Abstract
Carotid intima media thickness (IMT) is a strong, independent predictor of cardiovascular events in both the general population and among those with end-stage renal disease (ESRD), but it is unknown whether changes in IMT or other ultrasound-measured indicators of atherosclerosis over time provide additional prognostic information. The progression of atherosclerosis with carotid ultrasound was followed in a cohort of 135 ESRD patients, 103 of whom had a repeat ultrasound after 15 mo of follow-up. The number of plaques and the proportion of patients with severe atherosclerosis increased substantially during the follow-up period, but IMT, common carotid artery diameter, common carotid artery wall-to-lumen ratio, and cross-sectional area, did not change. The rate of formation of new plaques was a strong, independent predictor of incident cardiovascular events, even after adjusting for baseline plaque burden and other potential confounders. New plaque formation over time was independently predicted by background plaque burden and serum C-reactive protein (P = 0.004 and P = 0.02, respectively). Changes in IMT and the other ultrasound-measured indicators of atherosclerosis progression did not predict cardiovascular outcomes. Therefore, monitoring IMT over time is unlikely to provide additional prognostic information compared with a single measurement, but longitudinal ultrasound monitoring of plaque formation may be useful for cardiovascular risk stratification in the ESRD population.
Carotid ultrasonography (US) is one of the most used imaging technique for the assessment of atherosclerosis, and it is increasingly applied in patients with end-stage renal disease (ESRD) and in chronic kidney disease (CKD) in general. Carotid intima media thickness (IMT), the number of plaques and indicators of arterial remodeling, such as the internal diameter of the common carotid artery (CCAD), the wall/lumen ratio (CCAwlr), and cross-sectional area (CSA), are regarded as major US indicators for the staging of the atherosclerosis process.1 Increased carotid IMT, in particular, is considered to be not only a critical lesion predisposing to plaque formation but also a valid marker of the severity of atherosclerosis in critical vascular beds, such as the coronary artery territory.2 However, the strength of the association between carotid IMT and coronary plaques is quite variable in studies performed so far,3 and some experts emphasize that it remains uncertain whether an increase in IMT is a prerequisite for development of more advanced, event-driving lesions (i.e., atherosclerotic plaques). IMT emerged as a strong and independent predictor of cardiovascular events in variety of studies in the general population4 and in various disease states,3 including ESRD,5,6 but it is unknown whether changes over time in IMT or other US indicators of atherosclerosis bear prognostic value for cardiovascular complications. Although IMT was associated with the presence of plaques in the carotid arteries in cross-sectional studies in patients with CKD7 or ESRD,8 the interpretation of this association with regard to the evolution of the atherosclerosis process in these patients remains unclear because the issue has never been examined in longitudinal studies. Cross-sectional studies are inherently inadequate for studying the dynamics of the atherosclerosis process (i.e., do not allow establishing whether increased IMT heralds new plaque formation). A longitudinal study in the general population has documented that increased IMT signals propensity to new plaque formation,9 but to date there is no study investigating this problem in ESRD patients. The question is of importance because arterial lesions and risk factors for atherosclerosis in ESRD do not coincide with those in the general population.
This longitudinal study was designed to clarify two open questions related with the evolution of atherosclerosis in the ESRD population: 1) to establish whether IMT predicts the subsequent development of new carotid plaques; and 2) to assess whether repeated measurements of IMT and other US markers of atherosclerosis (CCAD, CSA, CCAwlr, and plaque burden) provide prognostic information for risk monitoring above and beyond a single assessment of these parameters in ESRD patients and whether this predictive power is independent of other strong risk markers such as left ventricular hypertrophy (LVH).
RESULTS
Nineteen patients were diabetics and 66 were habitual smokers (22 ± 18 cigarettes/d). Seventy-seven patients were being treated with various antihypertensive drugs (51 on mono-therapy with ACE inhibitors, AT-1 antagonists, calcium channel blockers, α- and β-blockers, and the remaining 26 on double or triple therapy with various combinations of these drugs). Sixty-seven patients were on treatment with erythropoietin, 19 with antiplatelet drugs, and 3 with statins. The main clinical, biochemical, Echo-color Doppler and echocardiographic data in the original study population and in the cohort who repeated the Echo-color Doppler study of the carotid arteries are detailed in Table 1. Serum cholesterol underwent a significant reduction during the follow-up. Otherwise, no significant change was recorded in the hemodynamic and biochemical variables listed in Table 1. As compared with the baseline study, IMT, CCAD, CCAwlr, and CSA remained almost unchanged at the second US study. In contrast, the total number of atherosclerotic plaques increased significantly (1 plaque per patient-yr), and the proportion of patients with severe atherosclerosis (>4 plaques) rose from 25% at baseline to 44% at the follow-up (P < 0.001). In parallel with these changes, LVMI showed an 8% increase (P < 0.01).
