Abstract
Podocyte adhesion to the glomerular basement membrane is required for proper function of the glomerular filtration barrier. However, the mechanism whereby podocytes adhere to collagen IV networks, a major component of the glomerular basement membrane, is poorly understood. The predominant collagen IV network is composed of triple helical protomers containing the α3α4α5 chains. The protomers connect via the trimeric noncollagenous (NC1) domains to form hexamers at the interface. Because the NC1 domains of this network can potentially support integrin-dependent cell adhesion, it was determined whether individual NC1 monomers or α3α4α5 hexamers support podocyte adhesion. It was found that, although human podocytes did not adhere to NC1 domains proper, they did adhere via integrin αvβ3 to a KRGDS motif located adjacent to α3NC1 domains. Because the KRGDS motif is a site of phosphorylation, its interactions with integrin αvβ3 may play a critical role in cell signaling in physiologic and pathologic states.
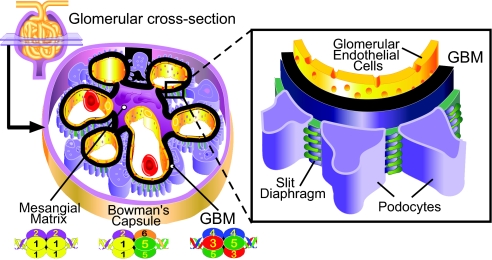
Glomerular development and function are dependent on cell matrix interactions that are mediated by binding of integrin receptors to extracellular matrix (ECM) proteins. The most abundant ECM proteins in the glomerulus are collagen IV networks. There are two networks in the glomerular basement membrane (GBM): the α1α1α2(IV) network and the major α3α4α5(IV) network (Figure 1). The networks are assembled from triple helical protomers characterized by three functional domains: a 7S domain at the N terminus, a long triple-helical collagenous domain in the middle of the molecule, and a trimeric noncollagenous (NC1) domain at the C terminus. Protomers self-assemble into networks by end-to-end associations that connect four 7S domains at one end and connect two NC1 trim-eric domains at the other end, forming an NC1 hexamer at the interface.1 These networks are essential for tissue development and function as they provide mechanical stability, a scaffold for assembly of other macromolecular components, and are ligands for integrins, receptors that mediate cell adhesion, migration, growth, and differentiation.
Figure 1.
Distribution of collagen IV networks in the glomerulus. Schematic diagram illustrating location of the collagen IV networks in the glomerulus. The figure was modified from reference (37).
Integrin binding sites have only been delineated for the α1α1α2(IV) networks.2 The principal receptors for these networks are integrins α1β1 and α10β1; however, integrin α10β1 expression is spatially and temporally restricted to chondrocytes and fetal muscle cells.3 The major collagen I binding receptor, integrin α2β1, also binds to the α1α1α2(IV) network, however, at much lower affinity than integrin α1β1.4 Finally, the laminin receptor integrin α3β1 has been reported to bind to the α1α1α2(IV) networks in certain cell types.5,6
The sites for integrin α1β1 and α2β1 binding to the α1α1α2 collagen IV network resides within the triple-helical domain,7–9 although integrin α1β1 also interacts with recombinant α1(IV) and α2(IV) NC1 domains.10,11 Integrins αvβ3 and αvβ5 have been shown to bind the α2(IV) NC1 domain.12,13 Although the integrin binding sites to the α1α1α2(IV) network have been extensively studied, it is not known which integrins bind to the triple-helical domain of the α3α4α5 collagen IV network. There is evidence that αvβ3 and α3β1 integrins bind monomeric α3 NC1 domain,13–16 but no integrin binding has thus far been shown for the α4NC1 and α5NC1 domains.13 Integrin-α3 NC1 domain interactions are the most studied because of the potential role of the α3NC1 domain as an antiangiogenic agent. Its binding to integrin αvβ3 is highly dependent on the RGD site located in the amino-terminus of the collagenous domain, whereas the interaction with integrin α3β1 is RGD-independent.15,16
The integrity of the glomerular filtration barrier requires normal interactions between the podocytes and the GBM (Figure 1). The predominant integrin expressed by podocytes is α3β1,17 and in vivo deletion of the α3 subunit, specifically in podocytes, results in a marked glomerular phenotype.18 Although integrins α1β1 and α2β1 are also expressed by podocytes, their role in normal glomerular development is likely to be less significant, as mice null for the α119 or α2 subunit (R. Zent and A. Pozzi, unpublished) only have a minor glomerular phenotype. Although αv integrins are expressed on podocytes,20,21 their role in glomerular development is unknown.
The mechanisms whereby podocytes interact with collagen IV networks in the GBM are unidentified. The only data available for the α1α1α2(IV) network are that rat podocytes adhere to it in an integrin α3β1- and α1β1-dependent manner,22 whereas nothing is known about the α3α4α5(IV) network. Whereas current technology is unavailable to purify the full α3α4α5(IV) network, recombinant α3(IV), α4(IV), α5(IV) NC1 monomers and native α3α4α5(IV) hexamers are available to determine integrin binding sites. Based on our data demonstrating that various cell types bind and adhere to collagen IV NC1 domains,15,16 we investigated how conditionally immortalized human podocytes interact with the NC1 domains of the α3α4α5(IV) network in either their monomeric or hexameric states. We demonstrate that podocytes do not adhere to NC1 domains per se; however, they do adhere to the KRGDS site of the collagenous domain located adjacent to the α3(IV) NC1 domain via integrin αvβ3. This integrin–RGD interaction, which occurs only in humans and nonhuman primates, may be important in mediating specific functions in the glomeruli of these species.
