Abstract
Background and objectives: Systemic inflammatory state is a hallmark of peritoneal dialysis (PD) patients, but its etiology remains obscure. Because circulating microbial products are an important cause of systemic immune activation in other conditions such as HIV infection, it was hypothesized that endotoxemia is a cause of systemic inflammatory state and atherosclerosis in PD patients.
Design, setting, participants, & measurements: Plasma lipopolysaccharide (LPS) levels in 30 consecutive new PD patients were measured. The result was compared with serum C-reactive protein (CRP) level, peritoneal transport status, history of pre-existing cardiovascular diseases, and carotid intima media thickness (IMT) by Doppler ultrasound.
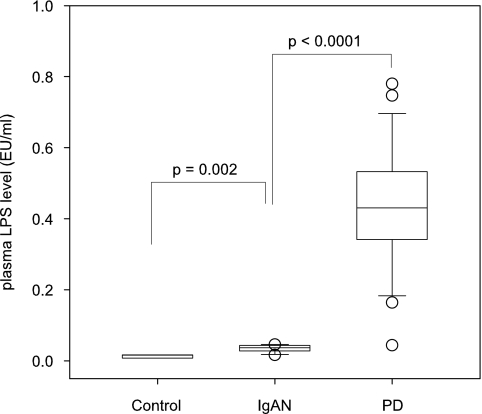
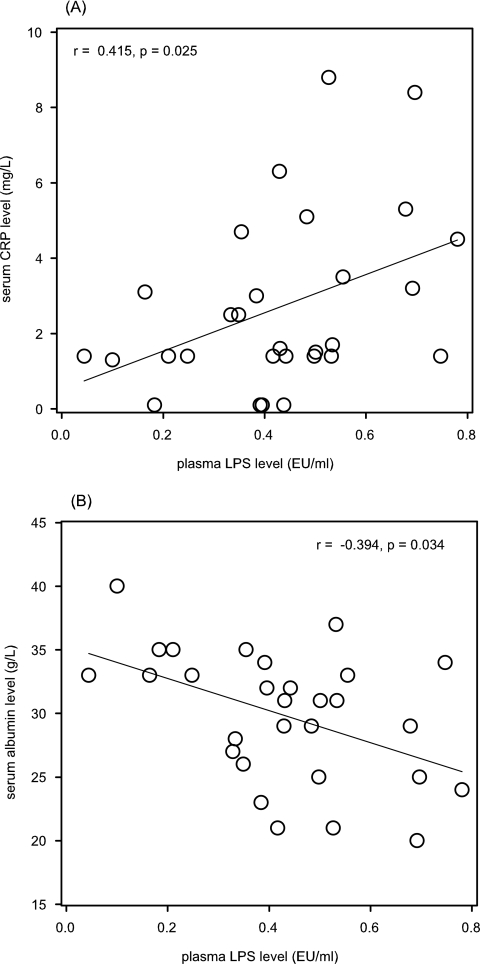
Results: Among the 30 PD patients, there were 17 men. The average age was 53.7 ± 15.1 yr. The average endotoxin concentration of PD patients was 0.44 ± 0.18 EU/ml, which was significantly higher than that of patients with chronic kidney disease secondary to Ig-A nephropathy (IgAN) (0.035 ± 0.009 EU/ml, P < 0.0001) and the controls (0.013 ± 0.007 EU/ml, P < 0.0001). In PD patients, plasma LPS concentration had a significant correlation with serum CRP (r = 0.415, P = 0.025) and serum albumin level (r = −0.394, P = 0.034). In contrast, plasma LPS level did not correlate with Charlson's Comorbidity Index, peritoneal transport characteristics, or nutritional indices. Patients with pre-existing cardiovascular disease (CVD) had higher plasma LPS level than those without CVD (0.53 ± 0.19 versus 0.36 ± 0.16 EU/ml, P = 0.016). Plasma LPS level correlated with carotid IMT (r = 0.438, P = 0.016).