Table 1.
Main clinical, biochemical, vascular, and echocardiographic data in the original study cohort and in patients who repeated the Echo-Color Doppler study of the carotid arteries
| Original Cohort (n = 135) | Patients Who Repeated Echo-Color Doppler Study (n = 103)
|
P (second vs first visit) | ||
|---|---|---|---|---|
| First Visit | Second Visit | |||
| Age, yr | 60.0 ± 16.0 | 58.1 ± 15.8 | 59.4 ± 15.8 | <0.001 |
| BMI, kg/m2 | 25.1 ± 4.4 | 24.7 ± 4.1 | 24.6 ± 4.2 | 0.79 |
| Males, n (%) | 79 (58) | 61 (59) | 61 (59) | 1.00 |
| Diabetics, n (%) | 19 (14) | 12 (12) | 12 (12) | 1.00 |
| Smokers, n (%) | 66 (49) | 50 (48) | 50 (48) | 1.00 |
| Patients on antihypertensive therapy, n (%) | 77 (57) | 62 (60) | 62 (60) | 1.00 |
| Systolic pressure, mmHg | 135 ± 21 | 136 ± 22 | 135 ± 24 | 0.62 |
| Diastolic pressure, mmHg | 75 ± 13 | 76 ± 13 | 74 ± 14 | 0.09 |
| Pulse pressure, mmHg | 60 ± 16 | 60 ± 16 | 61 ± 17 | 0.53 |
| Heart rate, beats/min | 83 ± 13 | 83 ± 13 | 82 ± 11 | 0.72 |
| Hemoglobin, g/L) | 105 ± 20 | 104 ± 18 | 103 ± 15 | 0.48 |
| Albumin, g/L | 38 ± 6 | 39 ± 6 | 38 ± 5 | 0.31 |
| Cholesterol, mg/dl | 5.46 ± 1.41 | 5.40 ± 1.32 | 4.40 ± 1.05 | <0.001 |
| Calcium * phosphate, mMol2/L2 | 4.44 ± 1.33 | 4.50 ± 1.31 | 4.35 ± 1.30 | 0.33 |
| C-reactive protein, mg/L | 8.4 (3.4–19.1) | 7.6 (3.4–16.0) | NA | — |
| Homocysteine, μMol/L | 28.0 (20.5–42.8) | 27.7 (20.7–43.9) | NA | — |
| Fibrinogen, mg/dl | 567 (476–675) | 567 (477–690) | NA | — |
| LVMI, g/m2.7 | 69.8 ± 19.0 | 67.3 ± 17.0 | 72.7 ± 20.9 | <0.001 |
| Intima media thickness, mm | 1.04 ± 0.23 | 1.02 ± 0.22 | 1.01 ± 0.20 | 0.24 |
| CCAD, mm | 6.88 ± 0.92 | 6.86 ± 0.92 | 6.86 ± 0.93 | 0.98 |
| CSA, mm2 | 12.1 ± 3.4 | 11.9 ± 3.4 | 11.8 ± 3.2 | 0.25 |
| CCAwlr | 0.32 ± 0.08 | 0.30 ± 0.07 | 0.30 ± 0.06 | 0.21 |
| No. of atherosclerotic plaques | 3 (1–5) | 3 (0–5) | 4 (1–6) | <0.001 |
| No. of patients with atherosclerotic plaques | ||||
| 0 | 29 (21%) | 26 (25%) | 19 (18%) | — |
| 1–4 | 66 (49%) | 51 (50%) | 39 (38%) | <0.001 |
| >4 | 40 (30%) | 31 (25%) | 45 (44%) | — |
NA, not available; LVMI, left ventricular mass index; CCAD, common carotid artery diameter; CCAwlr, common carotid artery wall to lumen ratio; CSA, cross-sectional area.
