Abstract
Background and objectives: Adherence to therapeutic guidelines for the treatment of hyponatremia becomes difficult when water diuresis emerges during therapy. The objective of this study was to assess the effectiveness and safety of desmopressin acetate as a therapeutic agent to avoid overcorrection of hyponatremia and to lower the plasma sodium concentration again after inadvertent overcorrection.
Design, setting, participants, & measurements: Retrospective chart review was conducted of all patients who were given desmopressin acetate during the treatment of hyponatremia during 6 yr in a 528-bed community teaching hospital.
Results: Six patients (group 1) were given desmopressin acetate after the 24-h limit of 12 mmol/L had already been reached or exceeded; correction was prevented from exceeding the 48-h limit of 18 mmol/L in five of the six. Fourteen patients (group 2) were given desmopressin acetate in anticipation of overcorrection after the plasma sodium concentration had increased by 1 to 12 mmol/L. In all 14 patients who were treated with desmopressin acetate as a preventive measure, correction was prevented from exceeding either the 24- or 48-h limits. After desmopressin acetate was administered, the plasma sodium concentration of 14 of the 20 patients fell by 2 to 9 mmol/L. In all six group 1 patients and in five of the group 2 patients, the plasma sodium concentration was actively lowered again by the concurrent administration of desmopressin acetate and 5% dextrose in water; no serious adverse consequences from this maneuver were observed.
Conclusion: Desmopressin acetate is effective in preventing and reversing inadvertent overcorrection of hyponatremia.
Overcorrection of chronic hyponatremia can cause major neuropathologic sequelae (1–6). For minimization of the risk for iatrogenic brain damage, it is recommended that the serum sodium be increased by no more than 12 mmol/L in 24 h and/or 18 mmol/L in 48 h (3). For avoidance of “overshooting the mark,” a therapeutic “target” of 8 mmol/L/d has been suggested (7), and in patients with liver disease, severe hypokalemia, or malnutrition, who are at high risk for osmotic demyelination, this should be viewed as an upper limit not to be exceeded rather than a therapeutic goal; however, inadvertent excessive correction of hyponatremia can occur if the renal diluting capacity returns during the course of treatment (8). Such a sequence commonly occurs in patients who are hyponatremic because of primary polydipsia (for which the diluting mechanism may be either normal or transiently impaired by nausea), hypovolemia (for which restoration of normovolemia removes the stimulus for antidiuretic hormone), hypopituitarism or adrenal insufficiency (for which cortisol replacement restores diluting ability), or medications (for which discontinuation of thiazide diuretics, selective serotonin reuptake inhibitors, carbamazepine, or desmopressin permits the excretion of maximally dilute urine).
Experimental studies in rats have shown that re-induction of hyponatremia with hypotonic fluids after rapid and excessive correction of hyponatremia reduces the risk for brain injury and death (9,10). Therapeutic re-lowering of the plasma sodium concentration (PNa) after inadvertent overcorrection has been used in isolated case reports using hypotonic fluids and desmopressin acetate (DDAVP), a vasopressin analogue used in the treatment of diabetes insipidus (11–13). On the basis of these findings, DDAVP has been recommended as a therapeutic adjunct in the treatment of hyponatremia (14); however, except for the case reports noted, there are no studies about the clinical efficacy of DDAVP in the management of hyponatremia.
Our study is intended to examine clinical outcomes associated with the use of DDAVP in patients who were treated for hyponatremia in a community teaching hospital. Specifically, we were interested in whether this strategy was successful in avoiding excessive correction and whether therapeutic re-lowering of the PNa had any unintended adverse outcomes.
Concise Methods
The list of all hospitalized patients who received DDAVP from August 2000 to August 2006 was obtained from hospital pharmacy records. Patients who received DDAVP as part of the treatment for hyponatremia were included in the study; patients who were given DDAVP for other reasons were excluded. Clinical chemistry results were retrieved from computerized laboratory archives, which included the date and time that the specimen was collected. Data regarding the time and dosage of DDAVP and the urine output (whenever available) before and after DDAVP administration were extracted from the medical record. Clinical outcomes were assessed by a review of daily progress notes and nursing records. In addition, all but one of the patients reported here were treated by one of the authors.
