Abstract
Age-related macular degeneration is the leading cause of irreversible blindness in the United States and often coexists with chronic kidney disease. Both conditions share common genetic and environmental risk factors. A total of 1183 participants aged 54+ were examined in the population-based, prospective cohort Blue Mountains Eye Study (Australia) to determine if chronic kidney disease increases the risk of age-related macular degeneration. Moderate chronic kidney disease (estimated glomerular filtration rate < 60 ml/min per 1.73 m2 based on the Cockcroft-Gault equation) was present in 24% of the population (286 of 1183). The 5-yr incidence of early age-related macular degeneration was 3.9% in participants with no/mild chronic kidney disease (35 of 897) and 17.5% in those with moderate chronic kidney disease (50 of 286). After adjusting for age, sex, cigarette smoking, hypertension, complement factor H polymorphism, and other risk factors, persons with moderate chronic kidney disease were 3 times more likely to develop early age-related macular degeneration than persons with no/mild chronic kidney disease (odds ratio = 3.2; 95% confidence interval, 1.8 to 5.7, P < 0.0001). Each SD (14.8 ml/min per 1.73 m2) decrease in Cockcroft-Gault estimated glomerular filtration rate was associated with a doubling of the adjusted risk for early age-related macular degeneration (odds ratio = 2.0; 95% confidence interval, 1.5 to 2.8, P < 0.0001). In conclusion, persons with chronic kidney disease have a higher risk of early age-related macular degeneration, suggesting the possibility of shared pathophysiologic mechanisms between the two conditions.
Chronic kidney disease (CKD) is a relatively common condition whose prevalence rises rapidly with age. CKD is estimated to affect over 50% of older persons in the United States1 and has important extrarenal systemic consequences, such as cardiovascular, metabolic, and hematologic abnormalities. However, the effects of CKD on the sensory organs, such as the eye, are not well understood.
Age-related macular degeneration (AMD) is another relatively common condition in older persons and is the leading cause of blindness and low vision in the United States.2 CKD and AMD may share common risk factors and pathophysiologic mechanisms.3 For example, vascular risk factors, such as hypertension and cigarette smoking, may increase risk of both conditions.4–7 CKD is known to accelerate the progression of atherosclerosis and increase propensity to oxidative stress,6 both of which are implicated in AMD pathogenesis.8,9 Systemic inflammatory processes may accelerate the course of CKD10 and contribute to the development of AMD.11 More recent evidence suggests that the two conditions have common susceptibility genes, such as complement factor H (CFH),12–14 complement C3,15,16 and apolipoprotein E (APOE).17,18 Finally, there are a number of rare inherited syndromes that involve both renal and ocular abnormalities.19
There have been no studies, however, that have examined if the two conditions are associated independently of age and common risk factors. In particular, there are no prospective studies addressing the clinically relevant question of whether persons with CKD are more likely to develop AMD. The purpose of this report is to describe prospectively the relationship of CKD with incident AMD.
RESULTS
The 1152 participants excluded from analyses did not differ from those included (n = 1183) on sex but were older (mean age 67.1 yr versus 62.0 yr), more likely to be current smokers (14.9% versus 10.8%), have hypertension (68.3% versus 57.2%), and higher serum creatinine (1.10 mg/dl versus 1.01 mg/dl). The rate of incident early AMD among included (7.2%) and excluded (7.0%) participants was similar.
Persons with moderate CKD were older, less likely to be current smokers, and more likely to have raised blood pressure and fibrinogen levels (Table 1). There were 102 participants with stage I (No CKD, eGFRCG ≥ 90 ml/min per 1.73 m2), 795 with stage II (mild CKD, eGFRCG 60 to 89 ml/min per 1.73 m2), 280 with stage III (moderate CKD, eGFRCG 30 to 59 ml/min per 1.73 m2), and 6 with stage IV (severe CKD, eGFRCG < 30 ml/min per 1.73 m2).
Table 1.
