Abstract
Background and objectives: For addressing the influence of muscle mass on serum and urinary creatinine and serum cystatin C, body composition was assessed by skinfold thickness measurement and bioelectrical impedance analyses.
Design, setting, participants, & measurements: A total of 170 healthy individuals (92 women, 78 men) were classified as sedentary or with mild or moderate/intense physical activity. Blood, 24-h urine samples, and 24-h food recall were obtained from all individuals.
Results: Serum and urinary creatinine correlated significantly with body weight, but the level of correlation with lean mass was even greater. There was no significant correlation between body weight and lean mass with cystatin C. Individuals with moderate/intense physical activity presented significantly lower mean body mass index (23.1 ± 2.5 versus 25.7 ± 3.9 kg/m2) and higher lean mass (55.3 ± 10.0 versus 48.5 ± 10.4%), serum creatinine (1.04 ± 0.12 versus 0.95 ± 0.17 mg/dl), urinary creatinine (1437 ± 471 versus 1231 ± 430 mg/24 h), protein intake (1.4 ± 0.6 versus 1.1 ± 0.6 g/kg per d), and meat intake (0.7 ± 0.3 versus 0.5 ± 0.4 g/kg per d) than the sedentary individuals. Conversely, mean serum cystatin did not differ between these two groups. A multivariate analysis of covariance showed that lean mass was significantly related to serum and urinary creatinine but not with cystatin, even after adjustment for protein/meat intake and physical activity.
Conclusions: Cystatin C may represent a more adequate alternative to assess renal function in individuals with higher muscle mass when mild kidney impairment is suspected.
Accurate renal function measurements are important in the diagnosis and treatment of kidney diseases, adjustment of drug dosages, and decision-making regarding when to initiate renal replacement therapy. Serum creatinine is the most commonly used indicator of renal function, but its measurement suffers from a variety of analytical interferences and significant standardization problems (1,2).
Serum creatinine can be affected by age, gender, ethnicity, dietary protein intake, and lean mass and may remain within the reference range despite marked renal impairment in patients with low muscle mass. Consequently, the sensitivity of serum creatinine for the early detection of kidney disease is poor and not a good predictor when analyzing the elderly (3,4). Conversely, theoretically, serum creatinine may be falsely increased in individuals with higher muscle mass and normal renal function.
The GFR represents the best overall assessment of kidney function, but the gold standard techniques for the measurement of GFR, such as inulin clearance, [125I]iothalamate, 51Cr-EDTA, 99mTc-diethylenetriaminepentaacetic acid, and iohexol are too labor-intensive and costly for routine clinical use (5,6), so creatinine clearance is used instead.
To rid the need of 24-h urine collections, several serum creatinine–based prediction formulas have been proposed to predict GFR (7–16). The equations of Cockcroft and Gault (7,8) and the one derived from the Modification of Diet in Renal Disease (MDRD) study (10) are the most widely accepted; however, the competence of such formulas to predict GFR in patients with normal values of serum creatinine is debated.
Despite the important influence of muscle mass on serum creatinine, the different equations used to predict GFR do not include parameters of body composition such as lean mass. Human body mass can be partitioned into two main compartments: Fat and lean (fat-free) mass. The latter comprises body cell mass (BCM), bone mass, and extracellular water. The gold standard techniques for the measurement of body composition include hydrodensitometry, computed tomography, magnetic resonance imaging, dual-photon absorptiometry, neutron activation analysis, total body potassium counting, and isotope dilution (17,18). Nevertheless, in clinical practice, the indirect, low-cost, noninvasive methods of determining human body composition, such as bioelectrical impedance and skinfold thickness, are used instead (17). Muscle mass is extremely variable among elderly individuals and in children (4,19–22) and can be substantially modified by physical exercise (23).