The patients included in the final analysis were divided into two groups on the basis of the timing of DDAVP administration. Group 1 patients were given DDAVP because the increase in PNa had already reached or exceeded the 24-h limit of 12 mmol/L. Group 2 patients were given DDAVP before the increase in PNa had reached 12 mmol/L. Data are expressed as means ± SD or as ranges.
Results
Eighty-nine patients were identified as having been given DDAVP. Three of the medical records were not available for review. Of the 86 medical records that were reviewed, 65 were excluded because the indication for giving DDAVP was diabetes insipidus or bleeding diatheses. Twenty-one patients who were given DDAVP as part of the treatment for hyponatremia were identified (all of whom had been treated between 2004 and 2006), and 20 of these cases are included in the analysis. One patient with thiazide-induced hyponatremia (PNa 106 mmol/L) was excluded because DDAVP was given late (at 51 h) in response to a single laboratory value (129 mmol/L) that was ultimately thought to be spurious because it was preceded and followed, without plausible explanation, by values that were 7 mmol/L lower; without the spurious result, correction in this patient was 8 mmol/L at 24 h and 16 mmol/L at 48 h, and the clinical course was uneventful.
All but one of the patients were treated by the authors, with attention to maintaining a correction rate <12 mmol/L in 24 h and 18 mmol/L in 48 h. These limits or a limit of 8 mmol/L/d in high-risk patients were often cited in the nephrology consultants’ progress notes. The patients were treated in the course of the nephrologists’ regular clinical practice, without a formal protocol. Patient 5, a 24-yr-old woman who presented with 5 d of intractable vomiting and a PNa of 129 mmol/L after a tonsillectomy, was not treated by any of the authors. The prescribing physician indicated in his progress notes that he intended to re-lower the PNa because of rapid correction.
The cause of hyponatremia was multifactorial in most cases (Table 1) and, with two exceptions, the cause of water retention was eliminated during the course of hospitalization. Thiazide diuretics and selective serotonin reuptake inhibitors, alone or in combination, were causative factors in 11 cases. Patient 8 had inappropriate antidiuretic hormone secretion that was subsequently found to be caused by small cell lung cancer (Table 1); this patient did not have a reversible cause for hyponatremia and DDAVP (which was administered after a brief infusion of hypertonic saline) did not seem to influence the outcome. Patient 20 had acute renal failure, and at the time that DDAVP was given, the serum creatinine was 3.5 mg/dl; because repeated urine osmolalities disclosed fixed isosthenuria and urine output did not change in response to DDAVP, it is doubtful that the drug affected the pace of correction of hyponatremia.
Table 1.