Conclusions: It was found that endotoxemia was probably common in PD patients, and the degree of circulating endotoxemia might be related to the severity of systemic inflammation and features of atherosclerosis. This result suggests that endotoxemia may have a contributory role to the systemic inflammatory state and accelerated atherosclerosis in PD patients.
Patients with chronic kidney disease (CKD) are at high risk of developing CVD (1,2). Longitudinal studies have established that CVD events occur more frequently than renal events in CKD, and CVD mortality rates are in fact higher than the rates of reaching ESRD (3). CVD shares many similar risk factors with CKD, such as diabetes and hypertension (4). However, after accounting for traditional risk factors, CKD remains an independent risk factor for CVD (5). It is now recognized that systemic inflammation plays a key role in atherosclerosis (6) and is an important contributor to CVD morbidity and mortality in CKD patients (7).
Around 30 to 50% of CKD or dialysis patients have serologic evidence of an activated inflammatory response (8–10). The mechanisms of systemic inflammation in CKD is complicated and include decreased renal clearance of pro-inflammatory cytokines, comorbidity (e.g. autoimmune disease), accumulation of advanced glycation end-products, persistent infections, and patient-specific processes such as clotted access grafts (7). However, the principal underlying causes of immune activation in CKD remains elusive. It is long postulated that there exists an infectious risk factor of atherogenesis and CVD, but the nature of such process remains obscure. It is now recognized that endotoxemia constitutes a strong risk factor of early atherogenesis in subjects with chronic or recurrent bacterial infections (11). Epidemiologic studies show that even a low-level endotoxemia constitutes a strong risk factor for the development of atherosclerosis (12). Circulating LPS is bioactive in vivo and correlates with measures of innate and adaptive immune activation (13), and an epidemiologic study suggests that the atherogenic potential of endotoxemia is affected by concomitant immune activation (14). It is recently shown that circulating microbial products, probably derived from the gastrointestinal tract (15), are a cause of HIV-related systemic immune activation. A recent study (16) shows that infusion of LPS led to a significant decrease in peripheral endothelial progenitor cells, which represents a strong predictor of CVD (17).
There is also early evidence that circulating microbial products, probably derived from the gastrointestinal tract, are not uncommon in CKD patients. The intestinal mucosa barrier is impaired and bacterial translocation occurs in experimental uremia (18). Translocation of bowel flora is a cause of gram-negative peritonitis in PD patients (19). We hypothesize that circulating microbial products contribute to the persistent inflammatory state and represents a reversible CVD risk factor in PD patients.
Patients and Methods
Patient selection
The Clinical Research Ethical Committee of the Chinese University of Hong Kong approved this study. We studied 30 new PD patients. These were subjects with CKD who participated in a previous study of our group (20) on the relation between carotid IMT, inflammation, and premature atherosclerosis. They progressed to dialysis-dependent renal failure and were put on PD. A blood test for circulating LPS level and a standard peritoneal permeability test (PET) (21) were performed within 2 mo after the commencement of PD, when the patient was in a euvolemic state. On the day before PET, 24-h urine and dialysate collection was performed for assessment of nutritional status. The presence of diabetes and a history of CVD at initiation of dialysis were recorded. Pre-existing CVD was defined as angina, class III to IV congestive heart failure, a history of previous myocardial infarction, cerebrovascular accident, or amputation for vascular disease. The modified Charlson's Comorbidity Index was used to calculate a comorbidity score (22). We further studied the circulating LPS level of ten patients with mild-to-moderate CKD secondary to IgAN, with average serum creatinine levels of 1.71 ± 1.32 mg/dl (151.3 ± 116.3 μmol/L), and six healthy subjects as control.
Circulating LPS level
The method of plasma LPS quantification has been described previously (15). Briefly, plasma samples were diluted to 20% with endotoxin-free water and then heated to 70°C for 10 min to inactivate plasma proteins. We then quantified plasma LPS with a commercially available Limulus Amebocyte assay (Cambrex, Verviers, Belgium) according to the manufacturer's protocol. The detection limit of this assay was 0.01 EU/ml. Samples with LPS level below the detection limit were taken as 0 EU/ml. All samples were run in duplicate and background subtracted.