Cystatin C, a low molecular weight basic protein (13 kD) that is freely filtered and metabolized after tubular reabsorption with only small amounts excreted in the urine, is an endogenous filtration marker that is being considered as a potential replacement for serum creatinine. Unlike serum creatinine, the serum concentration of cystatin remains constant up to 50 yr of age. It is commonly accepted that cystatin is produced at a constant rate in virtually all nucleated cells and that it is unaltered by inflammatory conditions. The advantages of using cystatin C as a filtration marker are less influence by age, gender, weight, and muscle mass than serum creatinine (24–31). An overall meta-analysis based on 46 studies performed on adults and children demonstrated, by means of receiver operating characteristic analysis, that cystatin C is superior to serum creatinine as a marker of kidney function (32). To address the influence of muscle mass on serum and urinary creatinine determination and serum cystatin C, we evaluated the body composition through bioelectrical impedance and skinfold thickness in healthy individuals with distinct levels of physical activity.
Concise Methods
A total of 206 healthy volunteers were recruited from the hospital staff and gymnastics schools. Selections were made using a questionnaire to exclude any carriers of kidney disease; those with relevant morbid conditions; or those taking anabolic steroids, creatine, vitamins, or any sort of dietary supplements. Spot urine samples were obtained, and screening tests using urine dipsticks were performed to exclude those who had positive protein, glucose, erythrocytes, nitrites, or leukocyte esterase tests. A total of 170 healthy adults (92 women and 78 men) were eligible to be enrolled in this study. A written consent was obtained from all participants, and the local ethics committee of the Universidade Federal de São Paulo approved the study.
All participants were subjected to an anthropometric evaluation and body composition assessment through skinfold thickness and bioelectrical impedance. They were then given collection containers and instructions for 24-h urine collection for creatinine and microalbuminuria determination. On the morning they completed the 24-h urine collection, a blood sample was drawn, and an additional fasting morning urine sample was collected at the laboratory for urinalysis and determination of retinol-binding protein (RBP). They were then asked to complete a 24-h food recall questionnaire to assess their food intake.
Biochemical Parameters
Serum creatinine, cystatin C, urea, and albumin were determined in serum samples. Creatinine was determined according to a modified Jaffé reaction (33) in Hitachi 912 (Roche Diagnostic System, Basel, Switzerland), by an isotope dilution mass spectrometry traceable method. Estimates of GFR were obtained using the Cockcroft-Gault (7,8) and re-expressed four-variable MDRD equations (10,34). Cystatin C was measured using a fully automated particle-enhanced immune turbidimetric method (DAKO Cystatin C Pet kit, Copenhagen, Denmark). Inter- and intra-assay variation, calculated from the control samples with assigned values of 0.97 and 3.36 mg/L, was 3.2 and 1.4%, respectively. Microalbuminuria was determined by ELISA and RBP by the immunoenzymometric method (35).
Measurements and Assessments
A 24-h food recall to assess daily energy intake and macronutrients (protein, lipids and carbohydrate) was undertaken by each participant. The amount of striated muscle intake (meat intake) was also assessed. Nutrient intakes were calculated with a computerized program developed in our department. The food table used in the program was from the US Department of Agriculture.
Anthropometric parameters included body weight, height, waist circumference, and body mass index (BMI). BMI was calculated as weight (kg)/height (m2). Body composition was assessed by two indirect methods: Skinfold thickness and bioelectrical impedance analysis (BIA). Skinfold measurements were performed by the same nutritionist in the nondominant arm at four sites—biceps, triceps, subscapular, and suprailiac—using a Lange skinfold caliper (Cambridge Instrument, Cambridge, MA). Three sets of measurements were averaged for each site. Body fat was calculated according to Siri's equation (36). BIA was performed with a portable device model BIA 101 Quantum, RJL Systems (Detroit, MI), and the software provided by the manufacturer calculated total body water, fat-free mass, BCM, and fat mass. BCM was defined as fat-free mass without bone mineral mass and extracellular water.
Physical Activity
The Baecke questionnaire (37), validated in a Brazilian population survey (38), was used to calculate the level of physical activity.