Results
Podocyte Adhesion to Recombinant NC1 Monomers of Collagen IV
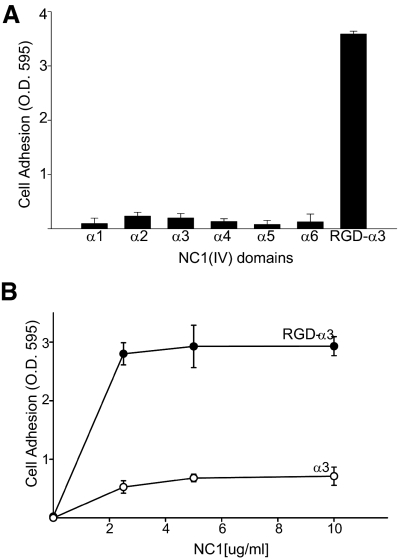
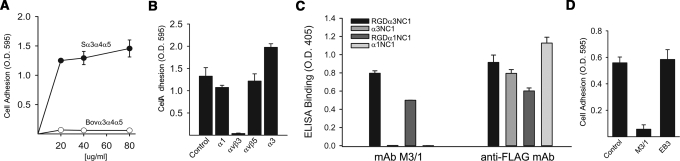
A conditionally immortalized human podocyte cell line23 was used to determine whether podocytes can adhere to recombinant human α1-α 6 NC1 monomers. Among these, podocytes only adhered to α3 NC1 that contained a KRGDS motif within the collagenous domain adjacent to the NC1 domain of the α3 chain of collagen IV (RGD-α3) (Figure 2A). As shown in Figure 2B, podocyte adhesion is significantly increased by the addition of the collagenous domain containing the KRGDS motif. Thus, this motif, which is only found in primates,16 was critical for podocyte adhesion.
Figure 2.
Podocytes adhere to recombinant human α3 NC1 domains of collagen IV containing a KRGDS site in the noncollagenous domain. (A) Adhesion assays of conditionally immortalized human podocytes were performed as described in “Concise Methods” on recombinant NC1 domains of collagen IV (20 μg/ml). (B) Adhesion assays were performed on different concentration of α3 and RGD-α3 NC1 domains. Values are the mean ± SD of triplicate wells. Three independent experiments were performed.
Figure 3.
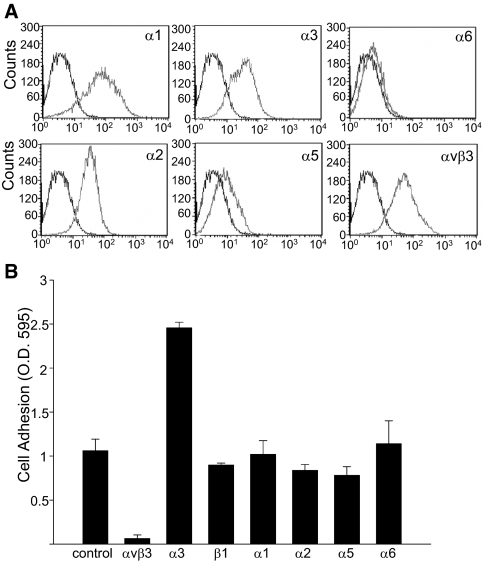
Podocytes adhere to recombinant RGD-α 3NC1 via integrin αvβ3. (A) Flow cytometry histograms of conditionally immortalized human podocytes showing integrin expression. (B) Podocytes adhesion to RGD-α 3 NC1 domain in the presence or absence of the indicated integrin-neutralizing antibodies at 10 μg/ml. Values are the mean ± SD of triplicate wells. Three independent experiments were performed.
To determine which integrins mediate podocyte adhesion to RGD-α3NC1, we analyzed integrin expression of conditionally immortalized human podocytes. We found integrins α1, α2, α3, and αvβ3 to be highly expressed, along with lower levels of α5 and α6 (Figure 3A). Using integrin-neutralizing antibodies, we determined that podocyte adhesion to RGD-α3NC1 was significantly inhibited by antibodies to integrin αvβ3 (Figure 3B). In addition, a blocking antibody to integrin α3β1 significantly increased adhesion to RGD-α3NC1, suggesting that integrin α3β1 transdominantly inhibits integrin αvβ3 activation, as previously shown.15
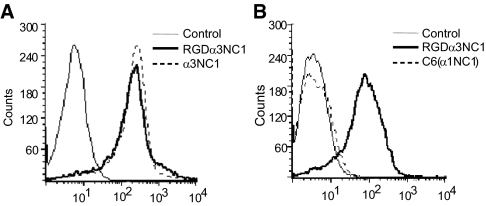
We next determined the binding of soluble α3NC1 domain and RGD-α3NC1 to podocytes, using flow cytometry methods previously described.15 Similar to our previous findings with endothelial cells, podocytes bound α3NC1 domains both containing and not containing the KRGDS motif equally (Figure 3A), indicating that the KRGDS site is not required for this binding. Binding specificity was confirmed by the absence of podocyte binding to C6, a control protein consisting of an α1NC1 scaffold with only a short fragment of α3NC1 (Figure 4B).24 The α3 NC1 sequence of C6 encodes the epitope for mAb EB3, which is used for detecting the NC1 fragment in the flow cytometry assays.15 Thus, similar to other cell types, both α3NC1 and RGD-α3NC1 domains bind to podocytes in solution. However, podocytes only adhere to immobilized α3NC1 domains that contain the KRGDS motif in an integrin αvβ3-dependent manner.