Patients given DDAVP during treatment for hyponatremiaa
| Patient | Age (yr)/Gender | Causative Factors of Hyponatremia | Precorrection PNa (mmol/L) | Rx With 3% NaCl | ΔPNa before DDAVP (mmol/L) | Time of DDAVP from Precorrection PNa (h) | Correction Rate before DDAVP (mmol/L per h) | Urine Output before DDAVP (ml/h) | UOsm before DDAVP (mOsm/kg) | ΔPNa at 24 h (mmol/L) | ΔPNa at 48 h (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | |||||||||||
| 1 | 44/F | PSYCH | 122 | No | 16 | 21 | 2.28 | 353 | 199 | 16 | 17 |
| 2 | 60/F | SSRI, THZ, ALC | 115b | No | 20 | 24 | 1.08 | 425 | 106 | 20 | 20 |
| 3 | 79/F | HYPOVOL | 109 | No | 12 | 16 | 0.78 | – | 63 | 12 | 12 |
| 4 | 68/F | SSRI, THZ | 108 | No | 17 | 17 | 0.83 | – | 191 | 17 | 16 |
| 5 | 24/F | POST-OP, N&V | 129 | No | 13 | 14 | 1.55 | – | – | 13 | 13 |
| 6 | 65/F | ALC | 112b | No | 13 | 23 | 1.56 | – | 254 | 13 | 13 |
| Group 2 | |||||||||||
| 7 | 42/M | SSRI, ALC | 115b | No | 6 | 5 | 1.26 | 302 | 72 | 7 | 7 |
| 8 | 66/F | SCCL | 110 | No | 7 | 5 | 1.53 | 148 | 342 | 9 | 14 |
| 9 | 78/M | SSRI, THZ | 111 | Yes | 1 | 8 | 0.18 | 266 | 284 | 7 | 17 |
| 10 | 34/F | HYPOVOL, ARF | 124 | No | 3 | 4 | 2.61 | 2796 | 340 | 7 | 16 |
| 11 | 59/F | ALC, HYPOVOL | 106 | Yes | 10 | 24 | 0.75 | – | 323 | 10 | 17 |
| 12 | 77/F | COPD, POLYDIP, THZ | 116 | Yes | 9 | 13 | 1.48 | 237 | – | 9 | 9 |
| 13 | 81/F | SSRI, PNEUM | 110 | Yes | 7 | 10 | 2.00 | 300 | 228 | 9 | 16 |
| 14 | 87/F | TRICYC, NSAID | 116 | No | 6 | 11 | 1.00 | 419 | – | 9 | 9 |
| 15 | 48/F | COPD, PNEUM | 110b | Yes | 6 | 18 | 0.57 | 93 | 457 | 8 | 16 |
| 16 | 63/F | SSRI, CARB, PSYCH | 117 | No | 6 | 4 | 1.50 | 270 | 83 | 12 | 15 |
| 17 | 61/F | PSYCH, SSRI | 118 | No | 11 | 23 | 1.00 | 378 | 163 | 11 | 14 |
| 18 | 67/F | SSRI, HYPOVOL | 120 | No | 4 | 8 | 1.00 | 225 | 140 | 8 | 15 |
| 19 | 54/F | COPD, HYPOX | 117 | No | 11 | 12 | 0.96 | – | 397 | 11 | 15 |
| 20 | 84/M | THZ, ARF | 120 | No | 6 | 10 | 1.62 | 380 | 308 | 8 | 8 |
ALC, acute alcoholism and alcohol withdrawal; ARF, acute renal failure; CARB, carbamazepine; COPD, chronic obstructive pulmonary disease exacerbation; DDAVP, desmopressin acetate; HYPOVOL, hypovolemia; HYPOX, hypoxemia; NSAID, nonsteroidal anti-inflammatory drug; N&V, nausea and vomiting; PNa, plasma sodium concentration; PNEUM, pneumonia; POLYDIP, polydipsia; POST-OP, postoperative; PSYCH, psychosis with self-induced water intoxication; SCCL, small cell carcinoma of the lung; SSRI, selective serotonin reuptake inhibitor; THZ, thiazide diuretic; TRICYC, tricyclic antidepressant.
Seizure associated with precorrection PNa.
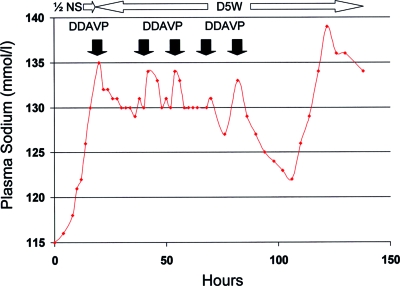
With two exceptions, DDAVP was administered in 1- to 2-μg doses given intravenously or subcutaneously; two patients were given single doses of 4 and 5 μg. Nine patients were given a single dose of DDAVP, eight were given two doses, two received three doses, and one was given five doses (Figure 1).
Figure 1.