Characteristics of persons with and without moderate chronic kidney disease (CKD)
| Moderate CKD (eGFRCG < 60 ml/min/1.73m2) (n = 286) | No or Mild CKD (eGFRCG ≥ 60 ml/min/1.73m2) (n = 897) | P | |
|---|---|---|---|
| Age, yr (SD; range) | 69.6 (5.7; 58–94) | 59.6 (6.2; 54–84) | <0.0001 |
| Male, % | 115 (40.2) | 382 (42.6) | 0.48 |
| Current smoker, % | 22 (7.7) | 106 (11.8) | 0.04 |
| Hypertension, % | 196 (68.5) | 480 (53.5) | <0.0001 |
| Diabetes, % | 27 (9.4) | 91 (10.1) | 0.87 |
| Systolic blood pressure, mmHg (SD) | 153.2 (20.9) | 146.1 (20.1) | <0.0001 |
| Diastolic blood pressure, mmHg (SD) | 83.3 (9.5) | 85.1 (9.1) | 0.005 |
| Fibrinogen, mg/dl (SD) | 372 (89) | 348 (81) | <0.0001 |
| White cell count, ×109 (SD) | 6.4 (1.9) | 6.3 (1.6) | 0.27 |
| Serum total cholesterol, mmol/L (SD) | 6.0 (1.1) | 6.0 (1.1) | 0.72 |
| Serum triglycerides, mmol/L (SD) | 1.5 (0.8) | 1.6 (0.8) | 0.26 |
| CFH Y402H polymorphism status | |||
| YY | 36 (12.6) | 118 (13.2) | |
| YH | 133 (46.5) | 409 (45.6) | |
| HH | 117 (40.9) | 370 (41.3) | 0.87b |
| Creatinine, mg/dl (SD) | 1.17 (0.37) | 0.96 (0.14) | <0.0001 |
| eGFRCG, ml/min/1.73m2 (SD)a | 52.5 (9.9) | 82.7 (18.4) | <0.0001 |
Estimated using Cockcroft-Gault equation.
P trend in category.
Of the 1183 persons at risk, 85 (7.2%) developed incident early AMD over 5 yr. The 5-yr incidence of early AMD was 1.0%, 4.3%, and 17.5% among participants with no (n = 102, 1 case), mild (n = 795, 34 cases), and moderate (n = 286, 50 cases) CKD, respectively. In further analyses, we combined the categories of no CKD and mild CKD because of the low number of incident AMD cases in those with no CKD.
Table 2 shows that the incidence of early AMD was 3.9% in participants with no/mild CKD versus 17.5% in those with moderate CKD. After adjusting for age, sex, and CFH Y402H polymorphism, persons with moderate CKD had a 3-fold higher odds of incident early AMD than persons with no/mild CKD (odds ratio [OR] = 3.2; 95% confidence interval [CI], 1.8 to 5.7, P < 0.0001). Additional multivariable adjustment in Model 2 did not change these results.
Table 2.
Chronic kidney disease and incident early AMD
| Chronic Kidney Disease Stage | Incident Early AMD
|
Odds Ratio (95% confidence interval)
|
||||
|---|---|---|---|---|---|---|
| No. at Risk | No. (%) Affected | Age-, Sex-, CFH Y402H Polymorphism-Adjusted | P | Multivariable Adjusteda | P | |
| No/mild (eGFRCG ≥ 60) | 897 | 35 (3.9) | 1.0 | — | 1.0 | — |
| Moderate (eGFRCG < 60) | 286 | 50 (17.5) | 3.2 (1.8–5.7) | <0.0001 | 3.0 (1.6–5.4) | 0.0004 |
| P | <0.0001 | |||||
AMD, age-related macular degeneration; eGFRCG, estimated glomerular filtration rate (in ml/min/1.73m2) using the Cockcroft-Gault formula.
Adjusted for age (continuous), sex, hypertension (present, absent), systolic blood pressure, diastolic blood pressure (both continuous), diabetes (present, absent), cigarette smoking (current, ex), pack-yr of cigarette smoking, white cell count, plasma fibrinogen, fasting plasma cholesterol, fasting plasma triglycerides (all continuous), CFH Y402H genotype (homozygous, heterozygous, noncarrier), and body mass index (continuous).