Figure 4.
Soluble α3 NC1 binding to human podocytes. (A) Flow cytometry histograms of podocytes incubated with 20 μg/ml of RGD-α 3 NC1 or α3 NC1, followed by PE-conjugated secondary antibody. Cells incubated with Mab EB3 are indicated as control. (B) Flow cytometry histograms of podocytes incubated with 10 μg/ml of α3 NC1 or C6 chimera (α1 NC1) followed by PE-conjugated secondary antibody. Three independent experiments were performed with similar results.
Characterization of Simian α3α 4α5 NC1 Hexamers of Collagen IV Networks
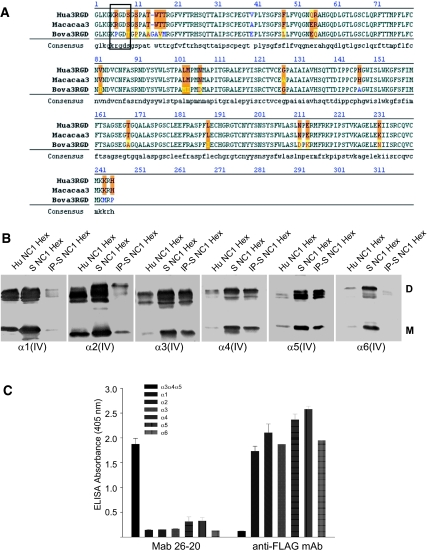
Although the above data demonstrated that human podocytes bind to monomeric RGD-α3NC1 domains, it was critical to determine the relevance of this binding to the native form of α3 collagen IV found in tissues. Within the collagen IV network, the RGDα3 NC1 monomer interacts with the α4(IV) and α5(IV) NC1 monomers to form α3α4α5(IV) NC1 trimers that interface with each other to form an α3α4α5(IV) NC1 hexamer.25 Because α3α 4α5(IV) NC1 hexamers represent only 15% of the total NC1 hexamers from human kidney cortex25,26 and human tissues are available in limited quantities, we purified NC1 hexamers of collagen IV from the kidney cortex of the nonhuman primate rhesus macaque monkey (Macaca mulatta). Simian NC1 domains are highly homologous to the human ones; in particular, the collagenous domain adjacent to the simian α3NC1 contains the KRGDS motif found in humans but is absent in other species, such as bovine (Figure 5A). The NC1 hexamers isolated from simian kidney cortex were found to contain α1-α6 NC1 domains (Figure 5B). Compared with human kidney cortex, simian cortex contained a relatively larger proportion of α3(IV), α4(IV), and α5(IV) NC1 domains, which were shown to be associated with each other by immunoprecipitation with mAb 26-20, which binds specifically to human and mouse α3α 4α5(IV) collagen;27 its lack of reactivity for NC1 monomers (Figure 5C) indicates that this antibody recognizes epitopes within the quaternary structure of α3α 4α5 NC1 hexamers. Therefore, the results indicate that simian kidney cortex contains α3α 4α5(IV) NC1 hexamers with similar composition and organization as NC1 hexamers from human kidneys.28
Figure 5.
Analysis of Simian type IV collagen NC1 hexamers. (A) Alignment of the human collagen IV α3 NC1domain with simian and bovine sequences. Orange shading denotes differences in amino acids between species. Conserved residues are indicated in black, homologous residues are indicated in yellow, and nonhomologous residues are indicated in either blue or red. The box around amino acids 5 to 9 shows the KRGDS site in simian and human sequences. (B) NC1 hexamers isolated from renal basement membranes by collagenase digestion were separated by SDS-PAGE on a 4% to 20% gradient gel under nonreducing conditions and blotted with monoclonal antibodies to α1 to α6 NC1 domains directly (lane 2) or after immunoprecipitation with Mab 26 to 20 (lane 3). Human NC1 hexamers are indicated as controls (lane 1). D indicates NC1 dimers (molecular mass, 44 to 50 kDa); M, NC1 monomers (molecular mass, 24 to 28 kDa). (C) ELISA indicating that Mab 26 to 20 specifically reacts with α3α 4α 5 NC1 hexamers but not with NC1 monomers.
