |
| white | 70 (238) |
| black | 16 (54) |
| Asian | 3 (9) |
| other | 11 (38) |
| Hispanic ethnicity (% [n])b | 14 (48) |
| Height percentile (mean ± SD) | 31 ± 28 |
| Weight percentile (mean ± SD) | 47 ± 33 |
| BMI percentile (mean ± SD) | 61 ± 30 |
| >90th (% [n]) | 24 (78) |
| <10th (% [n]) | 7 (23) |
| Primary CKD diagnosis (% [n]) | |
| glomerular | 22 (75) |
| genitourinary, cystic, hereditary | 67 (227) |
| other or missing | 11 (46) |
| Iohexol GFR (ml/min per 1.73 m2; mean ± SD) | 42 ± 14 |
| KDOQI CKD stage (% [n])c | |
| 1 | <1 (1) |
| 2 | 11 (39) |
| 3 | 64 (217) |
| 4 | 24 (82) |
| 5 | <1 (1) |
| Schwartz-estimated GFR (ml/min per 1.73 m2; mean ± SD) | 58 ± 18 |
| Duration of CKD (yr; mean ± SD) | 7 ± 5 |
| Hemoglobin (g/dl; mean ± SD) | 12.5 ± 1.5 |
| Anemia (% [n])d | 45 (154) |
| ESA use (% [n]) | 18 (61) |
| Iron supplement use (% [n]) | 32 (110) |
CKD, chronic kidney disease; KDOQI, Kidney Disease Outcomes Quality Initiative.
Missing data: n = 1 missing race, n = 6 missing ethnicity, n = 7 missing CKD duration.
Based on iohexol GFR.
Anemia defined as hemoglobin less than fifth percentile of normal (g/dl) for age and gender or if currently receiving an erythropoiesis-stimulating agent.
The primary objective was to describe the relationship between hemoglobin and GFR. ESA raise hemoglobin levels, and this effect had to be considered for the analysis. To avoid the potential of a selection bias resulting from the exclusion of patients who are receiving ESA, the hemoglobin values for patients who received ESA during the previous 30 d were left-censored. Considering the hemoglobin value for a child who received ESA as a left-censored value essentially replaces the observed hemoglobin value with a left-censored value. The left-censored value for each child who is receiving ESA is essentially the mean hemoglobin of the other observed children who are not receiving ESA but have the same values of other measured covariates, at any given GFR. The replacement is done in such a manner as to preserve the added uncertainty from hemoglobin's exact value being known only to be between zero and the observed left-censored value. This added uncertainty increases SE and therefore widens confidence intervals (CI) but often to a lesser extent than would occur if one excluded children who are receiving ESA. Valid analyses allowing for left-censoring of hemoglobin as a result of the use of ESA must assume that ESA use is random within levels of GFR and the measured covariates listed previously (i.e., two individuals with the same GFR and covariate values are equally likely to use ESA). A secondary analysis that excluded patients who are receiving ESA was also performed. This secondary analysis provided similar results, albeit less certain as a result of the loss of information caused by exclusion.