Each SD decrease in eGFRCG was associated with 2-fold (OR = 2.0; CI, 1.5 to 2.8, P < 0.0001) higher odds of incident early AMD, with similar associations in noncarriers/heterozygous and homozygous carriers of CFHY402H (Table 3). We observed the same association of decreasing kidney function with increasing risk of incident early AMD with eGFRMDRD or serum creatinine (Table 3). After adjustment in Model 2, each SD decrease in eGFRCG was associated with incident pigmentary abnormalities (n = 160, OR = 1.4; CI, 1.1 to 1.8), incident indistinct soft or reticular drusen (n = 69, OR = 2.4; CI, 1.6 to 3.4) but not with incident distinct soft drusen (n = 47, OR = 1.2; CI, 0.8 to 1.7). The adjusted relative risk was 2.7, and the population attributable risk percent of early AMD for moderate CKD was 29%.
Table 3.
Estimated glomerular filtration rate and incident early AMD
| Incident Early AMD
|
Odds Ratio (95% confidence interval)
|
|||||
|---|---|---|---|---|---|---|
| No. at Risk | No. (%) Affected | Age-, Sex-, CFH Y402H Genotype-Adjusted | P | Multivariable Adjusteda | P | |
| Per SD decrease eGFRCG | 1183 | 85 (7.2) | 2.0 (1.4–2.8) | <0.0001 | 2.0 (1.5–2.8) | <0.0001 |
| By CFH Y402H genotype | ||||||
| in noncarriers/heterozygotes (YY/YH) | 696 | 59 (8.5) | 1.8 (1.2–2.6) | 0.003 | 1.7 (1.2–2.6) | 0.008 |
| in homozygotes (HH) | 487 | 26 (5.3) | 2.5 (1.3–4.8) | 0.006 | 2.6 (1.3–5.3) | 0.009 |
| Per SD decrease eGFRMDRD | 1183 | 85 (7.2) | 1.6 (1.3–2.1) | 0.0002 | 1.6 (1.3–2.1) | 0.0001 |
| Per SD increase serum creatinine | 1.3 (1.1–1.5) | 0.008 | 1.3 (1.1–1.5) | 0.008 | ||
Units in either ml/min/1.73m2 (eGFR) or mg/dl (creatinine). AMD, age-related macular degeneration; eGFRCG and eGFRMDRD, estimated glomerular filtration rate (in ml/min/1.73m2) using the Cockcroft-Gault and MDRD formulae, respectively; SD, standard deviation (14.8 ml/min/1.73m2 for eGFRCG; 12.5 ml/min/1.73m2 for eGFRMDRD; 0.24 mg/dl for creatinine).
Adjusted for age (continuous), sex, hypertension (present, absent), systolic blood pressure, diastolic blood pressure (both continuous), diabetes (present, absent), cigarette smoking (current, ex), pack-yr of cigarette smoking, white cell count, plasma fibrinogen, fasting plasma cholesterol, fasting plasma triglycerides (all continuous), CFH Y402H genotype (homozygous, heterozygous, noncarrier), and body mass index (continuous).
Table 4 shows the results of analyses stratified by age. The association of worsening eGFRCG with incident early AMD was present in participants 66 to 75 yr of age and in those 76 to 94 yr of age, but not in those 54 to 65 yr of age. There were only 13 incident early AMD cases in the age group of 54 to 65 yr.
Table 4.
Chronic kidney disease and incident early AMD, stratified by age
| Age category | Incident Early AMD
|
Odds Ratio (95% confidence interval)
|
||
|---|---|---|---|---|
| No. at Risk | No. (%) Affected | Age-, Sex-, CFH Y402H Polymorphism-Adjusted | P | |
| 54–65 yr | ||||
| per SD decrease eGFRCG | 524 | 13 (2.5) | 1.0 (0.6–1.8) | 0.98 |
| 66–75 yr | ||||
| per SD decrease eGFRCG | 503 | 51 (10.1) | 1.9 (1.3–2.8) | 0.0004 |
| 76–94 yr | ||||
| per SD decrease eGFRCG | 156 | 21 (13.5) | 1.9 (1.2–3.2) | 0.008 |
AMD, age-related macular degeneration; eGFRCG, estimated glomerular filtration rate (in ml/min/1.73m2), using the Cockcroft-Gault formula.
