Abstract
Bone morphogenetic protein (BMP) 4 exerts multiple biological effects on kidney and ureter development. To examine the role of BMP4 in glomerular morphogenesis, we generated transgenic mice with altered BMP4 function in podocytes by conferring tissue-specificity with the nephrin (Nphs1) promoter. At birth, Tg(Nphs1-Nog) mice, which had loss of BMP4 function in podocytes, were found to have glomerular microaneurysms, collapsed glomerular capillary tufts, enlarged Bowman's capsules, and fewer normal proximal tubules. Conversely, Tg(Nphs1-Bmp4) mice, which had increased BMP4 function in podocytes, demonstrated defects in glomerular capillary formation, but podocytes were not appreciably affected. The Tg(Nphs1-Nog) and Tg(Nphs1-Bmp4) mice shared morphological characteristics with the previously reported podocyte-specific Vegf-A over-expressing and knockout mice, respectively. Consistent with the morphological similarity, in situ hybridization revealed an intense signal for podocyte expression of Vegf in Tg(Nphs1-Nog) mice, whereas the signal was markedly suppressed in Tg(Nphs1-Bmp4) mice. However, in vitro studies with metanephroi failed to demonstrate a direct interaction between BMP4 or Noggin and VEGF in podocytes. Instead, immunostaining showed that phosphorylated Smads, the mediators of BMP signaling, are present in endothelial and/or mesangial cells, but not in podocytes, within the developing glomeruli. Therefore, this study suggests that podocyte-derived BMP plays an important role in glomerular capillary formation, perhaps by acting on non-podocyte glomerular cells in a paracrine fashion.
Bone morphogenic protein 4 (BMP4), a member of the TGF-β superfamily of secretory signaling molecules, has important regulatory functions during embryonic development that include establishment of the basic embryonic body plan and morphogenesis of individual organs.1 Bmp4 homozygous null mutant mice are lethal around gastrulation, forming little or no mesoderm.2,3 Bmp4 heterozygous (Bmp4 +/−) mice display various kidney and urinary tract anomalies, including hypo/dysplastic kidneys, ectopic ureterovesical junction and double collecting system.4,5 These phenotypes in Bmp4 +/− mice, together with findings in studies using metanephric organ culture system, indicate that BMP4 has multiple biologic functions in the early stages of kidney and urinary tract development that include inhibition of ectopic budding of the ureter,5,6 stimulation of elongation of the branching ureter, and promotion of the growth of metanephric mesenchyme. Because of the multiple abnormalities developing during the early kidney formation in BMP knockouts, little is known about the function of BMP at its late stages. It is known, in this regard, that Bmp4 and Bmp7 are highly expressed in prospective podocytes and parietal epithelial cells of developing glomeruli, suggesting that BMP may play a role in glomerulogenesis.7
We recently generated transgenic mice (Nephrin-Noggin) with podocyte-selective expression of Noggin, an inhibitor of BMP2, 4, and 7.8 Histologic analysis at 2 and 10 mo of age revealed that transgenic mice expressing Noggin mRNA develop mesangial expansion. Moreover, some of the transgenic mice with high expression of Noggin mRNA had glomerular cysts.
Because we could not establish a transgenic line from these founder mice, we generated new Nephrin-Noggin transgenic mice to examine the effect of deletion in BMP on glomerular developmental structure. In addition, we generated transgenic mice with podocyte-selective overexpression of BMP4 (Nephrin-Bmp4). Examination of the phenotype in these transgenic mice revealed critical importance of a tight dosage regulation of podocyte BMP4 for the normal development of glomerular capillary tufts.