Patient 2, who presented with delirium as a result of a plasma sodium concentration (PNa) of 115 mmol/L and alcohol withdrawal, was given multiple doses of desmopressin acetate (DDAVP; filled arrows) in the intensive care unit after a spontaneous water diuresis had increased her PNa by 20 mmol/L over 21 h despite infusion of 0.45% saline (1/2 NS) at 150 ml/h. The final dose of DDAVP was given just before transfer from the intensive care unit, and it was followed by excessive re-lowering of the PNa because of the unintentional continuation of 5% dextrose in water (D5W). After the PNa had fallen from 133 to 122 mmol/L over 24 h (with no worsening of the patient's neurologic condition), a final water diuresis emerged, increasing the PNa to 138 over 17 h despite continued infusion of D5W at 150 ml/h without DDAVP.
In group 1, the lowest precorrection PNa was 115.8 ± 8.2 mmol/L (108 to 129 mmol/L), and the PNa had increased by 15.2 ± 3.1 mmol/L (12 to 20 mmol/L) before DDAVP was prescribed (Table 1). DDAVP was given because the PNa had already reached or exceeded the 24-h therapeutic limit of 12 mmol/L; therefore all but one of the group 1 patients had their PNa corrected by >12 mmol/L in the first 24 h; in patient 3, administration of DDAVP stopped any further correction for the next 24 h, such that the increase in PNa remained at 12 mmol/L and did not exceed the limit (Table 1). Correction at 48 h for group 1 patients was 14.6 ± 3.1 mmol/L (12 to 20 mmol/L), and administration of DDAVP prevented correction from exceeding the 48-h limit of 18 mmol/L in five (83%) of the six group 1 patients; the single exception (patient 2) had already exceeded this limit before DDAVP was given (Figure 1, Table 1).
In most group 1 cases, notes by the prescribing nephrologist indicated that DDAVP was given because of unintended overcorrection of hyponatremia that preceded the nephrology consultation. For example, patient 6, a 65-yr-old woman with liver disease, an alcohol withdrawal seizure, and a PNa of 112 mmol/L, was given DDAVP after treatment with isotonic saline and potassium chloride had increased her PNa by 13 mmol/L in 12 h. The consulting nephrologist stated, “She is at significant risk for osmotic demyelination because of her history of alcoholism. Thus, I would like to stop her increase in plasma sodium, and it may be beneficial to decrease her plasma sodium somewhat in this setting (e.g., to a level of 120 mmol/L or slightly below).”
In group 2, the lowest precorrection PNa was 115 ± 5.0 mmol/L (106 to 124 mmol/L); the increase in PNa before DDAVP was prescribed was 6.6 ± 2.9 mmol/L (1 to 11 mmol/L). These patients were given DDAVP for one or more of the following reasons: (1) The limit of 8 mmol/L had been reached or exceeded (n = 4), (2) the urine output was >250 ml/h (n = 6), or (3) the rate of increase in PNa was >1.5 mmol/L per h (n = 5; Table 1).
In most group 2 patients, notes by the prescribing nephrologist explicitly stated that DDAVP was given to prevent overcorrection of hyponatremia. For example, patient 8, a 66-yr-old woman who had a preexisting seizure disorder and presented with seizures and a PNa of 110 mmol/L, was given DDAVP after administration of 3% saline at 100 ml/h had increased the PNa by 7 mmol/L at the rate of 1.53 mmol/L per h (Table 1). The consulting nephrologist stated, “Our goal for correction of hyponatremia in acutely symptomatic patients has already been met… Our goal at this point is to avoid overcorrection.”