None of the prespecified interaction terms tested was significant (P > 0.10). We additionally adjusted for nuclear cataract as a biologic aging marker in Model 2 and found the results remained essentially unchanged. In unadjusted analyses, moderate CKD was associated with increased risk of incident late AMD (OR = 3.8; CI, 1.8 to 8.0, P = 0.0002). There were too few incident late AMD cases (n = 27) to perform further multivariable adjusted analyses.
DISCUSSION
CKD and AMD often coexist in the elderly, but it is not clear if they are linked in ways independent of age and common risk factors, such as hypertension. In this community-based cohort study, we demonstrate a strong, graded and consistent association of CKD and risk of early AMD that was independent of age and other known measured risk factors. In our study population, CKD accounted for a substantial portion of the population attributable risk of early AMD.
Although there are few data with which to compare our findings, our results are consistent with existing knowledge and supported by plausible biologic mechanisms. First, CKD and AMD share vascular risk factors, such as hypertension and cigarette smoking.4–7 Atherosclerosis of the choroidal circulation has been hypothesized as a pathogenic factor for AMD development, and atheroma-like deposits of lipid in Bruch's membrane have been found in eyes with AMD.8 Oxidative stress has also been implicated in AMD pathogenesis.7 As CKD accelerates, the progression of atherosclerosis and increases propensity to oxidative stress6; these processes may potentially explain the increased risk of AMD. Additionally, microvascular disease involving smaller vessels in both the renal and retinal circulations may also potentially play a role in explaining our findings, particularly as the microstructure at the interface of the capillary tuft, glomerular basement membrane, and the glomerular epithelial cells in the kidney is similar to the interface involving choriocapillaris, Bruch's membrane, and retinal pigment epithelium in the eye (the sites where AMD pathology occurs).15 Second, recent studies suggest that CKD and AMD share several common genetic susceptibility loci, in particular, those related to key complement pathway proteins.12–18,20,21 Genetic variants in complement factor H are associated with some forms of kidney disease, such as atypical hemolytic uremic syndrome,12,14 whereas C3 variants are associated with IgA nephropathy.16 Both complement factor H and C3 deficiency are associated with type II membranoproliferative glomerulonephritis,14,20,21 a disease characterized by kidney failure and early onset retinal drusen, which are structurally and compositionally identical to those that occur in AMD. Variants in these two key complement protein genes are thought to account for a significant proportion of AMD risk.13,15 Another non—complement-related gene, the APOE ɛ2 allele, has been linked to increased risk of both CKD and AMD, and part of this association may be mediated by its effects on lipid metabolism.17,18 However, in our study, adjusting for CFHY402H polymorphism, serum lipids, systemic inflammatory markers, hypertension, and diabetes did not attenuate the association between CKD and AMD, suggesting links between the two diseases that are not entirely explained by these factors. We did not adjust for other inflammatory markers, such as C-reactive protein or other complement protein variants associated with CKD.
Our study has several strengths: prospective follow-up of a population-based sample, masked and standard methods to ascertain AMD, which have been used successfully to detect associations with other AMD risk factors,22,23 and well-documented data on potential confounders of CKD and incident AMD. Study limitations are potentially important. First, we estimated GFR from formulas, which could result in misclassification, although such misclassification is likely random with respect to AMD. Additionally, we found consistently strong associations with the Cockcroft-Gault, Modified Diet Renal Disease (MDRD) equations, and serum creatinine, suggesting little, if any, bias from misclassification. Second, a proportion of participants from our study were excluded because of missing data; this may have introduced selection bias because they differed from included participants on several characteristics. Nonetheless, excluded participants had a similar incidence of early AMD, suggesting that such potential selection bias is unlikely to completely explain our findings. Third, although we adjusted for age continuously and in stratified analyses, residual confounding from age and other unmeasured factors may remain. The results remained unchanged after additionally adjusting for a biologic aging marker (nuclear cataract), arguing against inadequate age adjustment. Finally, the number of participants who developed incident late AMD was too few for multivariable adjusted analyses, although in unadjusted analyses the same association was present. This limitation does not materially change our conclusions, as early AMD is the main precursor of progression to late AMD.