Podocyte Adhesion to α3α 4α5(IV) NC1 Hexamers
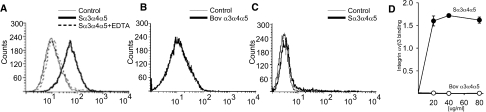
We determined whether simian α3α 4α5(IV) NC1 hexamers bind in solution to human podocytes, uszing flow cytometric analysis. As shown in Figure 6A, these hexamers bound significantly to podocytes, and this binding was blocked by EDTA, suggesting it was integrin-dependent. In contrast, hexamers of bovine origin, which lack the KRGDS site, did not bind to podocytes (Figure 6B), indicating that the KRGDS site is required for the interaction. To determine whether integrin αvβ3 was required for the binding of simian α3α 4α5(IV) NC1, we used mouse CT26 cells, which lack integrin αvβ3. Indeed, there was no significant binding of the simian α3α 4α5 NC1 hexamers to CT26 cells (Figure 6C). To further confirm that integrin αvβ3 binds simian, but not bovine, α3α 4α5(IV) NC1 hexamers, solid phase assays using purified integrins were performed. As shown in Figure 6D, purified integrin αvβ3 was able to bind simian, but not bovine, α3α 4α5(IV) NC1 hexamers.
Figure 6.
Simian but not bovine α3α4α5 NC1 hexamers bind to podocytes. (A) Flow cytometry histograms of podocytes incubated with simian type IV collagen NC1 hexamers (100 μg/ml) in the presence or absence of 10 mM EDTA. (B) Flow cytometry histogram of podocytes incubated with bovine type IV collagen NC1 hexamers (100 μg/ml). (C) Flow cytometry histograms of mouse carcinoma CT-26 cells (αvβ3-negative) incubated with simian α3α4α5 NC1 hexamer. Cells incubated with Mab 26 to 20 alone were used as control. (D) Simian (S) and bovine (Bov) α3α4α5 NC1 hexamers binding to purified integrin αvβ3. Immobilized integrin αvβ3 (1 μg/ml) was incubated with NC1 hexamers at increasing concentrations, and bound proteins were detected with Mab 26 to 20. Specific binding was calculated as the difference between the absorbance of samples with and without integrin. The data represent the mean and SD of 3 wells. The experiment was repeated twice with similar results.
To determine whether podocytes adhered specifically to immobilized α3α 4α5(IV) NC1 hexamers, mAb 26-20 was used to capture simian and bovine α3α 4α5 NC1 hexamers onto an ELISA plate, thus avoiding potentially confounding influences from the more abundant α1α 1α 2(IV) NC1 hexamers. Similar to the binding in solution, podocytes adhered to the immobilized simian, but not bovine, α3α 4α5(IV) hexamers, suggesting that the KRGDS site is required for adhesion (Figure 7A). Moreover, inhibition of podocyte adhesion to α3α 4α5(IV) NC1 hexamers by specific integrin blocking antibodies demonstrated that this interaction was mediated by integrin αvβ3 (Figure 7B). Furthermore, it verifies that a blocking antibody to integrin α3β1 transactivates integrin αvβ3 as described previously15 and seen in Figure 3B.
Figure 7.
Podocyte adhesion to simian α3α4α5 NC1 hexamer is integrin αvβ3-dependent. (A) Podocyte adhesion to α3α4α5 NC1 simian and bovine hexamers immobilized on 96-well plates coated with Mab 26 to 20 (5 μg/ml). (B) Podocyte adhesion to simian NC1 hexamers (80 μg/ml) in the presence of neutralizing antibodies to integrins (20 μg/ml). (C) ELISA assay indicating the specificity of M3/1. The ELISA plates were coated with the following recombinant proteins: RGD-α3 NC1, α3 NC1, which lacks the N-terminal 12 amino acids including RGD site, α1 NC1; and RGD-α1 NC1, which contains 22 N-terminal amino acids derived from collagenous and α3 NC1 domain. The proteins were detected with Mabs M3/1 or M2 against the FLAG epitope carried by all of the recombinant proteins. (D) Podocyte adhesion to simian NC1 hexamer (80 μg/ml) is blocked by mAb M3/1, specific for the RGD region of α3 NC1 domain, but not by mAb EB3, which binds to α3NC1 residues 127 to 141. Cell adhesion was measured as described in “Concise Methods.” The data represent the mean absorbance and SD of triplicate wells. The experiment was repeated 3 times with similar results.
The role of the KRGDS site was then investigated by using mAb M3/1, a monoclonal antibody raised to a peptide containing the KRGDS region of the α3 NC1 domain. By ELISA, mAb M3/1 bound to either RGD-α3NC1 or RGD-α1NC1 domains, but not to the same NC1 domains lacking the KRGDS site (Figure 7C), indicating the specificity of the Mab M3/1 antibody for the KRGDS epitope. This mAb completely blocked podocyte adhesion to simian α3α 4α5(IV) NC1 hexamers, indicating that the KRGDS motif is the binding site for integrin αvβ3 (Figure 7D).