Study of Peritoneal Transport
PET was performed by the method of Twardowski (21). Briefly, a 4-h dwell study was carried out with 2 L of dextrose 2.5% dialysis fluid (Dianeal, Baxter-Travenol, Deerfield, IL). Dialysate creatinine and glucose levels at 0, 2, and 4 h, and plasma creatinine and glucose levels at 2 h were measured. Drainage and ultrafiltration volumes at 4 h were documented. Dialysate-to-plasma ratios of creatinine (D/P) at 0, 2, and 4 h were calculated after correction of glucose interference. Mass transfer area coefficients of creatinine normalized for body surface area were calculated by the formula described by Krediet (23). Body surface area was determined from body weight and height by nomogram (24).
Peritoneal Protein and Albumin Excretion Rate
During the PET, dialysate albumin and total protein concentrations at 4 h were determined by a fully automated analyzer (Konelab 60, Thermo Clinical Labsystems) as described previously (25), and the concentrations were adjusted for the dialysate creatinine concentration. In all measurements, creatinine concentration in dialysate was corrected for glucose interference according to a formula provided by our laboratory (26).
Dialysis Adequacy, Nutrition, and Inflammation Markers
On the day before PET, 24 h-urine and dialysate collection was performed to calculate total Kt/V. Normalized protein nitrogen appearance was calculated by the modified Bergstrom's formula (27). Serum CRP was measured by the Tina-quant CRP (Latex) ultrasensitive assay (Roche Diagnostics GmbH, Mannheim, Germany).
B-mode Carotid Doppler Examination
As described in our previous study (20,25), Doppler ultrasonographic examinations were performed using an ATL HDI 5000 ultrasound scanner (Bothell, WA). A trained sonographer scanned the right and left common carotid arteries, the carotid bulbs, and the first 2 cm of the internal and external carotid arteries. For each location, the sonographer visualized the vessel in multiple planes and then focused on the interfaces required to measure IMT as well as any areas of calcified or ulcerated plaque. Carotid plaques were defined as echogenic structures showing protrusion into the lumen with focal widening that was 50% greater than the IMT of adjacent sites. All measurements were performed by technicians who were blinded to the clinical details and laboratory results of the patients.
Statistical Analyses
Statistical analyses was performed by SPSS for Windows software version 11.0 (SPSS Inc., Chicago, IL). Results were expressed as mean ± SD unless otherwise specified. Comparisons between groups were performed by χ2 test or Mann-Whitney U test as appropriate. Correlation between continuous variables was examined by Spearman's rank correlation coefficient. Factors independently associated with plasma LPS level were further explored by a multiple linear regression model with backwards-stepwise analysis. A P value of less than 0.05 was considered significant. All probabilities were two-tailed.
Results
We studied a total of 30 patients. The demographic and baseline clinical data are summarized in Table 1. The average endotoxin concentration of PD patients was 0.44 ± 0.18 EU/ml, which was significantly higher than that of patients with CKD secondary to IgAN (0.035 ± 0.009 EU/ml, P < 0.0001) and the controls (0.013 ± 0.007 EU/ml, P < 0.0001) (Figure 1).
Table 1.
Demographics and clinical information
| Number of Patients | 30 |
| Sex (men:women) | 17:13 |
| Age (yr) | 53.7 ± 15.1 |
| Body height (cm) | 161.4 ± 10.2 |
| Body weight (kg) | 60.2 ± 11.2 |
| Renal diagnosis, number of cases (%) | |
| GN | 16 (53.3%) |
| diabetic nephropathy | 9 (30.0%) |
| polycystic kidney | 1 (3.3%) |
| obstructive uropathy | 1 (3.3%) |
| others/unknown | 3 (10%) |
| Major comorbidity, number of cases (%) | |
| diabetes | 11 (36.7%) |
| cardiovascular disease | 12 (40.0%) |
| Charlson's comorbidity index | 5.0 ± 2.6 |
| Fasting lipid profile (mmol/l) | |
| total cholesterol | 5.04 ± 0.93 |
| LDL cholesterol | 2.87 ± 0.99 |
| total triglyceride | 1.66 ± 0.96 |
| Serum C-reactive protein (mg/dl) | 2.71 ± 2.31 |
Figure 1.