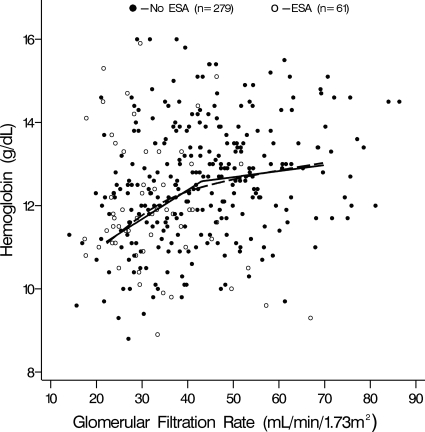
With this in mind, a nonparametric smoothing spline was overlaid on a scatter plot of GFR and hemoglobin (Figure 1). The smoothing spline is a flexible line that can be thought of as smoothly connecting a series of averaged hemoglobin measurements; each average is calculated within a narrow range of continuous GFR values (12). The smoothing spline demonstrated evidence of a “GFR threshold,” below which hemoglobin and GFR were positively associated. Given this, the data were described using a two-piece linear spline “threshold” regression model. The model took the form Hi = b0 + b1(GFR − c)/5 × I(GFR < c) + b2(GFR − c)/5 × I(GFR ≥ c) + bj covariatej, where Hi is the hemoglobin value for patient i, c is the threshold GFR, b0 is the mean hemoglobin for those with a GFR equal to c, b1 is the difference in hemoglobin for a 5-unit difference in GFR given GFR is below c, I(GFR < c) is an indicator of GFR being below c, and b2 is the difference in hemoglobin for a 5-unit difference in GFR given GFR is ≥c, and bj is the difference in hemoglobin for a 1-unit difference in covariate j. Potential covariates for the model included age, gender, race, and primary cause of CKD categorized as glomerular and nonglomerular (genitourinary, cystic, hereditary, and other nonglomerular). The threshold, c, was allowed to vary across the observed range of GFR, between 20 and 60 ml/min per 1.73 m2. The optimal value for c and 95% CI were determined using a profile likelihood (13). All statistical models were run using SAS 9.1 (SAS Institute, Cary, NC).
Figure 1.
Hemoglobin versus GFR in children with chronic kidney disease (CKD): Linear threshold model (solid line) and nonparametric smoothing model (dashed line) describing relationship of hemoglobin concentration and GFR in 340 pediatric patients with CKD.
Results
Characteristics of the 340 children in this study are listed in Table 1. Mean age was 11 ± 4 yr; 61% were male; and 70% were white, 16% were black, and 14% were of Hispanic ethnicity. Of the underlying causes for CKD, 22% were due to glomerular disease, 67% were due to genitourinary/cystic/hereditary diseases, and 11% had other or unknown causes. The mean iohexol GFR was 42 ± 14 ml/min per 1.73 m2, and the mean Schwartz-estimated GFR was 58 ± 18 ml/min per 1.73 m2. The mean hemoglobin was 12.5 ± 1.5 g/dl, and 154 (45%) children were classified as anemic. Prevalence ratios of anemia by KDOQI CKD stage are provided in Table 2. Among the 154 anemic children, 93 (60%) were not receiving ESA. Of the 61 children receiving ESA, 29 (48%) had a hemoglobin level ≥5th percentile; 14% of children had a hemoglobin level <11 g/dl, the lower limit recommended by the KDOQI Clinical Practice Guidelines for anemia in CKD (14). Of these children, 27% were receiving ESA.
Table 2.
Prevalence ratio of anemia by KDOQI CKD stagea
| CKD Stageb | n | % Anemic | Prevalence Ratio | 95% CI |
|---|---|---|---|---|
| 2 | 39 | 21 | 1.0 | – |
| 3 | 217 | 39 | 1.9 | 1.0 to 3.6 |
| 4 | 82 | 73 | 3.6 | 1.9 to 6.7 |
CKD stage 2 reference. CI, confidence interval.
Based on iohexol GFR.
Profile likelihood methods determined a GFR of 43 ml/min per 1.73 m2 (95% CI 28 to 52) to be the optimal threshold in the threshold model. This unadjusted model (Table 3) had a significantly improved fit of the data as compared with a linear regression (nonthreshold) model (likelihood ratio statistic = 7.25; P < 0.01). Below the GFR threshold of 43 ml/min per 1.73 m2, hemoglobin showed a statistically significant decrease of 0.3 g/dl for every 5-ml/min per 1.73 m2 decrease in GFR (95% CI −0.5 to −0.2; Figure 1, Table 3). Above the threshold, the decline in hemoglobin was less: 0.1 g/dl for every 5-ml/min per 1.73 m2 decrease in GFR (95% CI −0.2 to 0.0). As observed in Figure 1, the shapes of the flexible nonparametric smoothing spline and the two-piece linear spline regression model are almost identical. The close overlap of these lines provides visual confirmation that the description of the data by a linear-threshold regression model is accurate.
Table 3.