RESULTS
Glomerular Cysts in Bmp4 +/− Mice
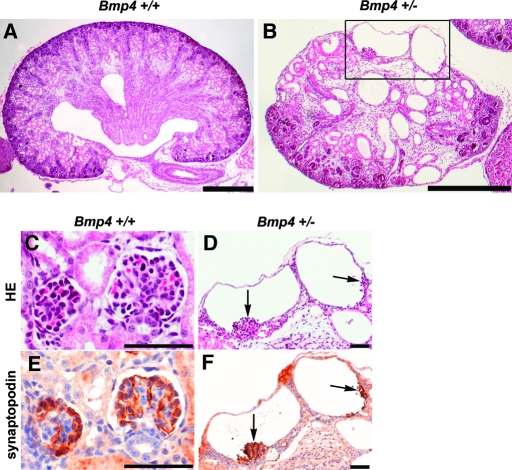
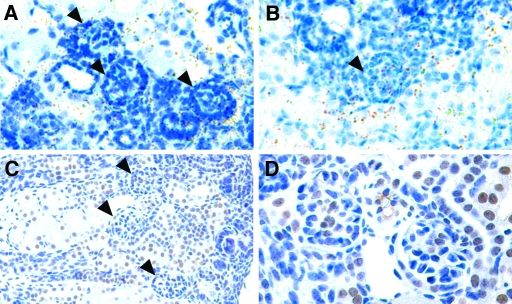
We previously reported that some Bmp4 +/− mice develop small multicystic dysplastic kidneys containing regions devoid of nephrogenic components as well as regions filled with multiple cysts and stromal mesenchymal cells.6 In this study, examination of the renal parenchyma (Figure 1) revealed that some cysts in Bmp4 +/− mice contained collapsed capillary tufts (Figure 1D). Furthermore, immunostaining found that the tufts were positive for synaptopodin (Figure 1F), a podocyte-specific protein. These observations suggest that at least some cysts are derived from glomeruli.
Figure 1.
Multicystic dysplastic kidney in Bmp4 +/− mice at birth. (A and B) Sagittal sections of kidneys from Bmp4 +/+ (A) and Bmp4 +/− (B) mice at birth (hematoxylin and eosin staining). Compared with wild-type kidney (A), kidney from Bmp4 +/− mice (B) was small. Areas are devoid of nephrogenic components but with cysts and stromal mesenchymal cells. (C through F) Glomerular morphology of Bmp4 +/+ (C and E) and Bmp4 +/− (D and F) mice (hematoxylin and eosin staining [C and D]) and synaptopodin staining [E and F]). D corresponds to the boxed area in B. Wild-type mice showed normal glomerular structure (C) with intense synaptopodin staining on podocytes (E). Cysts in Bmp4 +/− mice had collapsed capillary tufts (D, arrows) and stained for synaptopodin (F), indicating podocytes in the cysts. C and E, and D and F are adjacent sections. Bars = 400 μm in A and B; 50 μm in C through F.
Because the mutant allele of Bmp4 +/− mice contains an inserted lacZ gene that is transcribed by the Bmp4 promoter, we followed the lacZ expression pattern during glomerular development. LacZ staining was first observed in the presumptive podocytes and parietal epithelial cells in S-shaped bodies and became more intense at the capillary loop stage (Figure 2B). In the mature glomeruli, podocyte lacZ staining was undetectable (Figure 2, A, C, and D). Collectively, these findings indicate that transient elevation in Bmp4 promoter activity occurs in developing podocytes, thereby playing an important role in glomerulogenesis.
Figure 2.