Administration of DDAVP to group 2 patients maintained correction rates below the 24-h limit in 93% and below the 48-h limit in 100% of cases, and in no case did correction exceed either limit; however, in two of the 14 cases, DDAVP was unlikely to have been responsible for this outcome. Correction at 24 h averaged 8.9 ± 1.6 mmol/L (7 to 12 mmol/L). The one patient whose correction reached the limit of 12 mmol/L had been given two doses of DDAVP, 15 h apart. Because the duration of action of DDAVP is usually approximately 12 h (15), this “near miss” was probably caused by a dosing interval that allowed reemergence of water diuresis. Correction at 48 h in group 2 patients averaged 13.4 ± 3.5 mmol/L (7 to 17 mmol/L).
In patients who received hypertonic saline, correction at 24 h ranged from 7 to 10 mmol/L and correction at 48 h ranged from 9 to 17 mmol/L. In patient 9, a man with hyponatremia associated with thiazides, a selective serotonin reuptake inhibitor, and acute urinary retention, hypertonic saline and DDAVP were prescribed simultaneously after correction by only 1 mmol/L (from 111 to 112 mmol/L) after a large urine output was recognized by the nephrologist; this strategy resulted in correction by 7 mmol/L at 24 h and 17 mmol/L at 48 h (Table 1).
A few of the patients had urine osmolalities obtained shortly before the administration of DDAVP (patients 2, 3, 7, 16, 17, and 18; Table 1), and these values documented the emergence of a near maximal water diuresis with osmolalities <150 mOsm/kg. In most cases, the excretion of dilute urine was documented after brief infusions of isotonic saline. Other patients had determinations of urine osmolality early in their course, and these may not reflect the actual urine concentration immediately before DDAVP administration. Urine osmolalities that were obtained after DDAVP were difficult to interpret because of uncertainty regarding the timing of the sample. Urine volumes were seldom charted on an hourly basis, so the effect of DDAVP on urine output was not well documented in the medical record in most cases. In patient 2, who was treated in the intensive care unit, urine volumes fell by a factor of 4 after each dose of DDAVP (Figure 1).
All six of the patients in group 1 were given 5% dextrose in water (D5W) after DDAVP in a deliberate effort to re-lower their PNa (Table 2). This resulted in a decrease in PNa from its peak value before DDAVP to a value that was 4 to 9 mmol/L lower than the peak. The rate of re-lowering of the PNa in group 1 was 0.6 ± 0.2 mmol/L per h (0.30 to 0.89 mmol/L per h). The PNa of patient 2 was re-lowered on five separate occasions (Figure 1); each time, excretion of large volumes of dilute urine rapidly increased the PNa before the administration of DDAVP. Patient 3, a 79-yr-old woman who had been awake and alert on presentation with a PNa of 109 mmol/L and had remained so after initial correction to 121 mmol/L and subsequent re-lowering to 112 mmol/L, was noted to be confused with a PNa of 115 mmol/L, the day after her PNa had reached its re-lowered nadir of 112 mmol/L; her mental status normalized the next day. It is unclear whether this patient's transient confusion was a delayed response to the initial rapid correction of her PNa (12 mmol/L in 6 h) that had occurred 2 d earlier, was caused by the subsequent re-lowering of her PNa, or was an adverse reaction to the promethazine that had been prescribed for nausea. None of the other group 1 patients exhibited any worsening of their neurologic symptoms before, during, or after the re-lowering of their PNa, and all were discharged from the hospital at their baseline level of health.
Table 2.
