Abstract
Background and objectives: Failure to mature (primary failure) of new fistulas remains a major obstacle to increasing the proportion of dialysis patients with fistulas. This failure rate is higher in women than in men, higher in older than in younger patients, and higher in forearm than in upper arm fistulas. These disparities in the frequency of failure to mature may be due in part to marginal vessels in the high-risk groups and should be reduced by routine preoperative vascular mapping.
Design, setting, participants, & measurements: A prospective, computerized database was queried retrospectively to evaluate the frequency of primary fistula failure in 205 hemodialysis patients for whom preoperative mapping was obtained. The association between clinical characteristics and risk for primary fistula failure was analyzed by univariate and multiple variable regression analysis.
Results: The overall primary fistula failure rate was 40% (82 of 205 patients). On multiple variable logistic regression, three clinical factors were associated with an increased risk for failure to mature among patients who underwent preoperative vascular mapping: Female gender, age ≥65 yr, and forearm location. The primary fistula failure rate varied from 22% in younger men with an upper arm fistula to 78% in older women with a forearm fistula. Dynamic preoperative vascular measurements (change in peak systolic velocity and resistive index after tight fist clenching) did not differ between patients with mature and immature forearm fistulas.
Conclusion: Disparities in fistula maturation persist despite the use of routine preoperative vascular mapping.
The major hurdle to increasing arteriovenous fistula use in hemodialysis patients is the high frequency of fistulas that fail to mature. These fistulas are never usable for dialysis (primary failures) (1–3). The failure rate is not uniform, varying greatly among different patient subsets. A higher risk for failure to mature has been observed in older patients, women, nonwhite patients, and patients with cardiovascular disease (4,5). These differences are reflected in the prevalence rates of fistulas in US hemodialysis patients, which are lower in women, black patients, and older patients (6). Moreover, forearm fistulas are more likely than upper arm fistulas to fail to mature (5).
Fistula maturation requires adequate arterial inflow, adequate venous outflow, and the ability of the vein to dilate and increase blood flow sufficiently to permit reproducible cannulation for dialysis and deliver an adequate dosage of dialysis (1). One plausible explanation for the disparities in fistula success among dialysis patients is that the high-risk groups are more likely to have marginal vessels. If so, then one might expect that careful selection of arteries and veins that are most suitable for fistula creation would reduce the primary failure rate of new fistulas and minimize the disparities among patient subgroups in the likelihood of failure to mature. The desire to increase fistula use in the United States has driven widespread implementation of preoperative vascular mapping to assist the surgeons in optimizing fistula success. Certainly, this approach has dramatically increased fistula placement at several medical centers (7–10); however, there has been little systematic effort to determine whether the introduction of routine preoperative mapping reduces the primary failure rate of new fistulas, particularly in patient subsets that are known to be at high risk for this complication.
To evaluate this question, we evaluated the fistula maturation rate in hemodialysis patients at our medical center who received a new fistula after preoperative vascular mapping. A prospective, computerized access database was queried retrospectively to derive a comprehensive list of all fistulas placed, as well as their clinical outcomes.
Concise Methods
Vascular Access Procedures
Nephrologists at the University of Alabama at Birmingham oversee the medical care of approximately 450 hemodialysis patients at five outpatient units in metropolitan Birmingham. University of Alabama at Birmingham transplant surgeons place all new vascular accesses (fistulas and grafts) in these patients. Before 1999, the surgeons determined the type of access to be placed and its location by performing a clinical evaluation that included assessment of the vessels with a tourniquet but without the benefit of any imaging studies (5). After a pilot study determined that preoperative vascular mapping frequently changed the access plans of the surgeons (11), routine mapping was implemented, with the results made available to the surgeons before access creation (1,7). The criteria for fistula creation included a minimum arterial diameter of 2.0 mm, a minimum venous diameter of 2.5 mm, and absence of stenosis or thrombosis in the draining vein. Forearm fistulas were created preferentially, but upper arm fistulas were placed when the forearm vessels were inadequate. In the subset of patients who received a forearm fistula, spectral waveforms were recorded during tight fist clenching for 3 min and after fist relaxation, as described previously (12). The radial artery waveforms were used to calculate peak systolic velocity (PSV) and end-diastolic velocity (EDV). The resistive index (RI), a measure of downstream flow resistance, was calculated as (PSV − EDV)/PSV.