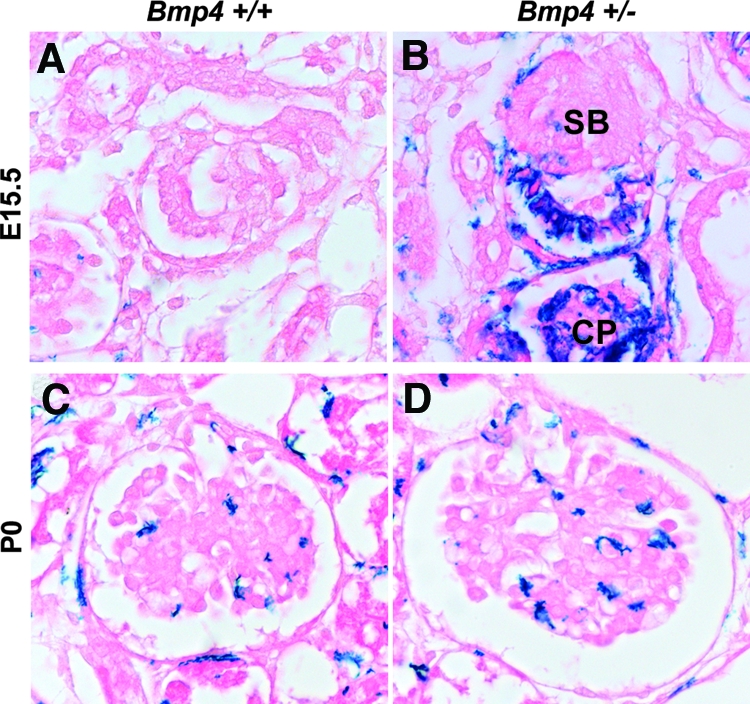
Bmp4 promoter activity during glomerular development in Bmp4 +/− mice. (A and C) Background lacZ staining in wild-type mice at E15.5 (A) or P0 (C). (B) In Bmp4 +/− mice, lacZ driven by Bmp4 promoter was first detected in future podocytes and Bowman's epithelial cells at S-shaped stage (SB) and capillary loop stage (CP) at E15.5. (D) Only background LacZ staining was detected in mature glomeruli at P0. Magnification, ×400.
Glomerular Abnormalities in Nephrin-Noggin Mice
To investigate further the role of BMP4 in glomerular development, we generated Nephrin-Noggin mice. PCR analysis of all transgenic and nontransgenic mice at birth or embryonic day 16.5 (E16.5) revealed that 21 of 137 were transgenic. In situ hybridization analysis showed that seven mice expressed intense Noggin mRNA on podocytes (Figure 3, A and B). We analyzed in detail the mice intensely expressing Noggin. At birth, the average renal volume was 5.76 ± 0.71 m3 in Nephrin-Noggin (n = 4), which was significantly smaller than that in wild-type littermates (9.34 ± 0.82 m3; n = 4). Histologic analysis revealed that 30 to 40% of glomeruli in Nephrin-Noggin mice had globally collapsed glomerular capillary tufts and dilated Bowman's capsule (Figure 4, B and D), which were similar to those observed in Bmp4 +/− (Figures 1D and 4D). Several glomeruli had microaneurysms (Figure 5, J and L). Further morphologic analyses of serial sections of these cystic glomeruli found that these glomeruli connected to rudimentary proximal tubules (Figure 4, E and F). Complementing this finding was the observation that the tubule population in Nephrin-Noggin mice was markedly and disproportionately lower when compared with the population of glomeruli (Figure 4B). Thus, glomerular density in Nephrin-Noggin (n = 4) was, on average, 18.2 ± 0.9/mm2, which was significantly higher than that in wild-type littermates (14.3 ± 0.9/mm2, n = 4).
Figure 3.