Statistical Analyses
Results were reported as means ± SD. Comparisons were performed through ANOVA (among the three groups). The Pearson correlation coefficient was used to determine the correlation between parameters. A multivariate analysis of covariance was performed to determine the effect of body weight, fat-free mass, and BCM (independent variables) on serum and urinary creatinine and cystatin C (dependent variables), adjusting for physical activity, protein intake, and meat intake (covariates). For the purpose of this analysis, the variables without normal distribution were converted in logarithmic (log10) scale. The level of significance was defined as P < 0.05.
Results
A total of 170 healthy individuals (78 men and 92 women; 36.6 ± 13.6 yr of age (range 18 to 75 yr old) participated in the study. None of the participants presented altered urinalysis, microalbuminuria, or increased urinary RBP.
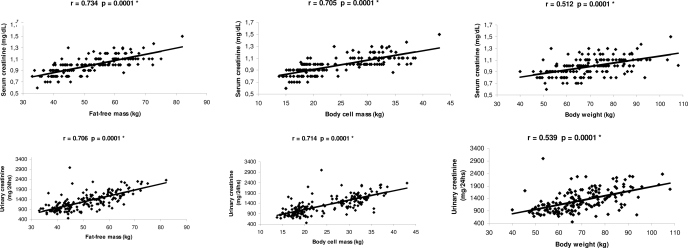
Correlations between fat-free mass, BCM, and body weight and serum and urinary creatinine are shown in Figure 1. Serum and urinary creatinine levels significantly correlated with body weight (r = 0.512 [95% confidence interval (CI) 0.391 to 0.615] and r = 0.539 [95% CI 0.422 to 0.638]; P = 0.0001), but the level of correlation was greater with fat-free mass (r = 0.734 [95% CI 0.656 to 0.797] and r = 0.706 [95% CI 0.620; 0.774]; P = 0.0001). There was no overlap between both CI.
Figure 1.
Correlation between fat-free mass, BCM, and body weight and urinary and serum creatinine.
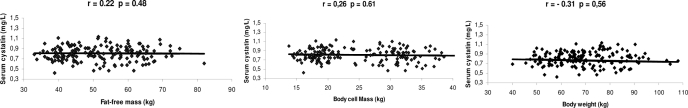
Correlations between fat-free mass, BCM, and body weight and serum cystatin C are shown in Figure 2. There was no significant correlation between body weight, fat-free mass, or BCM and serum cystatin C.
Figure 2.
Correlation between fat-free mass, BCM, and body weight and serum cystatin.
Mean daily energy intake of individuals according to their level of physical activity (sedentary or mild or moderate/intense activity) was shown to be significantly higher among the group with moderate/intense versus mild physical activity and versus sedentary individuals (36 ± 14 versus 28 ± 9 and versus 26 ± 10 kcal/kg per d; data not shown). Distribution of macronutrients (protein, lipids, and carbohydrates) was shown to be significantly higher as well: 1.4 ± 0.6 versus 1.2 ± 0.5 and versus 1.1 ± 0.6 and g/kg per d, 81 ± 42 versus 68 ± 32 and versus 63 ± 27 g/d, and 342 ± 173 versus 261 ± 106 and versus 218 ± 74 g/d, respectively. Meat intake was also significantly higher in individuals with moderate/intense physical activity versus mild and versus sedentary: 0.7 ± 0.3 versus 0.6 ± 0.4 and versus 0.5 ± 0.4 g/kg per d.
Table 1 shows the mean anthropometric, body composition, and renal function parameters according to the level of physical activity. Individuals with moderate/intense physical activity presented with a trend for lower body weight and a significantly lower BMI, higher muscle mass, and lower body fat content than the sedentary individuals or those with mild physical activity. Waist circumference was also significantly lower in individuals with moderate/intense activity when compared with sedentary individuals.
Table 1.