Comparison of plasma lipopolysaccharide (LPS) level between patients on peritoneal dialysis (PD), chronic kidney disease secondary to Ig-A nephropathy (IgAN) and controls. The boxes indicate median, 25th, and 75th percentile; whisker caps indicate 5th and 95th percentile; open circles indicate outliers. (Overall Kruskal-Wallis test, P < 0.0001.)
Plasma LPS and Inflammation
Plasma LPS level was not affected by diabetic status, average blood pressure, fasting total cholesterol, LDL cholesterol, or total triglyceride level (details not shown). There was, however, a significant correlation between plasma LPS level and serum CRP (r = 0.415, P = 0.025) and serum albumin level (r = −0.394, P = 0.034) (Figure 2). In contrast, plasma LPS level did not correlate with peritoneal transport characteristics, as represented by mass transfer area coefficients creatinine (r = 0.194, P = 0.3), or peritoneal permeability to albumin (r = 0.058, P = 0.8). Plasma LPS level did not correlate with total Kt/V (r = 0.133, P = 0.5), residual GFR (r = 0.001, P = 1.0), or normalized protein nitrogen appearance (r = 0.219, P = 0.25).
Figure 2.
Relation between plasma LPS level and (A) serum C-reactive protein (CRP), and (B) serum albumin level.
Plasma LPS and Atherosclerosis
Twelve patients had pre-existing CVD at the initiation of dialysis. They had higher plasma LPS levels than those without CVD (0.53 ± 0.19 versus 0.36 ± 0.16 EU/ml, P = 0.016). There was a significant correlation between plasma LPS level and carotid IMT (r = 0.438, P = 0.016) (Figure 3). By carotid ultrasound, 11 patients had significant carotid plaques. These patients also had higher plasma LPS levels than those without carotid plaque (0.55 ± 0.16 versus 0.36 ± 0.16 EU/ml, P = 0.005).
Figure 3.
Relation between plasma LPS level and intima-media thickness (IMT) of carotid artery as determined by Doppler ultrasound.
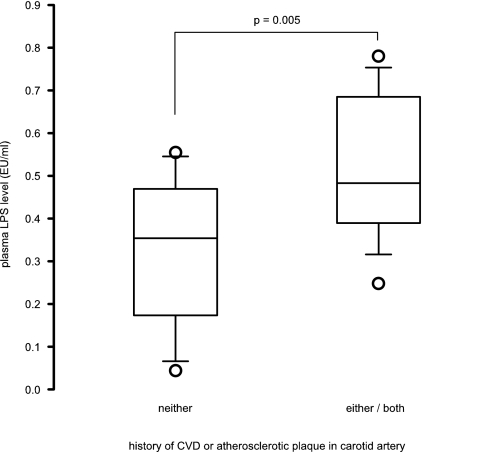
In total, 13 patients had neither pre-existing CVD nor carotid plaque from Doppler study. These patients had significantly lower plasma LPS levels than those with either (0.32 ± 0.17 versus 0.51 ± 0.16 EU/ml, P = 0.005) (Figure 4). Multiple linear regression analysis showed that carotid IMT and serum albumin level were the only independent factors associated with plasma LPS level (Table 2).
Figure 4.
Comparison of plasma LPS level between patients with neither a history of cardiovascular disease (CVD) nor atherosclerotic plaque identified by carotid Doppler ultrasound and patients with either or both problems. The boxes indicate median, 25th, and 75th percentile; whisker caps indicate 5th and 95th percentile; open circles indicate outliers.