Hemoglobin (g/dl) versus GFR (ml/min per 1.73 m2) and covariates in a threshold regression model
| Parameter | Parameter Estimates (95% CI)
|
||
|---|---|---|---|
| Unadjusted(n = 340) | Age/Gender Adjusted(n = 340) | Fully Adjusted(n = 339) | |
| GFR threshold, c (ml/min per 1.73 m2) | 43 (28 to 52) | 43 (29 to 51) | 43 (NA)a |
| Y-intercept, mean hemoglobin at GFR threshold, c (g/dl) | 12.6 (12.3 to 12.9) | 12.6 (12.3 to 13.0) | 12.7 (12.5 to 13.1) |
| Parameter | |||
| GFR ≤c, per 5-ml/min per 1.73 m2 decrease | −0.3 (−0.5 to −0.2) | −0.4 (−0.5 to −0.2) | −0.3 (−0.5 to −0.2) |
| GFR >c, per 5-ml/min per 1.73 m2 decrease | −0.1 (−0.2 to 0.0) | −0.1 (−0.2 to 0.0) | −0.1 (−0.2 to 0.0) |
| Female gender | −0.1 (−0.5 to 0.2) | 0.0 (−0.4 to 0.3) | |
| Age, per 5-yr increase | 0.1 (−0.1 to 0.3) | 0.2 (0.0 to 0.4) | |
| Black versus nonblack race | −0.1 (−0.6 to 0.3) | ||
| Glomerular versus nonglomerular CKD | −0.9 (−1.3 to −0.5) | ||
95% CI was not attainable (NA): The lower bound was less than a GFR of 20 ml/min per 1.73 m2, and the upper bound was >60 ml/min per 1.73 m2, the bounds of the search for threshold, c, using profile likelihood.
These unadjusted estimates for the threshold value, c, as well as the slope of hemoglobin and GFR above and below the threshold were tested in multivariate models adjusting for other clinical characteristics (Table 3). In the final multivariate model controlling for GFR, gender, age, black race, and glomerular CKD diagnosis, hemoglobin levels increased on average 0.2 g/dl for each 5-yr increase in age; hemoglobin levels were on average 0.9 g/dl lower in children with a glomerular CKD diagnosis compared with those with a nonglomerular diagnosis. Female gender and black race did not show significant associations with hemoglobin level; the estimated decreases in hemoglobin as GFR declined were not significantly different from those described in unadjusted analysis.
Analyses that were restricted to patients who were not receiving ESA provided relatively similar parameter estimates but less precise inferences as a result of the loss of information caused by exclusion. In a fully adjusted subanalysis restricted to the 272 patients who were not receiving an ESA and for whom all covariates were available (same as Table 3, fully adjusted model), the GFR threshold was 37 ml/min per 1.73 m2. This threshold was lower than that of the nonrestricted analysis and suggested a selection bias in the restricted analysis; the exclusion of patients who were receiving ESA left relatively “anemia-resistant” patients for whom the GFR threshold is lower. For every 5-ml/min per 1.73 m2 decline in GFR below the threshold GFR, the hemoglobin declined by 0.3 g/dl (95% CI −0.5 to −0.1). Above the threshold GFR, the hemoglobin declined by 0.1 g/dl (95% CI −0.2 to −0.1).
Discussion
The burden of anemia in pediatric patients with CKD is illustrated within this cross-sectional analysis; more than one third of patients met the KDOQI Clinical Practice Guidelines definition for anemia in pediatric patients: A hemoglobin concentration less than the fifth percentile of normal when adjusted for age and gender (14). Given the prevalence of anemia in the pediatric CKD population and its associated deleterious health effects, further characterization of the relationship between hemoglobin and GFR can help both researchers and clinicians anticipate the development of anemia in this population and target therapy appropriately.
In this cross-sectional investigation of patients who are enrolled in the CKiD Study, we found a linear decline in hemoglobin below a threshold iohexol-determined GFR of 43 ml/min per 1.73 m2, independent of age, race, gender, and underlying diagnosis. These findings support those of previous epidemiologic studies that examined the association between hemoglobin and estimated GFR in children and adults. In a cross-sectional analysis of 12,055 adult ambulatory patients by Hsu et al. (5), the hematocrit was found to decrease progressively for male patients with an estimated creatinine clearance by the Cockcroft-Gault equation <60 ml/min and for female patients <40 ml/min. By examining 15,419 adult patients from the Third National Health and Nutrition Examination Survey (NHANES III), Astor et al. (4) found that below an MDRD-estimated GFR of 60 ml/min per 1.73 m2, a lower estimated GFR was associated with a lower hemoglobin level for men and women.