Much of the morbidity and mortality of CKD arises from its adverse effects on other organ systems, particularly the circulatory, endocrine, and skeletal systems.6 There is little knowledge of the effects of CKD on the sensory organs, such as the eye. We demonstrate that persons with CKD have a 3-fold higher risk of developing early AMD within 5 yr, and that a substantial portion of early AMD cases could be attributed to its association with CKD. This relationship suggests shared pathogenic factors and/or processes between CKD and AMD, which could have implications for the prevention and treatment of both conditions. Additional research and replication in other populations is clearly needed; but if our findings are confirmed, efforts at early detection and prevention of progression of CKD may have a potential unexpected benefit: in addition to reducing the burden of end-stage renal disease and cardiovascular disease, the burden of blindness from AMD may also be lessened.
CONCISE METHODS
Study Population
The Blue Mountains Eye Study is a population-based cohort study of predominantly white persons designed to identify risk factors for AMD and other age-related eye diseases. The detailed methodology of the Blue Mountains Eye Study has been reported elsewhere.24–26 In brief, participants were examined every 5 yr from 1992 to 2004: baseline in 1992 to 1994, the 2nd examination in 1997 to 1999, and the third examination in 2002 to 2004.
Our current analysis is based on participants who attended the 2nd and 3rd examinations. Of the 2335 who participated at the 2nd examination, we excluded 1152 (374 with preexisting AMD, 109 who did not have creatinine measurement, 109 with missing data on important variables such as smoking, blood pressure, and CFHY402H polymorphism, and 560 who did not return for the 3rd examination), leaving 1183 at risk of incident AMD over 5-yr of follow-up. The age range of the participants was 54 to 94 yr. This study was conducted according to the recommendations of the Declaration of Helsinki and approved by the Western Sydney Area Human Ethics Committee. Written, informed consent was obtained from all participants.
Assessment of CKD
Serum creatinine was measured within 4 h of fasting venous blood collection with a Hitachi 747 biochemistry analyzer (Roche reagents, modified kinetic Jaffe) at the 2nd examination concurrent with retinal photography. We used the Cockcroft-Gault (CG) equation27 corrected for body surface area, to obtain estimated GFR (eGFRCG) in ml/min per 1.73 m2. This equation is reported to be more accurate in the elderly,28 and in the general healthy population with normal range of kidney function.29
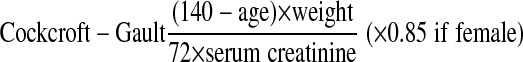 |
In sensitivity analyses, we also used the abbreviated 4-variable MDRD equation30 to obtain eGFRMDRD from serum creatinine.
 |
We defined CKD stages according to the National Kidney Foundation guidelines27 (i.e., no CKD: eGFRCG ≥ 90 ml/min per 1.73 m2; mild CKD, 60 ≤ eGFRCG <90 ml/min per 1.73 m2; moderate CKD, eGFRCG < 60 ml/min per 1.73 m2).
Assessment of AMD
We took two 30° stereoscopic color retinal photographs of the macula of both eyes, which were graded for presence of early and late AMD using the Wisconsin AMD Grading System.22,24,31 Graders were masked to participants’ CKD status, and the intergrader and intragrader reliability showed good agreement in identifying individual lesions.
The detailed methodology of AMD ascertainment in this population has been reported extensively elsewhere.22,24,31 Incident early AMD was defined as the absence of late AMD and presence of either: 1) large (>125-μm diameter) indistinct soft or reticular drusen or 2) both large distinct soft drusen and retinal pigmentary abnormalities (hyperpigmentation or hypopigmentation) at the 3rd examination in either eye of persons free of early AMD in both eyes at the 2nd examination.31 Similarly, incident late AMD was defined as the appearance of neovascular AMD or geographic atrophy at the 3rd examination in either eye of persons without AMD lesions in both eyes at the 2nd examination.31 A retinal specialist (P.M.) adjudicated all uncertain retinal pathology and confirmed all late AMD cases.