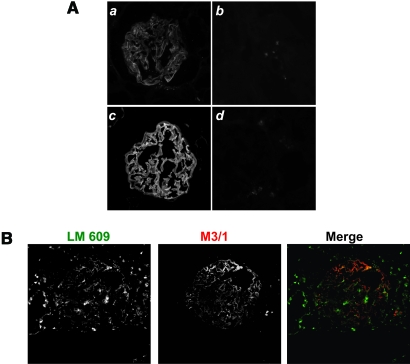
Typically, RGD sites within the triple helical collagenous domains of collagens are not accessible for integrin binding. To ascertain whether the KRGDS site of α3NC1 is accessible in the native collagen IV network of the GBM, frozen sections of human kidneys were stained with monoclonal antibody M3/1 under native conditions. By indirect immunofluorescence staining, mAb M3/1 bound in a linear fashion along the capillary loops (Figure 8A). This pattern was similar to that observed for mAb 8D1, a monoclonal antibody specific for α3NC1, which binds to accessible epitopes in the GBM. The binding of mAb M3/1 to human GBM was completely blocked by preincubation of the antibody with KRGDS-α1NC1, which contains the KRGDS motif, thus verifying that M3/1 binds specifically to KRGDS sites in the GBM. These results indicate that the KRGDS site is at least partially exposed in the native GBM and thus available for interaction with integrin αvβ3. To verify that integrin αvβ3 co-localizes with the KRGDS site on α3α 4α5(IV) network, immunofluorescent studies were performed on human kidneys using M3/1 and LM 609 (specific for integrin αvβ3) antibodies. As seen in Figure 8B, there is significant co localization of these antibodies suggesting that podocytes might be interacting with the KRGDS site in vivo.
Figure 8.
The KRGDS motif of α3NC1 domain is exposed in human GBM. (A) Human kidney sections stained with M3/1 (a), M3/1 preincubated with RGD-α1NC1 (b), 8D1 (c), and mouse isotype control (d). (B) Human kidneys co-immunostained with LM609 and M3/1 antibodies.
Discussion
The ability of podocytes to adhere to the NC1 domains of the α1α1α2 and α3α4α5 collagen IV networks was evaluated. Podocytes adhered only to immobilized α3(IV) NCI monomers or α3α4α5(IV) hexamers that contain a KRGDS site adjacent to the α3(IV) NC1 domain. The adhesion is mediated by integrin αvβ3, as shown in other cell types.15,16 Thus, podocytes do not adhere to the GBM by interactions with the NC1 domain proper of the α3α4α5(IV) network.
Although podocytes do not adhere to α3(IV) NC1 monomers, they do bind this domain in solution. This binding is dependent on a site found within the NC1domain proper, which becomes unavailable for binding in the α3α4α5(IV) NC1 hexamers. The binding to this site is mediated, at least in part, by integrin α3β1.15 Furthermore, as seen in other cells, antibody binding to integrin α3β1 transactivated integrin αvβ315,16 and increased podocyte adhesion to the KRGDS site. It is therefore possible that soluble monomeric α3(IV) NC1 domain binding to integrin α3β115 modulates podocyte adhesion to the native human GBM by increasing integrin αvβ3 avidity.
The observation that NC1 domains per se do not play a role in podocyte adhesion suggests that the binding sites reside within the triple-helical domain of the α3α4α5 networks. We identified one such site: the KRGDS site within the collagenous domain of the α3 chain located adjacent to the NC1 domain. RGD sites within a collagen triple helix are typically buried, and integrins can usually only bind to these RGD motifs after exposure by denaturation.29 However, in this case, the RGD motif is exposed in the native GBM. This RGD is located in a highly divergent hydrophilic triple helix-NC1 junction30 and embedded in a five-residue motif KRGDS that undergoes phosphorylation in vivo.21 Therefore, it is possible that, when this primate-exclusive motif in the native GBM networks undergoes phosphorylation, it becomes accessible to integrin αvβ3.21 It has been shown that phosphorylation of extracellular proteins promotes RGD-mediated cell attachment whereas dephosphorylation inhibits it.31 The RGD/phosphorylation motif is targeted by GPBP, a Ser/Thr kinase, which is expressed more abundantly in the human glomerulus than in other mammals that do not contain the RGD/phosphorylation motif.32
Interestingly, the KRGDS motif might play a role in certain human diseases. For example, increased GPBP expression has been associated with Goodpasture autoimmune pathogenesis.32 It has been speculated that increased phosphorylation of this motif could induce disturbance of quaternary structure, allowing exposure of cryptic epitopes located nearby in the primary structure. Furthermore, we recently demonstrated that modification of the critical arginine residue within this KRGDS motif by reactive carbonyl compounds inhibited αvβ3 integrin-mediated adhesion of podocytes.23 Possibly, increased levels of carbonyl compounds, known as a “carbonyl stress,” may contribute to the perturbation of podocyte–GBM interactions that contribute to diabetic nephropathy. The exposed KRGDS motif in native GBM supports the notion that this mechanism may be operable under diabetic conditions in vivo.