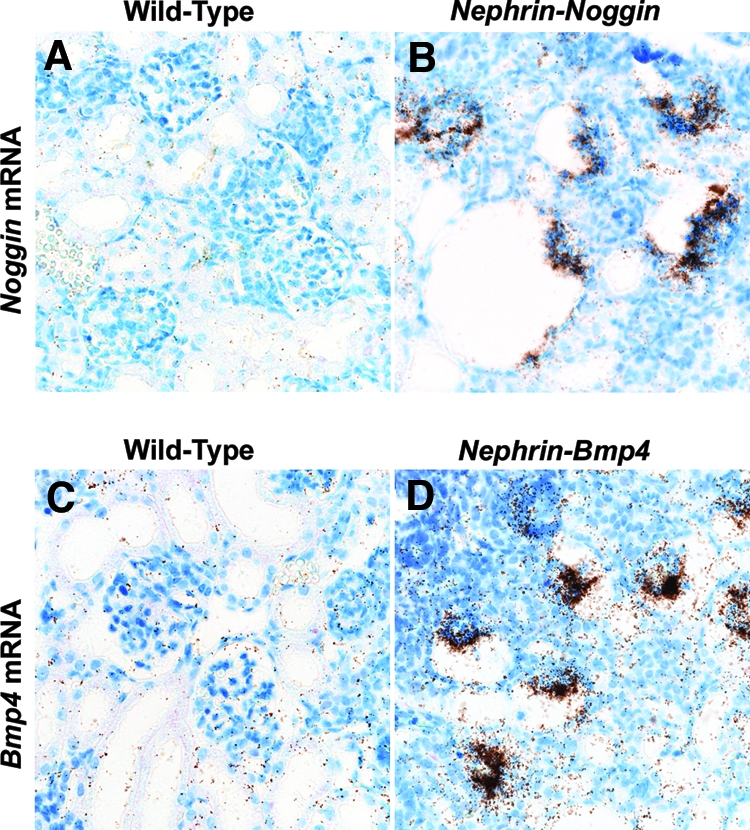
Podocyte-specific transgene expression in Nephrin-Noggin and Nephrin-Bmp4 mice. (A and B) In situ hybridization for Noggin mRNA in wild-type (A) and Nephrin-Noggin (B) mice at birth. No Noggin mRNA signal was observed in wild-type mice, whereas intense Noggin mRNA signal was observed in the podocytes of Nephrin-Noggin mice. (C and D) In situ hybridization for Bmp4 mRNA in wild-type (C) and Nephrin-Bmp4 (D) mice at birth. In mature glomeruli of wild-type mice, the Bmp4 mRNA was not detected (C). In Nephrin-Bmp4 mice, intense Bmp4 mRNA was observed on podocytes (D). Magnification, ×200.
Figure 4.
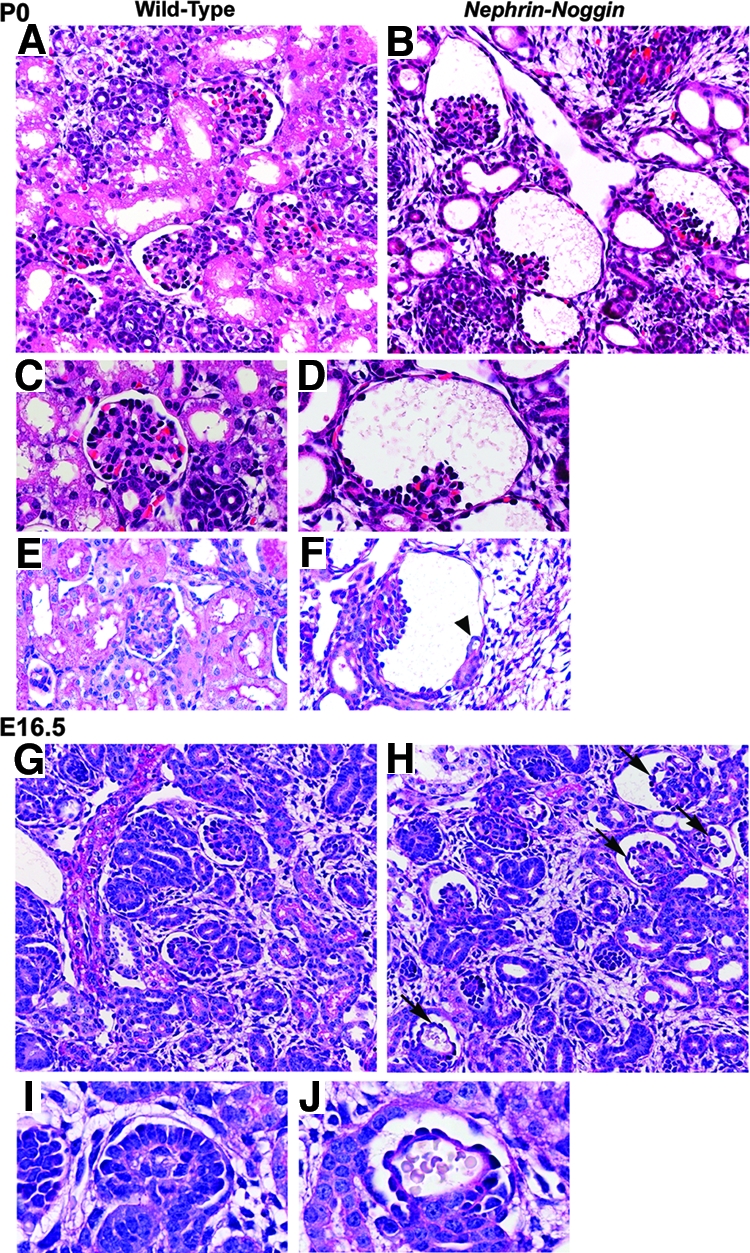
Histologic analysis of Nephrin-Noggin mice at P0 and E16.5. (A through F) Kidneys from wild-type (A, C, and E) and Nephrin-Noggin (B, D, and F) mice at P0 (periodic acid-Schiff staining). Nephrin-Noggin mice developed collapse of glomerular capillary tufts and dilation of Bowman's capsule (B, D, and F). Tubule population was decreased in Nephrin-Noggin mice (B) compared with wild-type mice (A). Some cystic glomeruli in Nephrin-Noggin mice had rudimentary tubules (arrowhead in F). (G though J) Embryonic kidneys at E16.5 from wild-type (G and I) and Nephrin-Noggin (H and J) mice (periodic acid-Schiff