New fistulas were evaluated for cannulation for dialysis 6 to 8 wk after their creation. Fistulas that were clinically immature underwent a postoperative ultrasound to assess for potentially correctible anatomic causes of immaturity, including vascular stenosis, accessory veins, or excessively deep location (13,14). When such an anatomic lesion was identified, it was treated radiologically or surgically in an attempt to promote fistula maturation.
A fistula was considered mature when it could be cannulated reproducibly for dialysis, using two needles and achieving a dialysis blood flow ≥300 ml/min, within 6 mo of its creation. Failure to mature (primary failure) was defined as the inability to meet this goal. The fistula outcome was considered indeterminate when the patient died, received a kidney transplant, or transferred to an outside dialysis unit before fistula outcome could be assessed.
Data Analysis
Two full-time access coordinators scheduled all vascular access procedures and recorded prospectively the procedures and complications in a computerized database (15). This database was queried retrospectively to identify all patients who received a new fistula. During the study period (January 1, 2001, to December 31, 2004), 285 fistulas were placed in hemodialysis patients with the benefit of preoperative vascular mapping. After exclusion of subsequent fistulas in patients who received two or more fistulas (n = 42) and fistulas with indeterminate outcomes (24 deaths, 13 transfers to outside dialysis facilities, and one patient who recovered kidney function), the remaining 205 patients constituted the study population. Approval was obtained from the local institutional review board to review the patients’ records for research purposes. Demographic and clinical parameters were determined for the patients by reviewing their electronic medical records. In addition, the sonographic diameters of the artery and vein used to create each fistula were recorded.
Univariate logistic regression analysis was used to determine the hazard ratios and their associated confidence intervals between patient variables and fistula outcome (mature versus failure to mature). Multiple variable logistic regression analysis was used to model which factors were independently associated with fistula outcome. Finally, the vascular diameters and dynamic measures were compared between patient subsets using t test.
Results
The clinical characteristics of the study population are summarized in Table 1. More than half of the patients were male, and approximately one quarter were ≥65 yr of age. The majority of patients were black, reflecting the demographic composition of our dialysis population. Diabetes, vascular disease, and congestive heart failure were common. A similar proportion of patients had a fistula placed in the upper arm and in the forearm.
Table 1.
Clinical features of the study population
| Clinical Parameter | n (%) |
|---|---|
| N patients | 205 |
| Males | 123 (60) |
| Black race | 176 (86) |
| Age ≥65 yr | 55 (27) |
| Diabetes | 106 (52) |
| Obesity* | 55 (27) |
| Coronary artery disease | 69 (34) |
| Peripheral vascular disease | 30 (15) |
| Congestive heart failure | 47 (23) |
| Fistula location | |
| upper arm | 113 (55) |
| forearm | 92 (45) |
aBody mass index (BMI) ≥30 kg/m2.
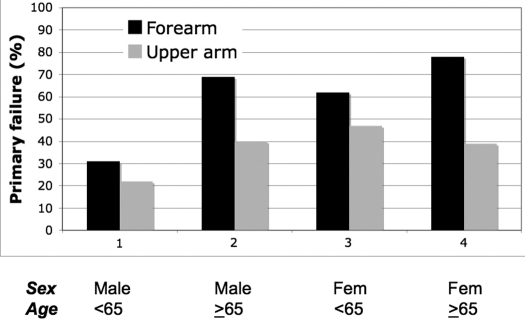
The overall primary failure rate of fistulas was 40% (82 of 205 patients). Univariate logistic regression analysis was performed to determine which factors were associated with primary fistula failure. Older age, female gender, and forearm location each were associated with a significantly higher risk for primary fistula failure (Table 2). In contrast, obesity, diabetes, coronary artery disease, peripheral vascular disease, congestive heart failure, and previous access were not significantly associated with fistula outcome. On multiple variable logistic regression modeling, patient age, gender, and fistula location were together predictive of primary fistula failure (Table 3). The impact of each of these variables on fistula outcome is illustrated in Figure 1. The primary failure rate varied greatly, from a low of 22% in younger men who received an upper arm fistula to a high of 78% in older women who received a forearm fistula.