Patients undergoing re-lowering of the PNaa
| Patient | Precorrection PNa (mmol/L) | Peak PNa before DDAVP (mmol/L) | Lowest PNa after DDAVP (mmol/L) | Amount of Re-lowering (mmol/L) | Time (h) | Rate of Re-lowering (mmol/L per h) | D5W Given after DDAVP |
|---|---|---|---|---|---|---|---|
| Group 1 | |||||||
| 1 | 122 | 138 | 134 | 4 | 4.50 | 0.89 | Yes |
| 2 | 115 | 135 | 129 | 6 | 20.00 | 0.30 | Yes |
| 3 | 109 | 121 | 112 | 9 | 17.50 | 0.51 | Yes |
| 4 | 108 | 125 | 118 | 7 | 8.25 | 0.85 | Yes |
| 5 | 129 | 142 | 134 | 8 | 14.50 | 0.55 | Yes |
| 6 | 112 | 124 | 120 | 4 | 10.00 | 0.40 | Yes |
| Group 2 | |||||||
| 7 | 115 | 122 | 118 | 4 | 17.00 | 0.16 | Yes |
| 8 | 110 | 117 | 114 | 3 | 7.00 | 0.43 | No |
| 10 | 124 | 127 | 124 | 3 | 22.00 | 0.14 | Yes |
| 13 | 110 | 117 | 115 | 2 | 2.00 | 1.00 | Yes |
| 14 | 116 | 125 | 118 | 7 | 18.00 | 0.39 | Yes |
| 17 | 118 | 129 | 127 | 2 | 16.00 | 0.22 | No |
| 19 | 117 | 128 | 125 | 3 | 6.00 | 0.50 | No |
| 20 | 120 | 126 | 122 | 4 | 2.50 | 1.60 | Yes |
D5W, 5% dextrose in water.
Five patients in group 2 were also given D5W after DDAVP in a deliberate effort to re-lower their PNa (Table 2). In these five patients, the PNa decreased from its peak value before DDAVP at a rate of 0.66 ± 0.39 mmol/L to a value that was 2 to 7 mmol/L lower than the peak. An additional three group 2 patients experienced a slight reduction in PNa ranging from 2 to 3 mmol/L after DDAVP. Patient 20, a critically ill 84-yr-old man with severe cardiomyopathy and sepsis, died of shock after a 3-wk hospitalization when his PNa was 135 mmol/L. None of the other group 2 patients exhibited any worsening of their neurologic or physical condition before, during, or after the re-lowering of their PNa, and all were discharged from the hospital at their baseline level of health.
Discussion
These findings show that administration of DDAVP can be a successful strategy to avoid inadvertent overcorrection of hyponatremia and that therapeutic re-lowering of the PNa using DDAVP and hypotonic fluids seems to be well tolerated. DDAVP was administered to 14 of our patients (group 2) as a preventive measure, before excessive correction had occurred. Because the PNa of 12 of these patients was noted to be increasing rapidly because of a large output of dilute urine, it is highly likely that correction would have exceeded 12 mmol/L in 24 h if there had been no intervention. Correction was prevented from exceeding either the 24- or 48-h limits in all these patients.
Six of our patients (group 1) were given DDAVP after the increase in PNa had already exceeded the intended limit. Thus, for most of these patients, correction exceeding 12 mmol/L per d could not be avoided; however, correction was prevented from exceeding 18 mmol/48 h in all but one of these patients. Overall, administration of DDAVP, regardless of timing, was successful in preventing correction that exceeded the 48-h limit in 19 (95%) of 20 patients.
Inadvertent overcorrection of hyponatremia is common. We recently reviewed our experience with the administration of hypertonic saline at our hospital and found that the increase in PNa was considerably more than expected because of unanticipated water diureses that emerged during the course of therapy (8). The results of that study (which ended in 2004) were known to the nephrologists who treated the patients described here, and its findings prompted the use of DDAVP to prevent overcorrection of hyponatremia. In this study, five of the patients were treated with hypertonic saline and DDAVP either sequentially or concurrently, and overcorrection was prevented in all five.
On the basis of these findings, it may be advisable to use DDAVP in a more planned and proactive manner, routinely prescribing the drug when a preset therapeutic limit is reached. Alternatively, one could argue that early and routine administration of DDAVP given in combination with hypertonic saline might result in a more controlled rate of correction of hyponatremia, avoiding the unanticipated emergence of water diuresis in patients at high risk for overcorrection (e.g., patients with inappropriate antidiuretic hormone secretion as a result of antidepressants; hyponatremia caused by hypovolemia, low dietary solute intake, cortisol deficiency, DDAVP, or thiazide diuretics). Because the goal is a sustained, maximal antidiuresis rather than relief of symptoms related to polyuria (15), DDAVP should be readministered at 6- to 8-h intervals (more frequently than is used in the treatment of diabetes insipidus) until the PNa has been gradually corrected to near-normal levels with 3% saline. To do otherwise risks the re-emergence of water diuresis as the effect of DDAVP wears off, leading to inadvertent overcorrection.