staining). Overall structure of the kidney was comparable between wild-type (G) and Nephrin-Noggin (H) mice. At this stage, neither cystic glomeruli nor loss of tubules was observed in Nephrin-Noggin mice (H). Microaneurysms were formed in glomerular capillary tufts of Nephrin-Noggin mice (H and J, arrows), which were absent in wild-type mice (G and I). Magnifications: ×200 in A, B, G, and H; ×400 in C, D, E, F, I, and J.
Figure 5.
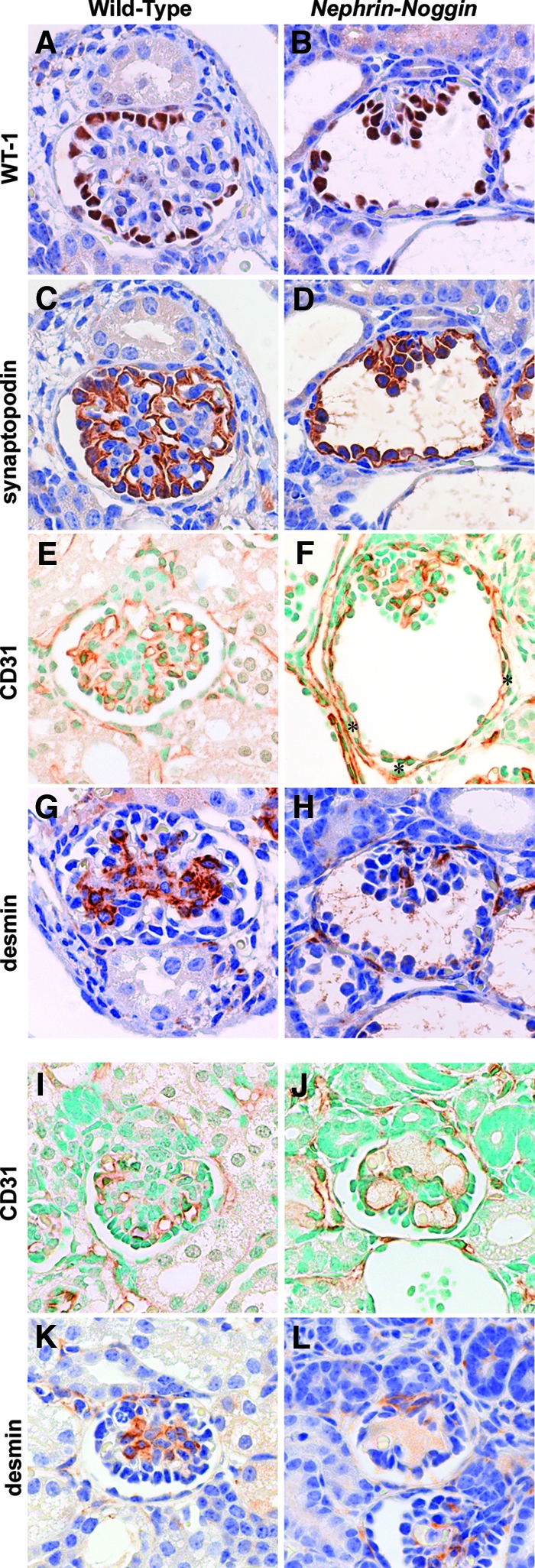
Immunohistologic analysis of Nephrin-Noggin mice. Immunostaining for WT-1 (A and B), synaptopodin (C and D), CD31 (E, F, I, and J), or desmin (G, H, K, and L) in glomeruli of wild-type (A, C, E G, I, and K) or Nephrin-Noggin (B, D, F, H, J, and L) mice at birth. (A through H) Glomeruli were from deep cortical area of kidneys of wild-type (A, C, E, and G) or Nephrin-Noggin (B, D, F, and H) mice. Glomeruli of Nephrin-Noggin mice showed cystic change with collapse of capillary tuft. In wild-type mice, intense WT-1 staining was confined to glomerular visceral epithelial cells (A), whereas it was observed in both visceral and parietal epithelial cells in Nephrin-Noggin mice (B). Similarly, synaptopodin was stained only in visceral epithelial cells in wild-type mice (C), whereas it was stained in both visceral and