In conclusion, we demonstrate that integrin αvβ3, which is expressed at low levels in podocytes under physiologic conditions, supports podocyte adhesion to the KRGDS site at the collagenous–NC1 domain junction of the α3NC1 domain, whereas the highly expressed integrin α3β1 does not. The αvβ3 interaction is not critical for normal renal development as deletion of the principal αv containing integrin, αvβ3, does not induce renal abnormalities. This contrasts with the severe renal phenotype observed in mice lacking integin α3. However, increased expression of integrin αvβ3 has been associated with diabetic nephropathy,5,33 and the KRGDS site is susceptible to “carbonyl stress.” Thus, it is possible that integrin αvβ3 interactions with this KRGDS/phosphorylation site might play a critical role in podocyte signaling in physiologic and pathologic states.
Concise Methods
Materials
The properties of antibodies used in this study are indicated in Table 1. Antibodies H11, H22, H31, H52, and H63 were a gift from Dr. Y Sado.34 M2 antibody (mAb) to FLAG peptide was purchased from Sigma (St. Louis, MO). mAb M3/1,29 EB325 26 to 2027 and 8D135 were purified from hybridoma supernatants. Antihuman integrin mAbs FB12, BHA2.1, P1B5, P1D6, LM609, or Alexa 488-LM609 and P1F6 were purchased from Chemicon (Temecula, CA). GoH3 was purchased from BD-Pharmingen (San Diego, CA) and AIIB2 from Developmental Studies Hybridoma Bank (Iowa City, IA). R-Phycoerythrin (PE) or fluorescein isothiocyanate (FITC) conjugated anti-mouse or rat IgG were from BD-Pharmingen. Purified integrin αvβ3 was purchased from Chemicon.
Table 1.
Properties of collagen IV and integrin mAbs used in this study
| mAb | Isotype | Specificity | Reference |
|---|---|---|---|
| H11 | rat IgG2a | α1NC1 collagen IV | Sado34 |
| H22 | rat IgG2a | α2NC1 collagen IV | Sado34 |
| H31 | rat IgG2a | α3NC1 collagen IV | Sado34 |
| EB3 | mouse IgG1 | α3NC1 collagen IV | Borza25 |
| 8D1 | mouse IgG1 | α3NC1 collagen IV; α3α4α5 NC1 hexamers | Zent35 |
| M3/1 | mouse IgG1 | RGDα3 NC1 collagen IV; RGDα3α4α5 NC1 hexamers | Revert29 |
| RH42 | rat IgG2a | α4 NC1 collagen IV; α3α4α5 NC1 hexamers | Heidet27 |
| H52 | rat IgG2b | α5NC1 collagen IV | Sado34 |
| H63 | rat IgG2a | α6NC1 collagen IV | Sado34 |
| 26–20 | mouse IgG1 | α3α4α5 NC1 collagen IV hexamers | Heidet27 |
| AIIB2 | rat IgG1 | β1integrin | Hybridoma Bank, Iowa |
| FB12 | mouse IgG1 | α1 integrin | Chemicon |
| BHA2.1 | mouse IgG1 | α2 integrin | Chemicon |
| P1B5 | mouse IgG1 | α3 integrin | Chemicon |
| P1D6 | mouse Ig1 | α5 integrin | Chemicon |
| GoH3 | rat IgG2a | α6 integrin | Pharmingen |
| LM609 | mouse IgG1 | αvβ3 integrin | Chemicon |
| P1F6 | mouse IgG1 | αvβ5 Integrin | Chemicon |
Cell Culture
Human conditionally immortalized podocytes were maintained as described previously23 and incubated at 37°C for 2 wk to induce differentiation.
Proteins
Recombinant type IV collagen NC1 domains were expressed and purified as described previously.13 NC1 hexamers were purified from simian or bovine kidney cortex as described.25
Cell Adhesion Assays
Cell adhesion assays were performed as described previously.36
Flow Cytometric Analysis
Flow cytometric assays were performed as described previously.15
Solid Phase Ligand Binding Assays
Solid phase ligand binding assays were performed as described previously.16
Enzyme-Linked Immunosorbent Assays
Enzyme-linked immunosorbent assay was as described previously.15
Immunoprecipitation and Western Blotting
mAb 26 to 20 (∼10 μg) was incubated with simian NC1 hexamers (5 to 10 μg) overnight. The antibody–antigen complexes were precipitated with protein G-agarose beads, solubilized in sample buffer, and resolved into component NC1 monomers and dimers by SDS-PAGE under nonreducing conditions. After transfer to Immobilon P and blocking with casein, α1-α6 NC1 domains were identified by immunoblotting with specific mAbs, as described previously.25
Indirect Immunofluorescence Staining
Frozen human kidney sections were fixed in acetone, blocked with 3% goat serum, and incubated with primary antibodies overnight, followed by a FITC-conjugated secondary antibody. To verify the specificity of binding to tissues, M3/1 antibody was preincubated with RGD-α1 NC1 then added to kidney sections. For co-localization studies of the integrin αv subunit and its binding site on the α3(IV) chain, acetone fixed human kidney sections were incubated sequentially with M3/1 antibody, rhodamine-conjugated anti-mouse antibody, and finally Alexa 488-conjugated LM609 antibody. The slides were mounted and examined under a Nikon Eclipse E800 fluorescence microscope. Photomicrographs were recorded with a charge-coupled device digital camera, using the same exposure settings for each primary antibody.