Correlates of New Plaque Formation
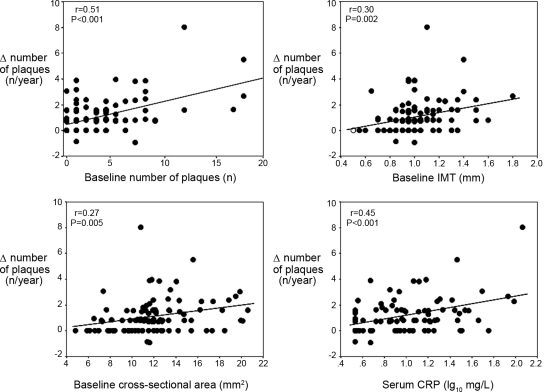
On univariate analysis, the formation rate of new atherosclerotic plaques was strongly and directly related to the baseline number of plaques (r = 0.51, P < 0.001), baseline IMT (r = 0.30, P = 0.002), CSA (r = 0.27, P = 0.005), and CCAwlr (r = 0.25, P = 0.01) as well as to serum C-reactive protein (CRP) (r = 0.45, P < 0.001) (Figure 1). New plaque formation was also related directly with smoking (r = 0.29, P = 0.003), male sex (r = 0.22, P = 0.02), fibrinogen (r = 0.27, P = 0.006), use of antiplatelet drugs (r = 0.20, P = 0.04), LVMI (r = 0.23, P = 0.02), and previous CV events (r = 0.20, P = 0.04). However, formation rate of new atherosclerosis plaques was largely unrelated to changes in IMT (r = −0.05, P = 0.60) and to changes in CCAD (r = −0.11, P = 0.247) or CSA (r = −0.09, P = 0.36). In a multiple regression model, including all univariate predictors of plaque formation, only the baseline number of plaques (β = 0.32, P = 0.004) and serum CRP (β = 0.24, P = 0.02) maintained an independent association with the formation of new plaques (Table 2). The predictive value of CCAwlr for plaque formation was tested in a multiple regression model excluding baseline IMT (to avoid co-linearity problems). In this analysis, CCA wall to lumen ratio was largely unrelated to plaque formation (β = 0.08, P = 0.44).
Figure 1.
Relationship between new atherosclerotic plaques and baseline number of plaques, baseline IMT, baseline cross-sectional area (CSA) and serum CRP. Data are Pearson Product Moment correlation coefficients and P values.
Table 2.
Multiple regression model of new plaque formation
| Standardized Regression Coefficient (β ) | P | |
|---|---|---|
| Baseline no. of plaques | 0.32 | 0.004 |
| CRP | 0.24 | 0.02 |
| Sex | 0.11 | 0.38 |
| Smoking | 0.09 | 0.41 |
| Baseline IMT | 0.14 | 0.51 |
| Baseline CSA | −0.13 | 0.56 |
| Use of antiplatelet drugs | 0.04 | 0.63 |
| Previous cardiovascular events | 0.04 | 0.64 |
| Fibrinogen | 0.04 | 0.67 |
| LVMI | 0.04 | 0.69 |
Multiple R = 0.61, R2 = 0.37, P < 0.001.
IMT, intima media thickness; CSA, cross-sectional area; LVMI, left ventricular mass index.
Survival Analysis
After the second Echo-color Doppler study, 40 patients died and 51 had fatal and nonfatal CV events. Changes in IMT, CCAD, CCAwlr, and CSA failed to predict death and CV events. In contrast, the formation rate of new atherosclerotic plaques was strongly and directly associated with all-cause mortality [HR (1 plaques/yr increase): 1.25; 95% confidence interval, 1.04 to 1.50, P = 0.02] and fatal and nonfatal CV events [HR (1 plaques/yr increase): 1.32; 95% confidence interval, 1.14 to 1.52, P < 0.001]. Accordingly, both mortality rate and CV events rate were higher in patients who formed new plaques (21 deaths per 100 patient-yr and 35 CV events per 100 patient-yr) than in those who did not (10 deaths per 100 patient-yr and 13 CV events per 100 patient-yr). The prognostic power of new atherosclerotic plaques for all-cause mortality was largely dependent on sex and smoking because this link lost substantial statistical significance after data adjustment for these two risk factors [Δ plaques-mortality link (sex-adjusted): P = 0.13; Δ plaques-mortality link (smoking-adjusted): P = 0.22)]. However, the association between the number of new atherosclerotic plaques and CV events was very little affected by (bivariate) adjustment for a series of potential confounders (Table 3), including demographic variables, traditional and nontraditional risk factors, and the baseline number of plaques, IMT, CCAD, CCAwlr, and CSA. The formation rate of new atherosclerotic plaques maintained an independent association with CV outcomes also in a multivariate Cox regression analysis (see “Concise Methods” and Table 4).