Anthropometric, body composition, and renal function parameters according to the level of physical activitya
| Parameter | Sedentary (n = 57) | Mild (n = 61) | Moderate/Intense (n = 52) | P |
|---|---|---|---|---|
| Age (yr) | 37.2 ± 14.0 | 39.5 ± 14.6 | 32.5 ± 10.9b | 0.0200 |
| Weight (kg) | 69.3 ± 14.2 | 70.5 ± 12.9 | 67.8 ± 11.5 | 0.5400 |
| BMI (kg/m2) | 25.7 ± 3.9 | 25.6 ± 4.1 | 23.1 ± 2.5b,c | 0.0001 |
| Waist circumference (cm) | 82.1 ± 10.3 | 80.6 ± 14.7 | 75.7 ± 10.9c | 0.0300 |
| Body fat (skinfold thickness; %) | 31.4 ± 8.4 | 30.7 ± 8.9 | 20.7 ± 8.8b,c | 0.0001 |
| Body fat | ||||
| % | 29.6 ± 7.4 | 27.7 ± 6.7 | 18.4 ± 6.2b,c | 0.0001 |
| kg | 20.7 ± 7.7 | 19.7 ± 6.9 | 12.5 ± 4.7b,c | 0.0001 |
| Fat-free mass | ||||
| % | 70.4 ± 7.4 | 72.3 ± 6.7 | 80.0 ± 11.9b,c | 0.0001 |
| kg | 48.5 ± 10.4 | 50.8 ± 9.6 | 55.3 ± 10.0b,c | 0.0002 |
| BCM (kg) | 22.8 ± 7.2 | 23.9 ± 6.7 | 27.4 ± 6.3b,c | 0.0020 |
| Serum cystatin (mg/L) | 0.82 ± 0.14 | 0.80 ± 0.14 | 0.79 ± 0.14 | 0.6000 |
| Serum urea (mg/dl) | 29.2 ± 8.1 | 29.1 ± 8.2 | 30.6 ± 8.2 | 0.5900 |
| Serum albumin (g/dl) | 4.46 ± 0.26 | 4.56 ± 0.26 | 4.70 ± 0.32b,c | 0.0001 |
| Serum creatinine (mg/dl) | 0.95 ± 0.17 | 0.96 ± 0.13 | 1.04 ± 0.12b,c | 0.0020 |
| Urinary creatinine (mg/24 h) | 1231 ± 430 | 1283 ± 400 | 1437 ± 471c | 0.0400 |
| Urinary RBP (mg/L) | 0.05 ± 0.03 | 0.06 ± 0.05 | 0.08 ± 0.05c | 0.0300 |
| Microalbuminuria (μg/min) | 6.7 ± 18.3 | 6.6 ± 10.8 | 7.2 ± 7.9 | 0.9600 |
| CrCl (ml/min per 1.73 m2) | 88.3 ± 17.3 | 87.5 ± 18.3 | 91.2 ± 22.2 | 0.5600 |
| CG (ml/min per 1.73 m2) | 93.0 ± 17.4 | 87.6 ± 15.0 | 89.0 ± 14.2 | 0.1500 |
| MDRD (ml/min per 1.73 m2) | 84.5 ± 17.7 | 79.6 ± 12.2 | 81.8 ± 13.2 | 0.1900 |
Data are means ± SD. BCM, body cell mass; BMI, body mass index; CG, Cockcroft-Gault; CrCl, creatinine clearance; MDRD, Modification of Diet in Renal Disease
P < 0.05 versus mild.
P < 0.05 versussedentary.
Individuals with moderate/intense physical activity presented a significantly higher serum creatinine and albumin compared with the other two groups and a significantly higher urinary creatinine than the sedentary individuals. There were no differences in serum cystatin or urea, microalbuminuria, and measured creatinine clearance among these groups. Individuals with moderate/intense physical activity presented with a trend for lower estimated creatinine clearance and GFR (Cockcroft-Gault and MDRD equation) than sedentary individuals.