Because the use of DDAVP in the management of hyponatremia was new to our hospital, each dose of the drug was typically prescribed in response to changes in PNa (and, in a few cases, urine output) that were measured at frequent intervals. The disadvantages of this approach are well illustrated in Figure 1. In patient 2, D5W was prescribed to re-lower the PNa to a more desirable level; however, the repeated re-emergence of water diureses as the antidiuretic effect of DDAVP abated resulted in episodic rapid increases in the PNa followed by rapid re-lowering of the PNa. Even in an ICU setting, delays in the recognition of changes in urine output or PNa and delays in the administration of DDAVP led to undesirable peaks and valleys of the PNa; therefore, we believe that DDAVP should be given parenterally at 6- to 8-h intervals to ensure greater stability of the PNa (15). Patient 2 also illustrates two other potential pitfalls in the use of DDAVP (Figure 1). First, continued administration of D5W after the administration of DDAVP must be very carefully supervised by experienced clinicians; this may be a significant issue in teaching hospitals, where administration of D5W by trainees can re-lower the PNa by more than intended. Second, it is important to continue DDAVP until the PNa has nearly normalized; to do otherwise allows a large increase in the PNa over a short time.
There are alternatives to giving DDAVP to avoid overcorrection of hyponatremia, but they have limitations. Rapid intravenous administration of electrolyte-free water such as D5W can lead to hyperglycemia (which lowers the plasma sodium by shifting water out of cells) and glycosuria, particularly in patients who are under stress, who use glucose slowly; electrolyte-free water losses from a glucose-induced osmotic diuresis will exacerbate the problem, and they cannot be eliminated with DDAVP. Avoiding overcorrection with oral intake is difficult in patients who are sick or who have altered mental status. Moreover, hypoosmolality suppresses thirst so that patients may reject water that is offered to them. Finally, attempting to match urinary water losses with intravenous or orally administered electrolyte-free water requires intensive monitoring of fluid balance that is often impractical. Some patients in this series were given DDAVP after attempts to match urinary water losses with hypotonic fluid had failed to control the rate of correction of hyponatremia. For these reasons, administration of DDAVP may be a more attractive strategy.
After the administration of DDAVP, 14 of our patients experienced a re-lowering of their PNa. On theoretical grounds, one would expect that a decrease in PNa under these circumstances would not cause cerebral edema. During the adaptation to hyponatremia, organic osmolytes are lost from the brain, and these are recovered very slowly after correction of hyponatremia (16). Thus, the solute-depleted brain can actually become dehydrated when the PNa is raised too rapidly, yet many clinicians are concerned that re-lowering of the PNa might provoke seizures or cause worsening of the patient's neurologic condition. All 14 patients whose PNa was re-lowered tolerated the decrease in PNa without major incident. Because this was a noncontrolled study, we cannot comment on what the outcome would have been had DDAVP not been given or if the PNa had not been re-lowered.
It should be emphasized that DDAVP should be given with extreme caution to patients with psychogenic polydipsia. Once DDAVP is given, the patient has a nonsuppressible antidiuresis and will be unable to excrete ingested water. Life-threatening self-induced water intoxication could potentially result; therefore, administration of DDAVP must be carefully supervised, and total fluid intake must be carefully controlled.