Assessment of Other Variables
We measured systolic and diastolic blood pressure using a single mercury sphygmomanometer with appropriate adult cuff size, after participants were seated for at least 10 min. We followed the 2003 World Health Organization (WHO)/ International Society of Hypertension (ISH) guidelines to define hypertension as WHO/ ISH category grade 2 or 3, i.e., a systolic blood pressure ≥ 160 mmHg or diastolic blood pressure ≥ 100 mmHg at examination or current use of antihypertensive medications. Diabetes was defined as a physician diagnosis of diabetes, or a fasting blood sugar ≥ 7 mmol/L. Smoking status was determined from history as never smoked, past smoker, and current smoker (which included those who had ceased smoking within the last 12 mo). Body mass index was calculated from measured height and weight. We genotyped the CFH (Try402 −> His402 polymorphism (rs 1061170, CFHY402H) using a Taqman assay (Applied Biosystems, Foster City, CA). Measurement of other variables is described elsewhere.22,24,25
Statistical Analyses
We examined the frequency of early and late AMD according to CKD categories. Because there were very few cases of AMD in those with no CKD, we classified CKD as no/mild and moderate for analyses. We used logistic regression to determine the adjusted odds of incident early and late AMD. Next we modeled eGFRCG continuously and determined the adjusted odds of incident AMD per SD, SD decrease in eGFRCG. On visual inspection of the distribution and quantile-quantile plot, eGFRCG was normally distributed with a mean of 70.1 ml/min per 1.73 m2 (SD, 14.8; range, 15.3 to 136.1 ml/min per 1.73 m2). eGFRMDRD and serum creatinine were also normally distributed. We performed analyses stratified by CFHY402H genotype in a recessive model for H (i.e., a dominant model for Y), where we combined noncarriers and heterozygotes for the risk allele (YY/YH) into one strata and homozygotes (HH) in the other. This was done because of small numbers of noncarriers. We repeated analyses with incident pigmentary abnormalities, large indistinct soft or reticular drusen, and large distinct soft drusen as outcome variables. We adjusted for age (continuous), sex, and CFHY402H polymorphism (YY, YH, and HH) in Model 1. We additionally adjusted for cigarette smoking (current, ex, or never), pack-yr of cigarette smoking (<20 pack-yr, ≥20 pack-yr <40, and ≥40 pack-yr), hypertension (yes, no), diabetes (yes, no), fibrinogen, white cell count, serum total cholesterol, triglycerides, and body mass index (all continuous) in Model 2. Model 2 fitted the data well (Hosmer-Lemeshow goodness of fit, c = 0.78). We tested for multiplicative interaction between eGFRCG, CFHY402H polymorphism, age, sex, hypertension, and diabetes on risk of early AMD in Model 2 with GFRCG modeled continuously. We repeated analyses with eGFRMDRD and serum creatinine. We also performed analyses in groups stratified by age. We calculated population attributable risk percent as PCKD × (Relative Risk − 1)/[PCKD × (Relative Risk − 1) + 1] × 100%, where PCKD refers to prevalence of moderate CKD. We estimated the adjusted relative risk from the adjusted OR from multivariable Model 2 using the method suggested by Zhang and Yu.32 SAS v9.1 (SAS Institute, Cary, NC) was used.
DISCLOSURES
All authors have no competing conflicts of interest, financial or otherwise, to declare. The principal author, Gerald Liew, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
This work was supported by the Australian National Health & Medical Research Council, Canberra Australia (Grant nos. 153948, 302068, 974159, and 211069), Australian RADGAC grant (1992–94), and the National Eye Institute (Grant no. EY015810).