Table 2 shows the results of the multivariate analysis of covariance showing that body weight, fat-free mass, and BCM were significantly related with serum creatinine (R2 = 0.37 and R2 = 0.54 and R2 = 0.56, P < 0.001) and with urinary creatinine (R2 = 0.34 and R2 = 0.48 and R2 = 0.51, P < 0.001, respectively), even after adjustment for physical activity and protein and meat intake. Conversely, there was no significant association between body weight, fat-free mass, or BCM and serum cystatin C in this model.
Table 2.
Multivariate analysis of covariance adjusted for physical activity, total protein, and meat intake
| Dependent Variables | Independent Variablesa | R2 | P |
|---|---|---|---|
| Serum creatininea | Body weight | 0.37 | 0.001 |
| Fat-free mass | 0.54 | 0.001 | |
| BCM | 0.55 | 0.001 | |
| Urinary creatininea | Body weight | 0.34 | 0.001 |
| Fat-free mass | 0.48 | 0.001 | |
| BCM | 0.51 | 0.001 | |
| Serum cystatin | Body weight | 0.01 | 0.904 |
| Fat-free mass | 0.01 | 0.803 | |
| BCM | 0.01 | 0.892 |
Variables converted in logarithmic (log10) scale.
Discussion
Serum creatinine is influenced by gender, age, and the amount of muscle mass; however, formulas that predict GFR take into account gender, age and weight but not muscle mass (7–16). To date, no study has evaluated the influence of muscle mass on serum and urinary creatinine and serum cystatin C in healthy individuals with distinct levels of physical activity.
In this study, although serum and urinary creatinine were significantly correlated with body weight, the level of correlation with lean (fat-free) mass and BCM was even greater. Conversely, there was no significant correlation between body weight, fat-free mass, or BCM and serum cystatin C.
The group with moderate/intense physical activity presented a significantly higher mean serum creatinine compared with the group with mild activity or the sedentary group and higher urinary creatinine than the latter. Mean values of measured creatinine clearance did not differ between these groups. Nevertheless, individuals with moderate/intense physical activity presented with a trend for lower estimated creatinine clearance (Cockcroft-Gault) and GFR (MDRD equation) than sedentary individuals, although not reaching statistical significance. Individuals with higher physical activity presented lower body weight, BMI, waist circumference, and body fat content and higher muscle mass than the sedentary or those with mild physical activity. These results were expected because intense physical activity directly influences body composition, reducing the body fat and increasing the fat-free mass (23). Conversely, serum cystatin did not differ between groups. These findings further corroborate that cystatin C is not influenced by muscle mass (24–31).
Whether the capacity of cystatin C to predict GFR in healthy individuals is influenced by body composition has been controversial (39), with some investigators advocating some association (40,41) and others finding no association of cystatin C and lean body mass (24–31). As pointed out recently by Shlipack (39), although the findings of MacDonald et al. (41) indicated a potential influence of lean body mass on cystatin C, adjustment for body surface area could possibly have eliminated such influence. In addition, dual-energy x-ray absorptiometry–determined lean body mass also may not reflect BCM well in patients with chronic kidney disease because of changes in hydration.
In this series, urinary creatinine was also significantly higher in the individuals with moderate/intense physical activity than the sedentary. Indeed, creatinine excretion is also influenced by muscle mass, because creatinine formation occurs almost exclusively in the muscle; therefore, theoretically, the urinary excretion is the most specific index to define muscle mass (42,43). Healthy men and women excrete approximately 1.5 and 1.2 g/d creatinine, respectively; however, these values can be modified according to the amount of muscle mass and diet. Food contributes with three distinct components that can alter the urinary excretion of creatinine: (1) Proteins that have arginine and glycine, precursors of creatine and guanidoacetate production; (2) creatine itself, which leads to a direct increase of the muscular creatine “pool,” thereby raising the urinary excretion of creatinine; and (3) dietary creatinine, which is excreted readily as soon as absorbed (43–45). In this study, individuals with moderate/intense physical activity presented higher urinary creatinine, probably as a result of the larger muscle mass and the higher mean protein and meat intake consumed by these individuals. Not only was the protein intake higher, but also the lipid and carbohydrate intake were higher when compared with sedentary individuals. It is noteworthy that, despite consuming more energy, individuals with moderate/intense physical activity tended to exhibit lighter weight and lower BMI, and the percentage of fat mass was significantly lower than that in the sedentary individuals. This might have been attributed to their higher fat-free mass, which requires and expends more energy (46).