Table 3.
Predictive power of new plaque formation rate for incident CV events as adjusted for other risk factors in bivariate Cox regression analyses
| Adjusted for | Hazard Ratio (95% CI), P (1 plaque/yr increase) |
|---|---|
| Age | 1.29 (1.12–1.50), <0.001 |
| Sex | 1.26 (1.08–1.47), 0.003 |
| BMI | 1.33 (1.14–1.55), <0.001 |
| Smoking | 1.23 (1.05–1.43), 0.01 |
| Diabetes | 1.31 (1.14–1.53), <0.001 |
| Previous CV events | 1.28 (1.10–1.48), 0.001 |
| Antihypertensive treatment | 1.31 (1.13–1.53), <0.001 |
| CRP | 1.41 (1.14–1.76), 0.002 |
| Homocysteine | 1.31 (1.13–1.51), <0.001 |
| Cholesterol | 1.32 (1.14–1.52), <0.001 |
| Hemoglobin | 1.31 (1.14–1.51), <0.001 |
| Albumin | 1.32 (1.14–1.54), <0.001 |
| Calcium*phosphate | 1.34 (1.15–1.56), <0.001 |
| Systolic pressure | 1.31 (1.13–1.53), <0.001 |
| Diastolic pressure | 1.37 (1.18–1.60), <0.001 |
| LVMI | 1.26 (1.08–1.47), 0.003 |
| Ejection fraction | 1.30 (1.11–1.53), 0.001 |
| Baseline no. of plaques | 1.24 (1.03–1.48), 0.02 |
| Baseline IMT | 1.27 (1.09–1.48), 0.002 |
| Baseline CCAD | 1.34 (1.15–1.57), <0.001 |
| Baseline CSA | 1.29 (1.10–1.51), 0.001 |
| Baseline CCAwlr | 1.29 (1.11–1.51), 0.001 |
| Changes in IMT | 1.31 (1.13–1.51), <0.001 |
| Changes in CCAD | 1.31 (1.14–1.52), <0.001 |
| Changes in CSA | 1.31 (1.14–1.51), <0.001 |
| Changes in CCAwlr | 1.31 (1.13–1.52), <0.001 |
CCAD, common carotid artery diameter; CCAwlr, common carotid artery wall to lumen ratio; CSA, cross-sectional area.
Table 4.
Multiple Cox regression analysis for incident CV events
| Units of Increase | Hazard Ratio (95% CI) | P | |
|---|---|---|---|
| Plaque formation | 1 plaque/yr | 1.22 (1.05–1.42) | 0.01 |
| Smoking | — | 2.18 (1.18–4.05) | 0.01 |
| Age | 1 yr | 1.02 (1.00–1.04) | 0.03 |
The final model was constructed by starting with the full set of variables listed in “Concise Methods” and by using a backward elimination strategy.
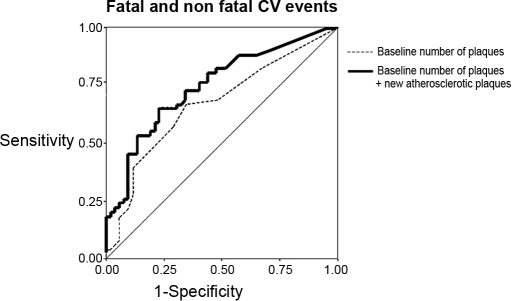
Prognostic Value of New Atherosclerosis Plaques: Receiver Operating Characteristic (ROC) Curve Analysis
The additional prognostic power for CV events provided by the formation rate of new atherosclerotic plaques beyond and above that of baseline number of plaques was investigated by ROC curves analysis. The area under the ROC curve for CV events as related to the baseline number of plaques alone was 0.67 ± 0.05 (P = 0.003). In an analysis including new plaque formation as a second predictive variable added to baseline number of plaques, the area under the ROC curve rose to 0.75 ± 0.05, and the gain in predictive power (+8%) was highly significant (P < 0.01) (Figure 2), suggesting that monitoring plaque burden is useful to better ascertain cardiovascular prognosis in ESRD patients.