Studies of GFR and anemia in a pediatric CKD population are few in number and limited by small sample size. In a group of 35 children with CKD, McGonigle et al. (15) described a direct linear correlation between hematocrit and creatinine clearance (r2 = 0.60, P < 0.001). In this study, anemia was not prevalent until the Schwartz-estimated GFR was <35 ml/min per 1.73 m2 (7,8,15). Chandra et al. (16) examined 48 pediatric patients with CKD and similarly described a linear correlation between hematocrit and creatinine (r2 = 0.34, P < 0.001). Anemia was noted to be prevalent when the GFR, estimated either via a creatinine clearance or the Schwartz formula, was <20 ml/min per 1.73 m2. To define more clearly the relationship between GFR and hemoglobin in the pediatric CKD population, our study used a larger sample size, more precise measurement of GFR, and statistical methods that explored the possibility of a GFR threshold.
The association between lower hemoglobin and lower GFR found in this analysis is consistent with theories of the physiology of erythropoietin production in the kidney (17). Anemia associated with kidney failure results from many factors, but inadequate erythropoietin production by diseased kidneys is known to be a primary contributor. It has been hypothesized on the basis of established principles of kidney physiology that the “signal” for erythropoietin production by peritubular fibroblasts in the renal cortex is directly related to the fractional reabsorption of sodium by tubular cells. Sodium reabsorption by the kidney tubular cells is the primary metabolic demand of the kidney and results in lower oxygen tension, which likely translates into erythropoietin production. Serum erythropoietin levels are inversely correlated with hematocrit: Lower red blood cell mass leads to lower oxygen tension and increased erythropoietin production. As GFR declines in CKD, the fractional sodium reabsorption declines, resulting in decreased oxygen use and an increase in kidney tissue oxygen pressure with attendant diminished erythropoietin production (17).
The association between GFR and hemoglobin has a biologic basis; however, the level of GFR at which erythropoietin production falls is not well defined, and the relative effect of the influence of underlying kidney pathology on erythropoietin production also is not clear. Although in this analysis we were unable to explore reliably a possible interaction of the relationship between GFR and hemoglobin by cause of CKD because of relatively small numbers, we anticipate being able to conduct such exploration in future analyses of longitudinal CKiD data. Many factors in addition to decreased erythropoietin production are known to contribute to anemia that is associated with CKD, such as iron deficiency, inflammation, and erythropoietin resistance. The correlation between hemoglobin and GFR in this study was 0.23; thus, GFR explains 5% of the variation in hemoglobin. Although this correlation is substantial for a biologic process that is known to be multifactorial, clearly other, unmeasured factors in this analysis may be contributing to the decline; this is further illustrated by the spread of hemoglobin values across any given GFR in Figure 1. The analysis presented here is limited by our ability to explain the heterogeneity of hemoglobin levels at a given GFR; however, the ongoing CKiD Study will provide opportunities to investigate known risk factors that contribute to anemia in this population, such as iron stores, inflammation, hyperparathyroidism, and less described risk factors, such as underlying cause of CKD, degree of tubulointerstitial fibrosis, and erythropoietin and IGF-1 levels. Such investigations will elucidate more precise targets for therapy in the prevention and treatment of anemia in CKD.