The Cockcroft-Gault formula was developed from 249 men with a range of creatinine clearance between 30 and 130 ml/min to estimate creatinine clearance on the basis of serum creatinine, age, gender, and body weight (7). The Cockcroft-Gault equation systematically overestimates GFR, because it was developed to estimate creatinine clearance and not GFR. Furthermore, because of the inclusion of weight in the numerator, the equation overestimates creatinine clearance in patients who are edematous or overweight (16,47) or in individuals with low creatinine production (malnourished patients). Conversely, it may underestimate creatinine clearance in individuals with high creatinine production as a result of higher muscle mass. In this study, the lower weight and the higher serum creatinine of individuals with moderate/intense physical activity might have contributed to the slightly lower mean value of the Cockcroft-Gault formula, although not achieving statistical significance, when compared with the sedentary individuals. Lim et al. (48) compared the acuity of some formulas used to estimate the GFR in healthy individuals with and without the adjustment for lean or fat-free mass and concluded that when the Cockcroft-Gault equation was corrected per fat-free mass, there was a significant increase in its acuity; however, in their study, muscle mass was not measured by BIA or skinfold thickness but roughly estimated through a formula.
The re-expressed four-variable MDRD equation (34) estimates GFR adjusted for body surface area on the basis of serum creatinine, age, gender, and race. As for the Cockcroft-Gault formula, the muscle mass is not considered. The overestimation of GFR by the MDRD formula in older populations (49) may reflect a relatively lower serum creatinine concentration, as a result of reduced muscle mass, compared with the middle-aged population in which the MDRD formula was derived. Conversely, the tendency of the formula to underestimate the GFR in the majority of the studies carried out in healthy individuals can be explained by their higher muscle mass contrasting with that of patients with chronic renal failure as a result of anorexia, chronic inflammation, and metabolic acidosis leading to muscle breakdown (50). In individuals with high creatinine production and higher muscle mass, underestimation of MDRD is expected. In this series, the higher serum creatinine of individuals with moderate/intense physical activity might have contributed to the slightly (although not significantly) lower mean value of MDRD compared with the sedentary individuals.
Finally, to separate the potential influence of protein and meat intake from the influence of fat-free mass itself, on serum/urinary creatinine, a multivariate analysis of covariance was performed in this study. Such analysis disclosed that fat-free (lean) mass remained significantly related to serum and urinary creatinine, even after adjustments for protein/meat intake and physical activity. Conversely, a significant association between fat-free mass with serum cystatin C was not depicted.
Conclusions
This study showed that muscle mass affects serum and urinary creatinine but not cystatin C; therefore, the use of cystatin may represent a more adequate alternative to assess renal function in healthy individuals with higher muscle mass and potential mild kidney impairment.
Disclosures
None.
Acknowledgments
Research was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (grant 03/13889-4), Coordenação de Aperfeiçoamento Pessoal de Nível Superior, and Fundação Oswaldo Ramos-Hospital do Rim e Hipertensão from Universidade Federal de São Paulo.
Part of this study was presented as an abstract at the annual meeting of the American Society of Nephrology; November 8 to 13, 2005; Philadelphia, PA.