This study greatly expands the published experience with the use of DDAVP to prevent or reverse overcorrection of hyponatremia; however, the study has several limitations. It was a retrospective, descriptive chart review that included a small number of patients. Records of input and output were incomplete or unavailable for many patients, and urine osmolalities, before and after DDAVP administration, were not obtained in most cases. Not all patients were treated in the same manner for their hyponatremia, and a variety of interventions were used to manage overcorrection. A variety of dosages of DDAVP, given by differing routes, were used, and the dosing schedule was not standardized. Although no obvious adverse neurologic outcomes were apparent, we cannot exclude subtle or rare neurologic impairment caused by re-lowering of the PNa. All of these limitations could be addressed in a properly designed prospective study.
Conclusions
Despite the limitations of a small retrospective study, we conclude that DDAVP is an effective and safe adjunct for treating patients who are at risk for excessively rapid correction of hyponatremia. Additional study is needed to define the optimum timing and dosing strategies for this intervention.
Disclosures
None.
Acknowledgments
Preliminary findings of this study were previously reported at the annual meeting of the National Kidney Foundation; Orlando, Florida; April 10–14, 2007.
We thank the Pharmacy and Health Information Management Departments at Rochester General Hospital.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Sterns RH, Riggs JE, Schochet SS Jr: Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med 314: 1535–1542, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Sterns RH: Severe symptomatic hyponatremia: Treatment and outcome. A study of 64 cases. Ann Intern Med 107: 656–664, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Sterns RH, Cappuccio JD, Silver SM, Cohen EP: Neurologic sequelae after treatment of severe hyponatremia: A multicenter perspective. J Am Soc Nephrol 4: 1522–1530, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Karp BI, Laureno R: Pontine and extrapontine myelinolysis: A neurologic disorder following rapid correction of hyponatremia. Medicine (Baltimore) 72: 359–373, 1993 [PubMed] [Google Scholar]
- 5.Ellis SJ: Severe hyponatraemia: Complications and treatment. QJM 88: 905–909, 1995 [PubMed] [Google Scholar]
- 6.Martin RJ: Central pontine and extrapontine myelinolysis: The osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry 75[Suppl 3]: iii22–iii28, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adrogue HJ, Madias NE: Hyponatremia. N Engl J Med 342: 1581–1589, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH: Hypertonic saline for hyponatremia: The risk of inadvertent overcorrection. Clin J Am Soc Nephrol 2: 1110–1117, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Soupart A, Penninckx R, Crenier L, Stenuit A, Perier O, Decaux G: Prevention of brain demyelination in rats after excessive correction of chronic hyponatremia by serum sodium lowering. Kidney Int 45: 193–200, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Soupart A, Penninckx R, Stenuit A, Perier O, Decaux G: Reinduction of hyponatremia improves survival in rats with myelinolysis-related neurologic symptoms. J Neuropathol Exp Neurol 55: 594–601, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Soupart A, Ngassa M, Decaux G: Therapeutic relowering of the serum sodium in a patient after excessive correction of hyponatremia. Clin Nephrol 51: 383–386, 1999 [PubMed] [Google Scholar]
- 12.Oya S, Tsutsumi K, Ueki K, Kirino T: Reinduction of hyponatremia to treat central pontine myelinolysis. Neurology 57: 1931–1932, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Goldszmidt MA, Iliescu EA: DDAVP to prevent rapid correction in hyponatremia. Clin Nephrol 53: 226–229, 2000 [PubMed] [Google Scholar]
- 14.Halperin ML, Kamel KS: A new look at an old problem: Therapy of chronic hyponatremia. Nat Clin Pract Nephrol 3: 2–3, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Rembratt A, Graugaard-Jensen C, Senderovitz T, Norgaard JP, Djurhuus JC: Pharmacokinetics and pharmacodynamics of desmopressin administered orally versus intravenously at daytime versus night-time in healthy men aged 55–70 years. Eur J Clin Pharmacol 60: 397–402, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Sterns RH, Silver SM: Brain volume regulation in response to hypo-osmolality and its correction. Am J Med 119[Suppl 1]: S12–S16, 2006 [DOI] [PubMed] [Google Scholar]