Disclosures
There are no competing financial interests with companies and any of the authors.
Acknowledgments
This work was supported by P01 DK65123, (D.R.A., B.G.H., R.Z., A.P., D.-B.B.), 4R37 DK18381 (B.G.H.), a National Research Service award 5F32 DK065375 (C.M.B.), R01-DK074359 (A.P.); R01-CA94849(AP); RO1-DK 69921 (R.Z.), a Merit award from the Department of Veterans Affairs (R.Z.) and SAF2006-N12520-C02–01 del Plan Nacional I+D (Spain) (J.S.). The Human Tissue Acquisition and Pathology Shared Resource is supported by the National Cancer Institute grant P30 CA68485.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Integrins and Matrix in the Glomerulus: Old Mysteries and New Insights,” on pages 650–651.
References
- 1.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Paulsson M: Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol 27: 93–127, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Gullberg DE, Lundgren-Akerlund E: Collagen-binding I domain integrins: what do they do? Prog Histochem Cytochem 37: 3–54, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Tulla M, Pentikainen OT, Viitasalo T, Kapyla J, Impola U, Nykvist P, Nissinen L, Johnson MS, Heino J: Selective binding of collagen subtypes by integrin alpha 1I, alpha 2I, and alpha 10I domains. J Biol Chem 276: 48206–48212, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Jin DK, Fish AJ, Wayner EA, Mauer M, Setty S, Tsilibary E, Kim Y: Distribution of integrin subunits in human diabetic kidneys. J Am Soc Nephrol 7: 2636–2645, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Krishnamurti U, Chen Y, Michael A, Kim Y, Fan WW, Wieslander J, Brunmark C, Rondeau E, Sraer JD, Delarue F, Tsilibary EC: Integrin-mediated interactions between primary/T-sv40 immortalized human glomerular epithelial cells and type IV collagen. Lab Invest 74: 650–657, 1996 [PubMed] [Google Scholar]
- 7.Kern A, Eble J, Golbik R, Kuhn K: Interaction of type IV collagen with the isolated integrins alpha 1 beta 1 and alpha 2 beta 1. Eur J Biochem 215: 151–159, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Eble JA, Golbik R, Mann K, Kuhn K: The alpha 1 beta 1 integrin recognition site of the basement membrane collagen molecule [alpha 1(IV)]2 alpha 2(IV). EMBO J 12: 4795–4802, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight CG, Morton LF, Peachey AR, Tuckwell DS, Farndale RW, Barnes MJ: The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J Biol Chem 275: 35–40, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Roth JM, Akalu A, Zelmanovich A, Policarpio D, Ng B, MacDonald S, Formenti S, Liebes L, Brooks PC: Recombinant alpha2(IV)NC1 domain inhibits tumor cell-extracellular matrix interactions, induces cellular senescence, and inhibits tumor growth in vivo. Am J Pathol 166: 901–911, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, Dhanabal M, Simons M, Post M, Kufe DW, Weichselbaum RR, Sukhatme VP, Kalluri R: Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res 60: 2520–2526, 2000 [PubMed] [Google Scholar]
- 12.Magnon C, Galaup A, Mullan B, Rouffiac V, Bouquet C, Bidart JM, Griscelli F, Opolon P, Perricaudet M: Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with alphavbeta3 and alphavbeta5 integrins. Cancer Res 65: 4353–4361, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP Jr, Hudson BG, Brooks PC: New functions for non-collagenous domains of human collagen type IV: novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J Biol Chem 275: 8051–8061, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R: Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J Biol Chem 275: 21340–21348, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Borza CM, Pozzi A, Borza DB, Pedchenko V, Hellmark T, Hudson BG, Zent R: Integrin alpha3beta1, a novel receptor for alpha3(IV) noncollagenous domain and a trans-dominant Inhibitor for integrin alphavbeta3. J Biol Chem 281: 20932–20939, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Pedchenko V, Zent R, Hudson BG: av{beta}3 and av{beta}5 integrins bind both the proximal RGD site and non-RGD motifs withinnoncollagenous (NC1) domain of the a3 chain of type IV collagen: Implication for themechanism of endothelial cell adhesion. J Biol Chem 279: 2772–2780, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Sterk LM, de Melker AA, Kramer D, Kuikman I, Chand A, Claessen N, Weening JJ, Sonnenberg A: Glomerular extracellular matrix components and integrins. Cell Adhes Commun 5: 177–192, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A: Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol 175: 33–39, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Moeckel G, Morrow JD, Cosgrove D, Harris RC, Fogo AB, Zent R, Pozzi A: Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury. Am J Pathol 165: 617–630, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patey N, Halbwachs-Mecarelli L, Droz D, Lesavre P, Noel LH: Distribution of integrin subunits in normal