We express thanks to Silvia Regina Moreira and Sonia Nishida for technical assistance.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Perrone RD, Madias NE, Levey AS: Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 38: 1933–1953, 1992 [PubMed] [Google Scholar]
- 2.Lawson N, Lang T, Broughton A, Prinsloo P, Turner C, Marenah C: Creatinine assays: Time for action? Ann Clin Biochem 39: 599–602, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Shemesh O, Golbetz H, Kriss JP, Myers BD: Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int 28: 830–838, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Goldberg TH, Finkelstein MS: Difficulties in estimating glomerular filtration rate in the elderly. Arch Intern Med 147: 1430–1433, 1987 [PubMed] [Google Scholar]
- 5.Bostom AG, Kronenberg F, Ritz E: Predictive performance of renal function equations for patients with chronic kidney disease and normal serum creatinine levels. J Am Soc Nephrol 13: 2140–2144, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Gaspari F, Perico N, Remuzzi G: Application of newer clearance techniques for the determination of glomerular filtration rate. Curr Opin Nephrol Hypertens 7: 675–680, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 8.Gault MH, Longerich LL, Harnett JD, Wesolowski C: Predicting glomerular function from adjusted serum creatinine. Nephron 62: 249–256, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Bjornsson TD: Use of serum creatinine concentrations to determine renal function. Clin Pharm 4: 200–222, 1979 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Gates GF: Creatinine clearance estimation from serum creatinine values: An analysis of three mathematical models of glomerular function. Am J Kidney Dis 5: 199–205, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Jelliffe RW, Jelliffe SM: A computer program for estimation of creatinine clearance from unstable serum creatinine levels, age, sex, and weight. Math Biosci 14: 17–24, 1972 [Google Scholar]
- 13.Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 14.Schwartz GJ, Gauthier B: A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 106: 522–526, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Brion LP, Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34: 571–590, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Lamb EJ, Tomson CR, Roderick PJ: Estimating kidney function in adults using formulae. Ann Clin Biochem 42: 321–345, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Kamimura MA, Draibe AS, Sigulem DM, Cuppari L: Methods of body composition assessment in patients undergoing hemodialysis. Rev Nutr Campinas 17(1): 97–105, 2004 [Google Scholar]
- 18.Lukaski HC: Methods for the assessment of human body composition: Traditional and new. Am J Clin Nutr 46: 537–556, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Brion LP, Boeck MA, Gauthier B, Nussbaum MP, Schwartz GJ: Estimation of glomerular filtration rate in anorectic adolescents. Pediatr Nephrol 3: 16–21, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Haycock GB: Creatinine, body size and renal function. Pediatr Nephrol 3: 22–24, 1989 [DOI] [PubMed] [Google Scholar]
- 21.Brion LP, Fleischman AR, McCarton C, Schwartz GJ: A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: Non-invasive assessment of body composition and growth. J Pediatr 109: 698–707, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Seikaly MG, Browne R, Bajaj G, Arant BS Jr: Limitations to body serum/serum creatinine ratio as an estimate of glomerular filtration in children. Pediatr Nephrol 10: 709–711, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP, Houmard JA, Bales CW, Kraus WE: Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—A randomized controlled study. Arch Intern Med 164: 31–39, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS: Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int 69: 399–405, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Filler G, Bökenkamp A, Hofmann W, Le Bricon T, Martínez-Brú C, Grubb A: Cystatin C as a marker of GFR: History, indications, and future research. Clin Biochem 38: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Kyhse-Andersen J, Schmidt C, Nordin G, Anderson B, Nilsson-Ehle P, Lindström V, Grubb A: Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem 40: 1921–1926, 1994 [PubMed] [Google Scholar]