Figure 2.
ROC curve analysis for incident CV events of baseline and new atherosclerotic plaques.
DISCUSSION
This study shows that new plaque formation in ESRD patients is largely independent of ongoing changes in IMT and other aspects of arterial remodeling and that this process is predicted only by the number of carotid plaques at baseline and by CRP. Furthermore, plaque formation rate is an independent predictor of incident CV events well beyond background plaque burden and other risk factors. Collectively, these findings indicate that in ESRD new plaque formation occurs independently of other anatomic aspects of the atherosclerotic process and that monitoring the evolution of plaque burden adds independent prognostic information for cardiovascular complications in this population.
Relationship Between IMT and Indicators of Vascular Remodeling and New Plaque Formation
Plaque formation is a fundamental step in the atherosclerosis process.10 Plaque growth initially leads to a compensatory arterial expansion (“positive remodeling”) at sites of lesions, a response pattern that is well demonstrated in animal models of coronary disease.11 The observation that luminal size is initially unaffected by plaque is well confirmed in necropsy studies in human coronary arteries.12 Yet the arterial remodeling process is bidirectional in nature. In other words, “negative remodeling” (i.e., shrinkage of external elastic membrane area at the lesion site) may lead to arterial narrowing at the site of a plaque lesion. Intimal thickening is considered as an early lesion in the pathogenesis of atherosclerosis. However, the role of increased IMT in the evolution of this process remains questionable.3 In the sole longitudinal study performed so far in the general population,9 baseline IMT predicted the subsequent development of carotid atherosclerotic plaques, an observation compatible with the hypothesis that ticker intima media might be an early response to insults conducive to arterial damage. Our study, which is the first longitudinal study in ESRD patients, confirms that high baseline IMT predicts a higher formation rate of new plaques. However, our data also show that new plaque formation in this population may occur independently of ongoing changes in other aspects of the atherosclerosis process. Indeed, plaque burden increased to an important extent (Table 1), whereas IMT and other indicators of the remodeling process (CCAD and CSA) remained unchanged. Indeed, IMT change over time was not associated with new plaque formation in our cohort. Remarkably, new plaque formation occurred in the face of declining serum cholesterol concentration and at constant calcium × phosphate product, CRP, and the baseline number of plaques being the sole independent predictors of progression of atherosclerosis. Thus, our longitudinal data identify inflammation and the extent of prior plaque burden as fundamental risk factors for the progression of arterial damage in the dialysis population.
Prognostic Value of Plaque Burden Monitoring in ESRD
IMT is a most consistent predictor of adverse outcomes in the general population4,13 and in the ESRD5,6 and CKD14 populations as well. Indeed, high IMT emerged as a strong risk factor in a variety of settings, from community studies to high risk conditions, such as coronary heart disease and ESRD.3,5,6 Whether progression or regression in IMT and other indicators of atherosclerosis has prognostic value for future cardiovascular complications is a very little investigated topic. A recent systematic review3 identified just one longitudinal study looking at this problem in the general population13 while the issue is still unexplored in ESRD patients. The question is of relevance because atherosclerosis is of particular severity in the dialysis population; therefore, the prognostic potential of serial carotid US studies needs to be specifically tested in this population. In the above-mentioned study,13 in 146 middle-aged men with coronary artery disease, a 0.003 mm/yr increase in IMT was related to a doubling in the risk of coronary artery disease events. In the present study, changes in IMT, CCAD, CCAwlr, and CSA were largely unrelated to clinical outcomes. The number of new plaques had no prognostic power for mortality after adjusting for gender and smoking, suggesting that these traditional risk factors explain much of the association between new plaque development and mortality. Yet the prediction power for incident CV events of new plaque formation was independent of sex, smoking, and other risk factors, indicating that this US parameter provides information on the risk of CV complications beyond that given by traditional risk factors. Importantly, change in plaque burden maintained an independent predictive power also in a model including LV mass, which is considered one of the strongest risk factors in ESRD. Furthermore, ROC curves analysis showed that change in plaque burden provides prognostic information beyond and above that of baseline number of plaques. Overall, our novel data indicate that plaque burden is an useful parameter for risk monitoring in the ESRD population.