Table 2.
Univariate analysis of factors associated with fistula failure to mature in patients with preoperative vascular mappinga
| Clinical Factor | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Age (≥65 versus <65 yr) | 1.98 | 1.10 to 3.70 | 0.040 |
| Gender (female versus male) | 2.29 | 1.29 to 4.10 | 0.005 |
| Diabetes | 1.19 | 0.68 to 2.07 | 0.550 |
| Coronary artery disease | 1.10 | 0.61 to 2.00 | 0.750 |
| Peripheral vascular disease | 1.29 | 0.86 to 1.93 | 0.250 |
| Congestive heart failure | 1.43 | 0.72 to 2.80 | 0.310 |
| Obesityb | 1.78 | 0.96 to 3.33 | 0.070 |
| Fistula location (forearm versus upper arm) | 1.74 | 1.00 to 3.06 | 0.050 |
| Previous access | 1.22 | 0.69 to 2.17 | 0.490 |
CI, confidence interval.
BMI ≥30 kg/m2.
Table 3.
Multiple variable logistic regression model of factors associated with fistula failure to mature in patients with preoperative vascular mapping
| Clinical Factor | Hazard Ratio | 95% CI |
|---|---|---|
| Age (≥65 versus <65 yr) | 1.98 | 1.03 to 3.80 |
| Gender (female versus male) | 2.42 | 1.32 to 4.45 |
| Fistula location(forearm versus upper arm) | 2.25 | 1.22 to 4.17 |
Figure 1.
Primary fistula failure, sorted by age, gender, and fistula location. Older age, female gender, and forearm location each were associated with a higher risk for failure to mature.
The preoperative vascular diameters were compared among patient subsets (Table 4). The arterial and venous diameters were similar in older and younger patients, regardless of whether forearm or upper arm fistulas were examined. The arterial diameters were significantly lower in women than in men, both for forearm and upper arm fistulas; however, the arterial diameters were similar in women whose fistulas failed to mature and in those with successful fistulas (2.4 ± 0.4 versus 2.3 ± 0.4 mm [P = 0.55] for forearm fistulas; 5.0 ± 1.0 versus 5.1 ± 1.4 mm [P = 0.86] for upper arm fistulas). The venous diameters did not differ between women and men.
Table 4.
Preoperative arterial and venous diameters by patient subgroup
| Parameter | Age ≥65 Yr | Age <65 Yr | P |
|---|---|---|---|
| Forearm fistula (mm) | |||
| artery diameter | 2.7 ± 0.5 | 2.6 ± 0.5 | 0.770 |
| vein diameter | 3.0 ± 0.5 | 3.0 ± 0.4 | 0.690 |
| Upper arm fistula (mm) | |||
| artery diameter | 4.8 ± 1.1 | 5.2 ± 1.2 | 0.280 |
| vein diameter | 4.3 ± 1.1 | 4.8 ± 1.5 | 0.100 |
| Female | Male | ||
| Forearm fistula (mm) | |||
| artery diameter | 2.4 ± 0.4 | 2.8 ± 0.5 | 0.001 |
| vein diameter | 3.1 ± 0.4 | 3.0 ± 0.4 | 0.240 |
| Upper arm fistula (mm) | |||
| artery diameter | 4.8 ± 1.2 | 5.4 ± 1.0 | 0.008 |
| vein diameter | 4.6 ± 1.6 | 4.6 ± 1.2 | 0.940 |
Dynamic vascular measurements were obtained for 72 patients who received a forearm fistula. This patient group had a mean age of 51 ± 15 yr; 67% were male, 86% were black, 44% had diabetes, 25% had coronary artery disease, 8% had peripheral vascular disease, 22% had congestive heart failure, and 28% were obese. The preoperative dynamic vascular measurements were compared between forearm fistulas that were successfully used for dialysis (mature) and those that failed to mature (Table 5). The absolute values of PSV, EDV, and RI measured during sustained fist clenching or after release of fist clenching did not differ significantly between immature and mature fistulas. Similarly, there was no difference in PSV or RI between successful and unsuccessful fistulas; however, the high variability in measurements among individual patients may have obscured small differences.