Table 2.
Summary of multiple linear regression model on factors associated with plasma lipopolysaccharide level
| Variable | B coefficient | 95% Confidence Interval | P value |
|---|---|---|---|
| Carotid IMT | 0.534 | 0.060 to 1.026 | 0.029 |
| Serum albumin | −0.016 | −0.004 to −0.029 | 0.01 |
| Model constant | 0.500 | −0.050 to 1.051 | 0.073 |
Discussion
In this study we found that endotoxemia was common in PD patients, and the degree of circulating endotoxemia was related to the severity of systemic inflammation and features of atherosclerosis. The correlation between endotoxemia and atherosclerosis was notably unrelated to other traditional cardiovascular risk factors.
The phenomenon of circulating endotoxemia in renal failure subjects has not been systemically studied previously. Our observation agrees with the previous study of Kiechl et al. (11) in subjects with recurrent infection and early atherosclerosis, as well as the study of Wiedermann et al. (12) in a general population with atherosclerosis. This latter study showed that subjects with circulating LPS levels beyond 50 pg/ml (90th percentile of normal population) faced a threefold risk of incident atherosclerosis. In the study presented here, 24 of the 30 PD patients (80%) had such a high circulating LPS level—an observation in line with the high prevalence of atherosclerosis in renal failure patients. In our study, the plasma LPS level was 0.44 ± 0.18 EU/ml, or 80 ± 36 pg/ml, which is higher than the median value of 14.3 pg/ml, as reported in patients with recurrent bacterial infection (12), but similar to the level observed in renal failure subjects undergoing hemodialysis (28) as well as patients with AIDS (15).
Our study supports the hypothesis that endotoxemia is related to accelerated atherosclerosis. Although there is a wealth of literature on this area, most of the published studies used endothelial or vascular dysfunction as a surrogate marker of atherosclerosis (13,29,30), whereas our study used carotid IMT—a more robust tool for quantifying atherosclerosis. In addition to an inverse correlation with serum albumin level (which is arguably a marker of inflammation rather than malnutrition), we did not find any correlation between circulating LPS levels and nutritional indices—another important component of the malnutrition-inflammation-atherosclerosis syndrome (31)—but our sample size was small and a clinically relevant correlation could have been missed. It remains possible that endotoxemia is part and parcel of the malnutrition-inflammation-atherosclerosis syndrome.
In our previous study (20), we showed that carotid IMT correlated with patient age, serum LDL level, Charlson's comorbid score, and serum CRP. Carotid IMT was also significantly higher in diabetic than nondiabetic subjects. Of note, although carotid IMT significantly correlated with both serum LPS (r = 0.415) in the study presented here and CRP (r = 0.279) in the previous study (20), the correlation coefficient was substantially higher in the former, suggesting that carotid IMT is more affected by LPS than by CRP. Because the sample size was small in this study, we did not perform multivariate analysis to determine the independent predictors of carotid IMT. On the basis of a multiple linear regression analysis of the data from the previous study (20), age, serum LDL level, serum CRP, and diabetic status were independently associated with carotid IMT (unpublished analysis from our previous study (20)).
Because of the limitation in the original study design, we cannot confirm the cause of endotoxemia in our patients. Because endotoxemia has been reported in hemodialysis patients (28,32), it seems probable that uremia per se is the cause. As to the source of endotoxin, occult infection is often implicated. However, none of the patients in this study had an indwelling vascular catheter at the time of the blood test or a history of peritonitis (as they were newly started on PD). Recently, Brenchley et al. (15) showed that microbial translocation from the gastrointestinal tract is the cause of endotoxemia and systemic immune activation in AIDS patients. Further study is needed to determine whether the same pathologic process exists in renal failure patients.
There are several other important inadequacies in our study and our result can only be considered preliminary. The sample size was small and the study was purely cross-sectional. Although we demonstrated a correlation between circulating LPS level and carotid IMT, which has been found to be an important predictor of CVD in dialysis (33–35) as well as predialysis renal failure patients (20), further prospective study is needed to determine whether a high circulating LPS level could predict CVD, and, if that is the case, whether the effect is mediated via arterial wall thickening as revealed in carotid IMT.