- 27.Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP: Serum cystatin C measured by automated immunoassay: A more sensitive marker of changes in GFR than serum creatinine. Kidney Int 47: 312–318, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Hoek FJ, Kemperman FA, Krediet RT: A comparison between Cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant 18: 2024–2031, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Tian S, Kusano E, Ohara T, Tabei K, Itoh Y, Kawai T, Asano Y: Cystatin C measurement and its practical use in patients with various renal diseases. Clin Nephrol 48: 104–108, 1997 [PubMed] [Google Scholar]
- 30.Vinge E, Lindergand B, Nilsson-Ehle P, Grubb A: Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 59: 587–592, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Laterza OF, Price CP, Scott MG: Cystatin C: An improved estimator of glomerular filtration rate? Clin Chem 48: 699–707, 2002 [PubMed] [Google Scholar]
- 32.Dharnidharka VR, Kwong C, Stevens G: Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis 40: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Bartels H, Böhmer M, Heierli C: Serum creatinine determination without protein precipitation. Clin Chim Acta 37: 193–197, 1972 [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Coresh J, Greene T: Expressing the MDRD study equation for estimating GFR with IDMS traceable (Gold Standard) serum creatinine values [Abstract]. J Am Soc Nephrol 16: 69A, 2005 [Google Scholar]
- 35.Pereira AB, Nishida SK, Vieira JG, Lombardi MT, Silva NS, Ajzen H, Ramos OL: Monoclonal antibody-based immunoenzymometric assays of retinol-binding protein. Clin Chem 39: 472–476, 1987 [PubMed] [Google Scholar]
- 36.Siri WE. Body composition from fluids spaces and density: Analysis of two methods. In: Techniques for Measuring Body Composition, edited by Brozec J, Henschel A, Washington, DC, National Research Council, 1961, pp 223–224
- 37.Baecke JA, Burema J, Frijters J: A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36: 936–942, 1982 [DOI] [PubMed] [Google Scholar]
- 38.Florindo AA, Latorre MR: Validation and reliability of the Baecke questionnaire for the evaluation of habitual physical activity in adult men. Rev Bras Med Esporte 9: 129–135, 2003 [Google Scholar]
- 39.Shlipak MF: Cystatin C as a marker of glomerular filtration rate in chronic kidney disease: Influence of body composition. Nat Clin Pract Nephrol 3: 188–189, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE: Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65: 1416–1421, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Macdonald J, Marcora S, Jibani M, Roberts G, Kumwenda M, Glover R, Barron J, Lemmey A: GFR estimation using cystatin C is not independent of body composition. Am J Kidney Dis 48: 712–719, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Proctor DN, O'Brien PC, Atkinson EJ, Nair KS: Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am J Physiol 277: 489–495, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S: Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am J Clin Nutr 37: 478–494, 1983 [DOI] [PubMed] [Google Scholar]
- 44.Crim MC, Calloway DH, Margen S: Creatine metabolism in man: Urinary creatine and creatinine excretions with creatine feeding. J Nutr 105: 428–438, 1975 [DOI] [PubMed] [Google Scholar]
- 45.Crim MC, Calloway DH, Margen S: Creatine metabolism in men: Creatine pool size and turnover in relation to creatine intake. J Nutr 106: 371–381, 1976 [Google Scholar]
- 46.Avesani CM, Kamimura MA, Draibe SA, Cuppari L: Is energy intake underestimated in nondialyzed chronic kidney disease patients? J Ren Nutr 15: 159–165, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Salazar DE, Corcoran GB: Predicting creatinine clearance and renal drug clearance in obese patients from estimated fat-free body mass. Am J Med 84: 1053–1060, 1988 [DOI] [PubMed] [Google Scholar]
- 48.Lim WH, Lim EE, McDonald S: Lean body mass-adjusted Cockcroft and Gault formula improves the estimation of glomerular filtration rate in subjects with normal-range serum creatinine. Nephrology 11: 250–256, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Lamb EJ, Webb MC, Simpson DE, Coakley AJ, Newman DJ, O'Riordan SE: Estimation of glomerular filtration rate in older patients with chronic renal insufficiency: Is the modification of diet in renal disease formula an improvement? J Am Geriatr Soc 51: 1012–1017, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Mitch WE. Nutritional therapy and progression of chronic renal insufficiency. In: Handbook of Nutrition and the Kidney, 3rd Ed., edited by Mitch WE, Klahr S, Philadelphia, Lippincott-Raven, 1998, pp 237–252