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P: Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 122: 477–485, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Rattner A, Nathans J: Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci 7: 860–872, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D, Sarnak MJ, Weiner DE: Cardiovascular disease and subsequent kidney disease. Arch Intern Med 167: 1130–1136, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Ejerblad E, Fored CM, Lindblad P, Fryzek J, Dickman PW, Elinder CG, McLaughlin JK, Nyren O: Association between smoking and chronic renal failure in a nationwide population-based case-control study. J Am Soc Nephrol 15: 2178–2185, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Freedman BI, DuBose TD Jr: Chronic kidney disease: cause and consequence of cardiovascular disease. Arch Intern Med 167: 1113–1115, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Peto T, Bird A, VanNewkirk MR: The epidemiology of age-related macular degeneration. Am J Ophthalmol 137: 486–495, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Friedman E: The role of the atherosclerotic process in the pathogenesis of age-related macular degeneration. Am J Ophthalmol 130: 658–663, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Snow KK, Seddon JM: Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol 6: 125–143, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Vidt DG: Inflammation in renal disease. Am J Cardiol 97: 20A–27A, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N: Association between C-reactive protein and age-related macular degeneration. JAMA 291: 704–710, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Zipfel PF, Heinen S, Jozsi M, Skerka C: Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol Immunol 43: 97–106, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Despriet DDG, Klaver CCW, Witteman JCM, Bergen AAB, Kardys I, de Maat MPM, Boekhoorn SS, Vingerling JR, Hofman A, Oostra BA, Uitterlinden AG, Stijnen T, van Duijn CM, de Jong PTVM: Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA 296: 301–309, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Dragon-Durey MA, Fremeaux-Bacchi V, Loirat C, Blouin J, Niaudet P, Deschenes G, Coppo P, Herman FW, Weiss L: Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol 15: 787–795, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, Armbrecht AM, Dhillon B, Deary IJ, Redmond E, Bird AC, Moore AT: Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med 357: 553–561, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Rambausek M, Rauterberg EW, Waldherr R, Demaine A, Krupp G, Ritz E: Evolution of IgA glomerulonephritis: relation to morphology, immunogenetics, and BP. Semin Nephrol 7: 370–373, 1987 [PubMed] [Google Scholar]
- 17.Hsu CC, Kao WH, Coresh J, Pankow JS, Marsh-Manzi J, Boerwinkle E, Bray MS: Apolipoprotein E and progression of chronic kidney disease. JAMA 293: 2892–2899, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Schmidt S, Klaver C, Saunders A, Postel E, De La Paz M, Agarwal A, Small K, Udar N, Ong J, Chalukya M, Nesburn A, Kenney C, Domurath R, Hogan M, Mah T, Conley Y, Ferrell R, Weeks D, de Jong PT, van Duijn C, Haines J, Pericak-Vance M, Gorin M: A pooled case-control study of the apolipoprotein E (APOE) gene in age-related maculopathy. Ophthalmic Genet 23: 209–223, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Matejas V, Al Gazali L, Amirlak I, Zenker M: A syndrome comprising childhood-onset glomerular kidney disease and ocular abnormalities with progressive loss of vision is caused by mutated LAMB2. Nephrol Dial Transplant 21: 3283–3286, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Abrera-Abeleda MA, Nishimura C, Smith JL, Sethi S, McRae JL, Murphy BF, Silvestri G, Skerka C, Jozsi M, Zipfel PF, Hageman GS, Smith RJ: Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease). J Med Genet 43: 582–589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appel GB, Cook HT, Hageman G, Jennette JC, Kashgarian M, Kirschfink M, Lambris JD, Lanning L, Lutz HU, Meri S, Rose NR, Salant DJ, Sethi S, Smith RJ, Smoyer W, Tully HF, Tully SP, Walker P, Welsh M, Wurzner R, Zipfel PF: Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol 16: 1392–1403, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Shankar A, Mitchell P, Rochtchina E, Tan J, Wang JJ: Association between circulating white blood cell count and long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Am J Epidemiol 165: 375–382, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Smith W, Mitchell P, Leeder SR: Smoking and age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol 114: 1518–1523, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Wang JJ, Rochtchina E, Lee AJ, Chia EM, Smith W, Cumming RG, Mitchell P: Ten-year incidence and progression of age-related maculopathy: the blue Mountains Eye Study. Ophthalmology 114: 92–98, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Cumming RG, Mitchell P, Leeder SR: Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med 337: 8–14, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Shankar A, Wang JJ, Rochtchina E, Yu MC, Kefford R, Mitchell P: Association between circulating white blood cell count and cancer mortality: a population-based cohort study. Arch Intern Med 166: 188–194, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G: National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Jones GRD, Lim E: The National Kidney Foundation Guideline on estimation of the glomerular filtration rate. Clin Biochem Rev 24: 95–98, 2003 [Google Scholar]
- 29.Bostom AG, Kronenberg F, Ritz E: Predictive performance of renal function equations for patients with chronic kidney disease and normal serum creatinine levels. J Am Soc Nephrol 13: 2140–2144, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Davis M, Magli Y, Segal P, Klein B, Hubbard L: The Wisconsin age-related maculopathy grading system. Ophthalmology 98: 1128–1134, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Yu KF: What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280: 1690–1691, 1998 [DOI] [PubMed] [Google Scholar]