Another aspect that is worth further investigation is the potential benefit of treatment. Recent reports suggest that endotoxin-related inflammation and vascular abnormality is reversible. Statin has been found to inhibit endotoxin-induced vascular inflammation, which may contribute to the therapeutic role in atherosclerotic diseases (29). Selective intestinal decontamination partially reverses the hyperdynamic circulatory state in cirrhotic patients (36). Before embarking on therapeutic trials, however, it seems logical to clarify the origin of endotoxemia in renal failure patients so that targeted therapy can be designed.
In summary, we found that endotoxemia was common in PD patients, and the degree of circulating endotoxemia was related to the severity of systemic inflammation and features of atherosclerosis. Our result suggests that endotoxemia may contribute to the systemic inflammatory state and accelerated atherosclerosis in PD patients.
Disclosures
None.
Acknowledgments
This study was supported in part by the CUHK Research Committee Funding (Direct Grant), Project ID 2041276 and CUHK research account 6901031. The authors declare no conflict of interest.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Weiner DE, Tabatabai S, Tighiouart H, Elsayed E, Bansal N, Griffith J, Salem DN, Levey AS, Sarnak MJ: Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis 48: 392–401, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Levin A: Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. Semin Dial 16: 101–105, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Parikh NI, Hwang SJ, Larson MG, Meigs JB, Levy D, Fox CS: Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch Intern Med 166: 1884–1891, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Ross R: Atherosclerosis: An inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Stenvinkel P: Inflammation in end-stage renal failure: Could it be treated? Nephrol Dial Transplant 17[Suppl 8]: S33–S38, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Stenvinkel P, Heimburger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Owen WF, Lowrie EG: C-reactive protein as an outcome predictor for maintenance hemodialysis patients. Kidney Int 54: 627–636, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Yeun JY, Kaysen GA: Acute phase proteins and peritoneal dialysate albumin loss are the main determinants of serum albumin in peritoneal dialysis patients. Am J Kidney Dis 30: 923–927, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Kiechl S, Egger G, Mayr M, Wiedermann CJ, Bonora E, Oberhollenzer F, Muggeo M, Xu Q, Wick G, Poewe W, Willeit J: Chronic infections and the risk of carotid atherosclerosis: Prospective results from a large population study. Circulation 103: 1064–1070, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J: Association of endotoxaemia with carotid atherosclerosis and cardiovascular disease: Prospective results from the Bruneck Study. J Am Coll Cardiol 34: 1975–1981, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Stoll LL, Denning GM, Weintraub NL: Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol 24: 2227–2236, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Wiedermann CJ, Kiechl S, Schratzberger P, Dunzendorfer S, Weiss G, Willeit J: The role of immune activation in endotoxin-induced atherogenesis. J Endotoxin Res 7: 322–326, 2001 [PubMed] [Google Scholar]
- 15.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC: Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12: 1365–1371, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Mayr FB, Spiel AO, Leitner JM, Firbas C, Sieghart W, Jilma B: Effects of low dose endotoxaemia on endothelial progenitor cells in humans. Atherosclerosis 191: e202–e206, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G: Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353: 999–1007, 2005 [DOI] [PubMed] [Google Scholar]