Study Limitations
Our study has limitations. First, it is well established that the primary negative effect of atherosclerosis in the arterial system is not the increase in artery thickness itself but rather its functional consequence, i.e., an increase in stiffness. Such an effect can be now reliably estimated by pulse wave velocity, which is a strong predictor of cardiovascular events in ESRD.15 Thus, it may be argued that functional rather than anatomic measurements are useful for prognostic purposes in these patients. Yet to date, there is no longitudinal study testing the value of changes in pulse wave velocity in risk monitoring in ESRD. In any case, we think that carotid US and PWV represent complementary rather than competitive techniques for the assessment of the vascular system. The second limitation is that, because of early mortality and censoring, the cohort that we considered in the follow-up study had a lower CV risk than the original cohort (survival cohort bias). This is an inherent limitation of all studies testing the predictive power for hard clinical endpoints of changes in clinical indicators over time. Yet it is of importance that changes in plaque burden maintained a strong predictive power for CV events in a cohort at relatively lower risk than the original cohort, such as the cohort of patients who survived beyond the second Echo-color Doppler study.
In ESRD, plaque formation may occur independently of other aspects of the atherosclerosis process. Prior plaque burden and inflammation are fundamental risk factors for the progression of arterial damage in this population. Plaque formation rate is the sole US marker bearing independent prognostic power for incident CV events. These findings indicate that plaque burden monitoring is useful to refine cardiovascular prognosis in ESRD patients. Whether repeated US studies are useful in clinical practice remains to be formally tested in a clinical trial.
CONCISE METHODS
Protocol
The protocol was in conformity to the ethical guidelines of our institution, and informed, written consent was obtained from each participant. All studies were performed between 8:00 a.m. and 1:00 p.m.
Original Study Cohort
The original study cohort was formed by 135 dialysis patients (age 60 ± 16 yr) [79 males and 56 females; 90 on hemodialysis (HD) and 45 on chronic ambulatory peritoneal dialysis (CAPD)]. Hemodialysis patients were being treated thrice weekly with standard bicarbonate dialysis (Na 138 mmol/L, HCO3 5 mmol/L, K 1.5 mmol/L, Ca 1.25 mmol/L, Mg 0.75 mmol/L) using either with cuprophan or semisynthetic membranes. The average urea Kt/V in these patients was 1.28 ± 0.28. The remaining 45 patients were on CAPD (weekly Kt/V 1.67 ± 0.33). At enrollment, no patient had active infections or was hospitalized for intercurrent inflammatory illness.
Patients Who Repeated the Carotid US Study
Twenty-seven patients of 135 who entered into this study died before the time at which the second carotid US study was performed, 4 patients underwent renal transplantation, and 1 patient could not repeat US for logistic reasons. Therefore, 103 patients were left for this longitudinal study (Table 1).
Follow-up
After the initial assessment and the first US study, patients were followed up by the nephrologists participating into the study. The study was purely observational; therefore, it did not contemplate changes in treatment policies. The second US study was performed from 11 to 19 mo (average, 15 mo) after the baseline study. The overall duration of the follow-up was 43 ± 14 mo. Since the scope of the present study was that of establishing the prognostic value of changes in atherosclerosis indicators, all survival analyses reported herein apply to the follow-up after the second US study (see “Statistical Analysis”), which was 28 ± 14 mo.
Endpoint Evaluation
During the follow-up cardiovascular events (ECG documented anginal episodes and myocardial infarction, heart failure, ECG documented arrhythmia, transient ischemic attacks, stroke, and other thrombotic events except arteriovenous fistula thromboses) and death were accurately recorded. Each death was reviewed and assigned an underlying cause by a panel of 5 physicians. As a part of the review process, all available medical information about each death was collected. This information always included study and hospitalization records. In the case of an out-of-hospital death, family members were interviewed by telephone to better ascertain the circumstances surrounding death. As alluded to before, for the purpose of establishing the prognostic value of progression in carotid atherosclerosis, only events (death and cardiovascular events) occurring after the second US study were considered.