human kidney. Cell Adhes Commun 2: 159–167, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Wada J, Kumar A, Liu Z, Ruoslahti E, Reichardt L, Marvaldi J, Kanwar YS: Cloning of mouse integrin alphaV cDNA and role of the alphaV-related matrix receptors in metanephric development. J Cell Biol 132: 1161–1176, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cybulsky AV, Carbonetto S, Huang Q, McTavish AJ, Cyr MD: Adhesion of rat glomerular epithelial cells to extracellular matrices: role of beta 1 integrins. Kidney Int 42: 1099–1106, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Pedchenko VK, Chetyrkin SV, Chuang P, Ham AJ, Saleem MA, Mathieson PW, Hudson BG, Voziyan PA: Mechanism of perturbation of integrin-mediated cell-matrix interactions by reactive carbonyl compounds and its implication for pathogenesis of diabetic nephropathy. Diabetes 54: 2952–2960, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Netzer KO, Leinonen A, Boutaud A, Borza DB, Todd P, Gunwar S, Langeveld JP, Hudson BG: The goodpasture autoantigen: mapping the major conformational epitope(s) of alpha3(IV) collagen to residues 17–31 and 127–141 of the NC1 domain. J Biol Chem 274: 11267–11274, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Borza DB, Bondar O, Todd P, Sundaramoorthy M, Sado Y, Ninomiya Y, Hudson BG: Quaternary organization of the goodpasture autoantigen, the alpha 3(IV) collagen chain: sequestration of two cryptic autoepitopes by intrapromoter interactions with the alpha4 and alpha5 NC1 domains. J Biol Chem 277: 40075–40083, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Borza DB, Bondar O, Colon S, Todd P, Sado Y, Neilson EG, Hudson BG: Goodpasture autoantibodies unmask cryptic epitopes by selectively dissociating autoantigen complexes lacking structural reinforcement: novel mechanisms for immune privilege and autoimmune pathogenesis. J Biol Chem 280: 27147–27154, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Heidet L, Borza DB, Jouin M, Sich M, Mattei MG, Sado Y, Hudson BG, Hastie N, Antignac C, Gubler MC: A human-mouse chimera of the alpha3alpha4alpha5(IV) collagen protomer rescues the renal phenotype in Col4a3-/- Alport mice. Am J Pathol 163: 1633–1644, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boutaud A, Borza DB, Bondar O, Gunwar S, Netzer KO, Singh N, Ninomiya Y, Sado Y, Noelken ME, Hudson BG: Type IV collagen of the glomerular basement membrane: evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J Biol Chem 275: 30716–30724, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Revert F, Penades JR, Plana M, Bernal D, Johansson C, Itarte E, Cervera J, Wieslander J, Quinones S, Saus J: Phosphorylation of the Goodpasture antigen by type A protein kinases. J Biol Chem 270: 13254–13261, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Quinones S, Bernal D, Garcia-Sogo M, Elena SF, Saus J: Exon/intron structure of the human alpha 3(IV) gene encompassing the Goodpasture antigen (alpha 3(IV)NC1): identification of a potentially antigenic region at the triple helix/NC1 domain junction. J Biol Chem 267: 19780–19784, 1992 [PubMed] [Google Scholar]
- 31.Ek-Rylander B, Flores M, Wendel M, Heinegard D, Andersson G: Dephosphorylation of osteopontin and bone sialoprotein by osteoclastic tartrate-resistant acid phosphatase: modulation of osteoclast adhesion in vitro. J Biol Chem 269: 14853–14856, 1994 [PubMed] [Google Scholar]
- 32.Raya A, Revert-Ros F, Martinez-Martinez P, Navarro S, Rosello E, Vieites B, Granero F, Forteza J, Saus J: Goodpasture antigen-binding protein, the kinase that phosphorylates the goodpasture antigen, is an alternatively spliced variant implicated in autoimmune pathogenesis. J Biol Chem 275: 40392–40399, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Boettiger D, Enomoto-Iwamoto M, Yoon HY, Hofer U, Menko AS, Chiquet-Ehrismann R: Regulation of integrin α5β1 affinity during myogenic differentiation. Dev Biol 169: 261–272, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Sado Y, Kagawa M, Kishiro Y, Naito I, Joh K, Ninomiya Y: Purification and characterization of human nephritogenic antigen that induces anti-GBM nephritis in rats. J Pathol 182: 225–232, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Zent R, Yan X, Su Y, Hudson BG, Borza DB, Moeckel GW, Qi Z, Sado Y, Breyer MD, Voziyan P, Pozzi A: Glomerular injury is exacerbated in diabetic integrin alpha1-null mice. Kidney Int 70: 460–470, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Chen D, Roberts R, Pohl M, Nigam S, Kreidberg J, Wang Z, Heino J, Ivaska J, Coffa S, Harris RC, Pozzi A, Zent R: Differential expression of collagen- and laminin-binding integrins mediates ureteric bud and inner medullary collecting duct cell tubulogenesis. Am J Physiol Renal Physiol 287: F602–F611, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Borza DB, Neilson EG, Hudson BG: Pathogenesis of Goodpasture syndrome. Semin Nephrol 23: 522–531, 2003 [DOI] [PubMed] [Google Scholar]