- 18.de Almeida Duarte JB, de Aguilar-Nascimento JE, Nascimento M, Nochi RJ Jr: Bacterial translocation in experimental uremia. Urol Res 32: 266–270, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Selgas R, Bajo MA, Jimenez C, Sanchez C, Del Peso G, Cacho G, Diaz C, Fernandez-Reyes MJ, De Alvaro F. Peritoneal dialysis in liver disorders. Perit Dial Int 16[Suppl 1]: S215–S219, 1996 [PubMed] [Google Scholar]
- 20.Szeto CC, Chow KM, Woo KS, Chook P, Kwan BC, Leung CB, Li PK: Carotid intima media thickness predicts cardiovascular diseases in Chinese pre-dialysis chronic kidney diseases patients. J Am Soc Nephrol 18: 1966–1972, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Twardowski ZJ, Nolph KD, Prowant B, Ryan L, Moore H, Nielsen MP: Peritoneal equilibration test. Perit Dial Bull 7: 138–147, 1987 [Google Scholar]
- 22.Beddhu S, Zeidel ML, Saul M, Seddon P, Samore MH, Stoddard GJ, Bruns FJ: The effects of comorbid conditions on the outcomes of patients undergoing peritoneal dialysis. Am J Med 112: 696–701, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Krediet RT, Boeschoten EW, Zuyderhoudt FMJ, Strackee J, Arisz L: Simple assessment of the efficacy of peritoneal transport in continuous ambulatory peritoneal dialysis patients. Blood Purif. 4: 194–203, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Noe DA: A body surface area nomogram based on the formula of Gehan and George. J Pharm Sci 80: 501–502, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Szeto CC, Chow KM, Lam CW, Cheung R, Kwan BC, Chung KY, Leung CB, Li PK: Peritoneal albumin excretion is a strong predictor of cardiovascular events in peritoneal dialysis patients: A prospective cohort study. Perit Dial Int 25: 445–452, 2005 [PubMed] [Google Scholar]
- 26.Mak TW, Cheung CK, Cheung CM, Leung CB, Lam CW, Lai KN: Interference of creatinine measurement in CAPD fluid was dependent on glucose and creatinine concentrations. Nephrol Dial Transplant 12: 184–186, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Bergstrom J, Heimburger O, Lindholm B: Calculation of the protein equivalent of total nitrogen appearance from urea appearance. Which formulas should be used? Perit Dial Int 18: 467–473, 1998 [PubMed] [Google Scholar]
- 28.Nisbeth U, Hallgren R, Eriksson O, Danielson BG: Endotoxemia in chronic renal failure. Nephron 45: 93–97, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Rice JB, Stoll LL, Li WG, Denning GM, Weydert J, Charipar E, Richenbacher WE, Miller FJ Jr, Weintraub NL: Low-level endotoxin induces potent inflammatory activation of human blood vessels: Inhibition by statins. Arterioscler Thromb Vasc Biol 23: 1576–1582, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Bannerman DD, Goldblum SE: Direct effects of endotoxin on the endothelium: barrier function and injury. Lab Invest 79: 1181–1199, 1999 [PubMed] [Google Scholar]
- 31.Pecoits-Filho R, Lindholm B, Stenvinkel P: The malnutrition, inflammation, and atherosclerosis (MIA) syndrome—The heart of the matter. Nephrol Dial Transplant 17[Suppl 11]: 28–31, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Markum HM, Suhardjono, Pohan HT, Suhendro, Lydia A, Inada K: Endotoxin in patients with terminal renal failure undergoing dialysis with re-processing dialyser. Acta Med Indones 36: 93–96, 2004 [PubMed] [Google Scholar]
- 33.Nishizawa Y, Shoji T, Maekawa K, Nagasue K, Okuno S, Kim M, Emoto M, Ishimura E, Nakatani T, Miki T, Inaba M: Intima-media thickness of carotid artery predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 41[Suppl 1]: S76–S79, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Kato A, Takita T, Maruyama Y, Kumagai H, Hishida A: Impact of carotid atherosclerosis on long-term mortality in chronic hemodialysis patients. Kidney Int 64: 1472–1479, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Ekart R, Hojs R, Hojs-Fabjan T, Balon BP: Predictive value of carotid intima media thickness in hemodialysis patients. Artif Organs 29: 615–619, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Rasaratnam B, Kaye D, Jennings G, Dudley F, Chin-Dusting J: The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis. A randomized trial. Ann Intern Med 139: 186–193, 2003 [DOI] [PubMed] [Google Scholar]