Echo-Color Doppler of the Carotid Arteries and Echocardiography
In all patients, carotid US studies were performed mid-week during the dialysis interval. Ultrasound measurements were made bilaterally and always using the same equipment by a single observer (F.A.B.) who was blinded to the clinical and biochemical data. All studies were performed with a Hewlett Packard Sonos 1500 using a 7.5-MHz high resolution probe, and images were stored in optic disks according to a standard protocol applied in our US laboratory.5 All measurements were made by a cardiologist (F.A.B.) at the time of examination (online) with the accuracy of the electronic calipers of the instrument to the nearest 0.1 mm. IMT was defined as a low level echo gray band, which does not project into the arterial lumen and was measured during end-diastole as the distance from the leading edge of the second echogenic line of the far walls of the distal segment of the common carotid artery, the carotid bifurcation, and the initial tract of internal carotid artery on both sides. Measurements were performed 0.5, 1, and 2 cm below and above the bifurcation (six measurements on each side), and the average measurement was taken as IMT. The CCAD was measured bilaterally 2 cm below the bifurcation during end diastole and the average measurement was taken as CCAD. The number of atherosclerotic plaques16 [either as faint gray echoes (soft plaques) or bright white echoes (calcified plaque) protruding into the lumen] detected in the bulbar area (from 2 cm below to 2 cm above the bifurcation) of the carotid arteries was recorded on both sides and summed up. IMT and CCAD measurements were always performed in plaque-free arterial segments. Cross-sectional area was calculated by the standard formula.17 CCAwlr was calculated by the formula: 2 × IMT/CCAD. Repeated studies in our laboratory showed that IMT, CCAD, CSA, and the number of atherosclerotic plaques represent reliable measurements in dialysis patients because the coefficient of variation of repeated measurements in our laboratory was 5.5%, 3.2%, 5.3%, and 2.0%, respectively. The formation rate of new atherosclerotic plaques was calculated by subtracting the total number of plaques at baseline from that observed at the second study and by factoring this difference for the time interval between the two studies.
The echocardiographic study was performed during the same day immediately after the carotid US study. The echocardiographic protocol applied in our laboratory, and details about left ventricular mass and left ventricular ejection fraction measurements were reported in full elsewhere.18
Biochemical Measurements
Fasting blood sampling was performed between 8:00 a.m. and 12:00 a.m. Serum lipids, albumin, hemoglobin, calcium and phosphate, CRP, fibrinogen, and homocysteine were measured by standard methods in the routine clinical laboratory.
Statistical Analysis
Data are expressed as mean ± SD (normally distributed data), median, and interquartile range (non-normally distributed data) or as percent frequencies, and within-subjects comparisons were made by paired t test, Wilcoxon rank test, or χ2 test, as appropriate.
The relationship between traditional and nontraditional risk factors with markers of the atherosclerosis process (IMT, plaques, CCAD, CCAwlr, and CSA) and changes in these indicators during follow-up was analyzed by univariate and multiple linear regression analyses. In this analysis, we considered a large series of risk factors, including Framingham risk factors (age, sex, body mass index, smoking, diabetes, arterial pressure and antihypertensive treatment, cholesterol, left ventricular mass and function), use of antiplatelet drugs and statins, factors peculiar to ESRD (hemoglobin, albumin, calcium, and phosphate), and emerging risk factors (CRP, fibrinogen and homocysteine). Significant univariate correlates of indicators of atherosclerosis and of their changes over time were then used to develop appropriate multiple linear regression models. For the description of these models, significant independent variables were then ordered according to their standardized effect. Data are expressed as standardized regression coefficient (β) and P value.
The relationship between US markers of atherosclerosis and all-cause mortality and incident CV events (fatal and nonfatal) was analyzed by univariate, bivariate (i.e., models adjusting for other risk factors considered one by one) and multivariate Cox regression. Because the scope of this analysis was prognostic rather than etiological, multivariate Cox regression analysis was run by a backward elimination strategy aimed at producing a parsimonious model (i.e., a model optimizing prediction of incident CV events based on a restricted number of variables19). Furthermore, we used ROC curves analysis to calculate the additional predictive value for CV outcomes of new atherosclerotic plaques beyond and above that provided by baseline number of plaques alone.