parietal epithelial cells in Nephrin-Noggin mice (D). Total number of WT-1–or synaptopodin-positive cells and their intensity in each cell were similar between the two strains of mice. (E) CD31 staining depicts glomerular and peritubular capillaries in wild-type mice. In Nephrin-Noggin mice, collapsed glomeruli contain CD31-positive cells. In addition, intense CD31 staining was ectopically observed just outside Bowman's capsule (F, asterisks). (G) Desmin staining depicts mesangial cells in wild-type mice. (H) The number of desmin-positive cells was markedly decreased in collapsed capillary tufts. (I through L) Glomeruli from the superficial cortex of wild-type (I and K) and Nephrin-Noggin (J and L) mice. Glomeruli of Nephrin-Noggin mice showed microaneurysms (J and H). They contained CD31-positive endothelial cells but almost no desmin-positive mesangial cells (L). Magnification, ×400.
At E16.5, although there was no appreciable glomerular cyst (Figure 4H), many glomeruli in Nephrin-Noggin mice showed microaneurysms (arrows in Figure 4, H and J), which were never observed in wild-type mice (Figure 4, A, C, G, and I). Furthermore, the tubule area in the superficial cortex was already lower in Nephrin-Noggin (0.15 ± 0.013; n = 4) than wild-type embryos (0.22 ± 0.017; n = 4).