This analysis of hemoglobin and GFR in the CKiD Study is unique in several respects. First, it benefits from the precise and accurate determination of GFR via the plasma disappearance of iohexol, rather than relying on GFR estimating equations in children, which are known to be less accurate and imprecise. In this study, the mean Schwartz-estimated GFR was 58 ± 18 ml/min per 1.73 m2, in comparison with the mean iohexol GFR of 42 ± 14 ml/min per 1.73 m2. This compares with the inaccuracy of the currently available GFR estimating equation in children recently described in the CKiD GFR Pilot Study; estimated GFR from the Schwartz height/creatinine formula overestimated iohexol GFR by 12.2 ml/min per 1.73 m2 (9). This overestimation has clinical implications in the context of this study; decline in hemoglobin or anemia may occur at higher GFR as estimated by the current Schwartz formula. Second, this study uses statistical approaches to allow a threshold in the relationship between hemoglobin and GFR, as well as the inclusion of patients whose hemoglobin levels are elevated as a result of the use of ESA. Treating these observations as left-censored allows the estimation of ESA-naive hemoglobin levels and avoids the bias that would be introduced if this subset of patients had been excluded from the analysis. Finally, this study benefits from the standardized data collection methods used by the CKiD Prospective Cohort Study, which improve the overall quality of the data. This analysis is limited to cross-sectional data; however, future longitudinal analyses, including within-subject changes, will be possible as the CKiD Study progresses and will allow further insight into the relationship between hemoglobin and GFR decline.
Disclosures
None.
Acknowledgments
The CKiD prospective cohort study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Neurologic Disorders and Stroke, National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (UO1-DK-66143, UO1-DK-66174, and UO1-DK-66116). J.J.F. is supported by a Mentored Patient-Oriented Career Development Award (K23-ES-016514). S.L.F. is also supported by a Midcareer Investigator Award (K24-DK-078737).
This study was presented as a poster at the annual meeting of the American Society of Pediatric Nephrology; April 29 through May 2, 2006; San Francisco, CA.
The CKiD prospective cohort study has clinical coordinating centers (principal investigators) at Children's Mercy Hospital and the University of Missouri-Kansas City (Bradley Warady, MD) and Johns Hopkins School of Medicine (Susan Furth, MD, PhD) and a data coordinating center at the Johns Hopkins Bloomberg School of Public Health (principal investigator Alvaro Muñoz, PhD).
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Centers for Medicare & Medicaid Services: 2005 Annual Report, End Stage Renal Disease Clinical Performance Measures Project, Baltimore, Department of Health and Human Services, Centers for Medicare & Medicaid Services, Office of Clinical Standards & Quality, December 2005
- 2.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–266, 2002 [PubMed] [Google Scholar]
- 3.Wong H, Mylrea K, Feber J, Drukker A, Filler G: Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int 70: 585–590, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Astor BC, Muntner P, Levin A, Eustace JA, Coresh J: Association of kidney function with anemia: The Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 162: 1401–1408, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, Bates DW, Kuperman GJ, Curhan GC: Relationship between hematocrit and renal function in men and women. Kidney Int 59: 725–731, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz GJ, Wong C, Muñoz A, Warady B: Design and methods of the chronic kidney disease in children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 8.Schwartz GJ, Gauthier B: A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 106: 522–526, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Furth S, Cole SR, Warady B, Muñoz A: Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 10.HE-B Basic Hematology Participant Summary, Northfield, IL, College of American Pathologists, 2007.
- 11.Nathan DG, Orkin SH: Nathan and Oski's Hematology of Infancy and Childhood, Philadelphia, Saunders, 1998
- 12.Steenland K, Deddens JA: A practical guide to dose-response analyses and risk assessment in occupational epidemiology. Epidemiology 15: 63–70, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Pawitan Y: In All Likelihood: Statistical Modeling and Inference Using Likelihood, New York, Oxford University Press, 2001
- 14.KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 47: S11–145, 2006 [DOI] [PubMed] [Google Scholar]
- 15.McGonigle RJ, Boineau FG, Beckman B, Ohene-Frempong K, Lewy JE, Shadduck RK, Fisher JW: Erythropoietin and inhibitors of in vitro erythropoiesis in the development of anemia in children with renal disease. J Lab Clin Med 105: 449–458, 1985 [PubMed] [Google Scholar]
- 16.Chandra M, Clemons GK, McVicar MI: Relation of serum erythropoietin levels to renal excretory function: Evidence for lowered set point for erythropoietin production in chronic renal failure. J Pediatr 113: 1015–1021, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Donnelly S: Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am J Kidney Dis 38: 415–425, 2001 [DOI] [PubMed] [Google Scholar]