All calculations were made using a standard statistical package (SPSS for Windows, version 9.0.1, Chicago, IL).
DISCLOSURES
No conflict of interest is related to this manuscript.
Acknowledgments
The authors thank Dr. Daniela Leonardis and Rocco Tripepi for their help in the study. This study was supported by the National Research Council of Italy and by Regione Calabria.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Roman MJ, Naqvi TZ, Gardin JM, Gerhard-Herman M, Jaff M, Mohler E: American Society of Echocardiography Report. Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society for Vascular Medicine and Biology. Vasc Med 11: 201–211, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Mancini GB: Carotid intima-media thickness as a measure of vascular target organ damage. Curr Hypertens Rep 2: 71–77, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bots ML, Baldassarre D, Simon A, de Groot E, O’Leary DH, Riley W, Kastelein JJ, Grobbee DE: Carotid intima-media thickness and coronary atherosclerosis: weak or strong relations? Eur Heart J 28: 398–406, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Rosvall M, Janzon L, Berglund G, Engstrom G, Hedblad B: Incident coronary events and case fatality in relation to common carotid intima-media thickness. J Intern Med 257: 430–437, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Benedetto FA, Mallamaci F, Tripepi G, Zoccali C: Prognostic value of ultrasonographic measurement of carotid intima media thickness in dialysis patients. J Am Soc Nephrol 12: 2458–2464, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Nishizawa Y, Shoji T, Maekawa K, Nagasue K, Okuno S, Kim M, Emoto M, Ishimura E, Nakatani T, Miki T, Inaba M: Intima-media thickness of carotid artery predicts cardiovascular mortality in hemodialysis patients[Abstract]. Am J Kidney Dis 41: S76–S79, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Leoncini G, Viazzi F, Parodi D, Ratto E, Vettoretti S, Vaccaro V, Ravera M, Deferrari G, Pontremoli R: Mild renal dysfunction and cardiovascular risk in hypertensive patients. J Am Soc Nephrol 15(Suppl 1): S88–S90, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Wang AY, Ho SS, Wang M, Liu EK, Ho S, Li PK, Lui SF, Sanderson JE: Cardiac valvular calcification as a marker of atherosclerosis and arterial calcification in end-stage renal disease. Arch Intern Med 165: 327–332, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Zureik M, Ducimetiere P, Touboul PJ, Courbon D, Bonithon-Kopp C, Berr C, Magne C: Common carotid intima-media thickness predicts occurrence of carotid atherosclerotic plaques: longitudinal results from the Aging Vascular Study (EVA) study. Arterioscler Thromb Vasc Biol 20: 1622–1629, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Schoenhagen P, Ziada KM, Vince DG, Nissen SE, Tuzcu EM: Arterial remodeling and coronary artery disease: the concept of “dilated” versus “obstructive” coronary atherosclerosis. J Am Coll Cardiol 38: 297–306, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong ML, Heistad DD, Marcus ML, Megan MB, Piegors DJ: Structural and hemodynamic response of peripheral arteries of macaque monkeys to atherogenic diet. Arteriosclerosis 5: 336–346, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ: Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 316: 1371–1375, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, Azen SP: The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 128: 262–269, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Szeto CC, Chow KM, Woo KS, Chook P, Ching-Ha, Kwan B, Leung CB, Kam-Tao Li P: Carotid intima media thickness predicts cardiovascular diseases in chinese predialysis patients with chronic kidney disease. J Am Soc Nephrol 18: 1966–1972, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99: 2434–2439, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Berglund GL: Ultrasound in clinical trials of atherosclerosis: introduction. J Intern Med 236: 551–553, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Khattar RS, Acharya DU, Kinsey C, Senior R, Lahiri A: Longitudinal association of ambulatory pulse pressure with left ventricular mass and vascular hypertrophy in essential hypertension. J Hypertens 15: 737–743, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Cataliotti A, Seminara G, Stancanelli B, Malatino LS: Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis. J Am Soc Nephrol 12: 2768–2774, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Ambler G, Brady AR, Royston P: Simplifying a prognostic model: a simulation study based on clinical data. Stat Med 21: 3803–3822, 2002 [DOI] [PubMed] [Google Scholar]