Table 5.
Preoperative dynamic measurements by forearm fistula outcome
| Dynamic Parameter | Immature | Mature | P |
|---|---|---|---|
| Pre PSV (Cm/s) | 57 ± 18 | 53 ± 22 | 0.41 |
| Post PSV (cm/s) | 60 ± 22 | 58 ± 19 | 0.70 |
| Pre EDV (cm/s) | 1.6 ± 2.8 | 1.4 ± 2.9 | 0.79 |
| Post EDV (cm/s) | 13 ± 9 | 13 ± 12 | 0.94 |
| ΔPSV (cm/s) | 3 ± 13 | 5 ± 16 | 0.57 |
| Pre RI | 0.96 ± 0.06 | 0.94 ± 0.16 | 0.35 |
| Post RI | 0.78 ± 0.12 | 0.80 ± 0.12 | 0.67 |
| ΔRI | −0.18 ± 0.12 | −0.14 ± 0.20 | 0.31 |
aData are means ± SD. Pre, values measured during sustained fist clenching; Post, values measured after release of fist clenching; PSV, peak systolic velocity; EDV, end-diastolic velocity; ΔPSV, Post PSV − Pre PSV; ΔRI, Post resistive index − Pre resistive index.
Discussion
The overall frequency of primary fistula failure remained high despite routine preoperative vascular mapping and attempts to salvage immature fistulas by radiologic or surgical interventions. There were striking variations in the primary fistula failure rate among patient subgroups: Failure to mature was more common in women than in men, in older than in younger patients, and in forearm than in upper arm fistulas. Moreover, these three clinical variables had a cumulative interaction on fistula outcomes. Thus, the failure to mature rate varied from a low of 22% in younger men who received an upper arm fistula to a high of 78% in older women who received a forearm fistula.
Women had a higher primary fistula failure rate than men and also had significantly smaller preoperative arterial diameters than men. Thus, one could postulate that the lower arterial diameters were responsible for the lower fistula success rate in female patients. This seems unlikely, given that the arterial diameters were not different in women whose fistulas failed to mature and in those with mature fistulas. In fact, among patients <65 yr of age, the primary failure rate was higher in women who received an upper arm fistula than in men who received a forearm fistula (47 versus 31%; Figure 1), even though the former had higher arterial diameters (Table 4). Moreover, the primary failure rate of forearm fistulas in the pediatric population is 33% despite the small vessel size (16).
These disparities suggest that successful fistula maturation is not determined exclusively by the diameters of the artery and vein used to create the fistula. Rather, it is likely that the ability of fistulas to dilate (vascular compliance) varies substantially by patient age and gender. How best to determine vascular compliance preoperatively remains a subject of investigation. Lockhart et al. (12) correlated preoperative changes in PSV after fist clenching with clinical fistula outcomes in 89 patients who received a forearm fistula. Neither the absolute PSV nor its change after fist clenching predicted fistula maturation in the overall group. In a post hoc analysis, a lack of increase in PSV was associated with a lower likelihood of fistula maturation in female but not in male patients. The lack of association between dynamic vascular measurements and clinical fistula outcomes in this study is in agreement with the previous pilot data from our institution (12). Another pilot study used strain-gauge plethysmography to measure venous distensibility in 17 patients who were scheduled for placement of a forearm fistula (17). Patients whose fistula matured had a higher distensibility score than those whose fistula failed to mature. Extensive multicenter studies are needed to assess the predictive value of various dynamic vascular measurements for clinical maturation of both forearm and upper arm fistulas.