Analysis of podocyte, endothelial, and mesangial cell populations in abnormal glomeruli with cysts or microaneurysms found that both types of abnormal glomeruli had similar or slightly fewer podocytes labeled by Wilms’ tumor 1 (WT-1; Figure 5B) or synaptopodin (Figure 5D) when compared with normal glomeruli of wild-type mice (Figure 5, A and C). Similarly, immunoreactivity of CD31, a marker of endothelial cells, was comparable between histologically abnormal glomeruli (Figure 5, F and J) and normal glomeruli (Figure 5, E and I). In contrast, mesangial cells, labeled by desmin, were remarkably decreased in glomeruli with collapsed capillary tufts (Figure 5H) or glomeruli with microaneurysms (Figure 5L). The number of desmin-positive cells in collapsed glomeruli in Nephrin-Noggin mice (n = 5) was, on average, 0.40 ± 0.17 per glomerulus, whereas it was 2.82 ± 0.51 in normal glomeruli of wild-type littermates (n = 5). These results suggest that the two abnormal phenotypes, collapse and microaneurysm, stem from a common abnormality, namely deficiency of mesangial cells.
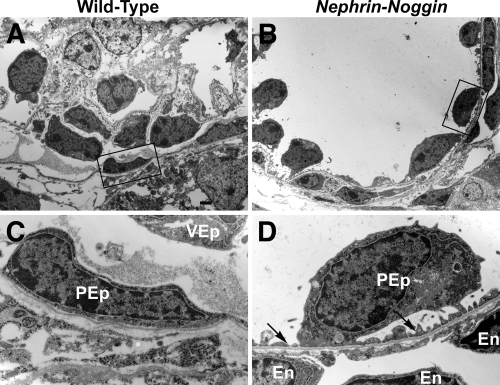
Additional immunostaining revealed other abnormalities in Nephrin-Noggin mice. In wild-type mice, WT-1 and synaptopodin signals were localized exclusively to the visceral epithelial cells (Figure 5, A and C). By contrast, distinct WT-1 and synaptopodin-positive signals were observed on Bowman's capsule in the cystic glomeruli of Nephrin-Noggin mice, suggesting abnormal localization of podocytes (Figure 5, B and D). Furthermore, CD31-positive endothelial cells were abnormally present just outside Bowman's capsule in Nephrin-Noggin mice (asterisks in Figure 5F) carrying ectopic WT-1 and synaptopodin-positive cells, a pattern never observed in wild-type mice (Figure 5, E, G, and K). These observations were further confirmed by electron microscopic analysis (Figure 6, B and D). Thus, the majority of parietal epithelial cells in cystic glomeruli had foot processes (arrows in Figure 6, A, C, and D) and were thus morphologically indistinguishable from normally localized podocytes (i.e., “parietal” podocyte).9,10 Notably, an abnormal capillary was formed just outside the basement membrane of Bowman's capsule with ectopic parietal podocytes. Because normal podocytes express intense Vegf11 and because formation and maintenance of glomerular capillary depend on podocyte-derived vascular endothelial growth factor (VEGF),11 we examined whether the podocytes on Bowman's capsule express Vegf. In situ hybridization confirmed intense expression for Vegf in these cells.
Figure 6.
Electron microscopic analysis of Nephrin-Noggin mice at birth. (A and B) Glomeruli from wild-type (A) and Nephrin-Noggin (B) mice. Boxed area in B shows parietal epithelial cells. (C and D) High-power field views. (A and C) A glomerulus from a wild-type mouse showing a normal visceral epithelial cell (VEp) and a parietal epithelial cell (PEp). Foot processes are observed only in visceral epithelial cells. A glomerulus from a Nephrin-Noggin mouse shows parietal epithelial cells with foot processes (arrows in D). An endothelial cell (En) is present just outside Bowman's capsule, which is not observed in wild-type mice (A and C). Bar = 1.25 μm.