This study has a number of limitations. First, the fistula outcomes were determined retrospectively; however, a prospective, computerized vascular access database was used to collect this information, giving a high level of confidence that the data were complete and accurate. Second, these observations were obtained from a single dialysis center, and they may not generalize to some dialysis centers. Third, because our dialysis population has very few white patients, it was not possible to draw meaningful conclusions on differences in fistula maturation between white and black patients; however, a recent multicenter, observational study observed a higher rate of primary fistula failure rate in nonwhite as compared with white patients (4). Fourth, we did not find a significant association between fistula maturation and peripheral vascular disease or coronary artery disease, in contrast to a large, multicenter, observational trial (4). The smaller sample size may have obscured this association in this investigation. Finally, the relatively high primary fistula failure rate at our teaching institution may in part reflect inferior clinical outcomes of fistulas placed by trainees.
Conclusions
Despite routine preoperative vascular mapping, disparities in fistula maturation persist. Specifically, a higher likelihood of primary fistula failure is observed in women, older patients, and forearm fistulas. These discrepancies suggest that functional properties of the vessels may contribute to fistula failure to mature even when the diameters are adequate.
Disclosures
None.
Acknowledgments
This research was supported in part by grant 1 K24 DK59818-01 from the National Institute of Diabetes and Digestive and Kidney Diseases to M.A.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Dixon BS: Why don’t fistulas mature? Kidney Int 70: 1413–1422, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Roy-Chaudhury P, Sukhatme VP, Cheung AK: Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J Am Soc Nephrol 17: 1112–1127, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Lok CE, Allon M, Moist LM, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Miller PE, Tolwani A, Luscy CP, Deierhoi MH, Bailey R, Redden DT, Allon M: Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int 56: 275–280, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Medicare and Medicaid Services: 2004 annual report: End-stage renal disease clinical performance measures project. Am J Kidney Dis 46: 1–100, 2005. 15983951 [Google Scholar]
- 7.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML: Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60: 2013–2020, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Ascher E, Gade P, Hingorani A, Gunduz Y, Fodera M, Yorkovich W: Changes in the practice of angioaccess surgery: Impact of dialysis outcomes quality initiative recommendations. J Vasc Surg 31: 84–92, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Silva MB, Hobson RW, Pappas PJ, Jamil Z, Araki CT, Goldberg MC, Gwertzman G, Padberg FT: A strategy for increasing use of autogenous hemodialysis access procedures: Impact of preoperative noninvasive evaluation. J Vasc Surg 27: 302–308, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Gibson KD, Caps MT, Kohler TR, Hatsukami TS, Gillen DL, Aldassy M, Sherrard DJ, Stehmann-Breen CO: Assessment of a policy to reduce placement of prosthetic hemodialysis access. Kidney Int 59: 2335–2345, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Robbin ML, Gallichio ML, Deierhoi MH, Young CJ, Weber TM, Allon M: US vascular mapping before hemodialysis access placement. Radiology 217: 83–88, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Lockhart ME, Robbin ML, Allon M: Preoperative sonographic radial artery evaluation and correlation with subsequent radiocephalic fistula outcome. J Ultrasound Med 23: 161–168, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Miller CD, Robbin ML, Allon M: Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int 63: 346–352, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Robbin ML, Chamberlain NE, Lockhart ME, Gallichio MH, Young CJ, Deierhoi MH, Allon M: Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 225: 59–64, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Allon M, Bailey R, Ballard R, Deierhoi MH, Hamrick K, Oser R, Rhynes VK, Robbin ML, Saddekni S, Zeigler ST: A multidisciplinary approach to hemodialysis access: Prospective evaluation. Kidney Int 53: 473–479, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Sheth RD, Brandt ML, Brewer ED, Nuchtern JG, Kale AS, Goldstein SL: Permanent hemodialysis vascular access survival in children and adolescents with end-stage renal disease. Kidney Int 62: 1864–1869, 2002 [DOI] [PubMed] [Google Scholar]
- 17.van der Linden J, Lameris TW, van den Meiracker AH, de Smet AA, Blankestijn PJ, van den Dorpel MA: Forearm venous distensibility predicts successful arteriovenous fistula. Am J Kidney Dis 47: 1013–1019, 2006 [DOI] [PubMed] [Google Scholar]