Lack of Glomerular Endothelial and Mesangial Cells in Nephrin-Bmp4 Mice
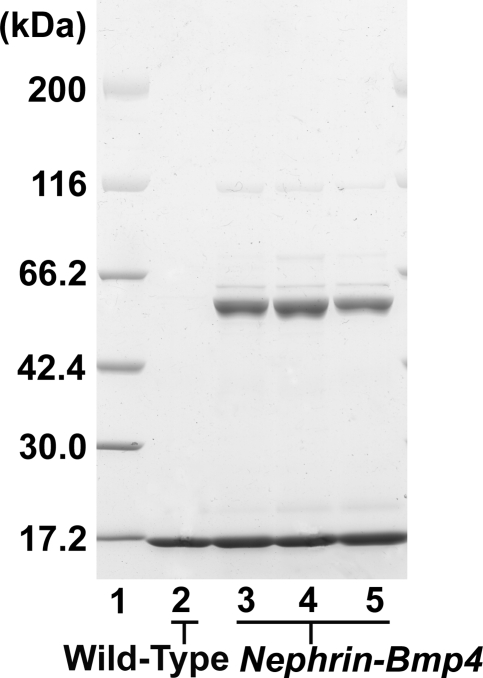
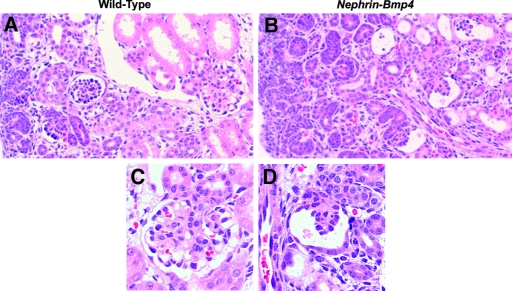
We next generated Nephrin-Bmp4 mice that overexpress Bmp4 in podocytes. Ten of 57 founder mice were found to carry the transgene. Renal biopsy was performed in the transgenic mice at 9 wk of age, and Bmp4 expression was analyzed by in situ hybridization. Bmp4 mRNA was detected in podocytes in three transgenic mice but not in the other transgenic or wild-type mice (data not shown). SDS-PAGE analysis of urine demonstrated albuminuria in these mice (Figure 7). These mice were mated with wild-type C57BL/6 mice to obtain transgenic offspring. In situ hybridization revealed that, at birth, podocytes expressed Bmp4 much more intensely in the transgenic mice (Figure 3D) than in wild-type mice (Figure 3C), although Bmp4 expression in other cells was comparable. The number of glomeruli in the Nephrin-Bmp4 mice was comparable to that in wild-type mice; however, compared with the mature glomeruli with open capillaries observed in the deep cortical areas of wild-type mice (Figure 8, A and C), the majority of glomeruli in the deep cortex of Nephrin-Bmp4 mice were small and almost completely devoid of capillary lumen (Figure 8, B and D), appearing as nonstructural clumps of cells in Bowman's space (Figure 8D). Immunostaining revealed that most of the cells in glomeruli of Nephrin-Bmp4 mice were WT-1–positive podocytes (Figure 9B) but contained almost no CD31-positive endothelial cells (Figure 9D) or desmin-positive mesangial cells (Figure 9, A, C, E, and F).
Figure 7.
SDS-PAGE analysis of urine from Nephrin-Bmp4 mice at 9 wk of age. Lane 1, molecular weight markers; lane 2, urine from a wild-type female mouse; lanes 3 through 5, urine from three Nephrin-Bmp4 founder mice. Nephrin-Bmp4 mice showed albuminuria.
Figure 8.
Histologic analysis of Nephrin-Bmp4 mice at birth.. (A and C) In wild-type mice, mature glomeruli were observed in deep cortical area. (B and D) In Nephrin-Bmp4 mice, development of glomerular capillary tuft was markedly impaired in the majority of glomeruli in deep cortical area with no or few identifiable capillary lumen (hematoxylin and eosin staining). Magnification: ×200 in A and B; ×400 in C and D.
Figure 9.
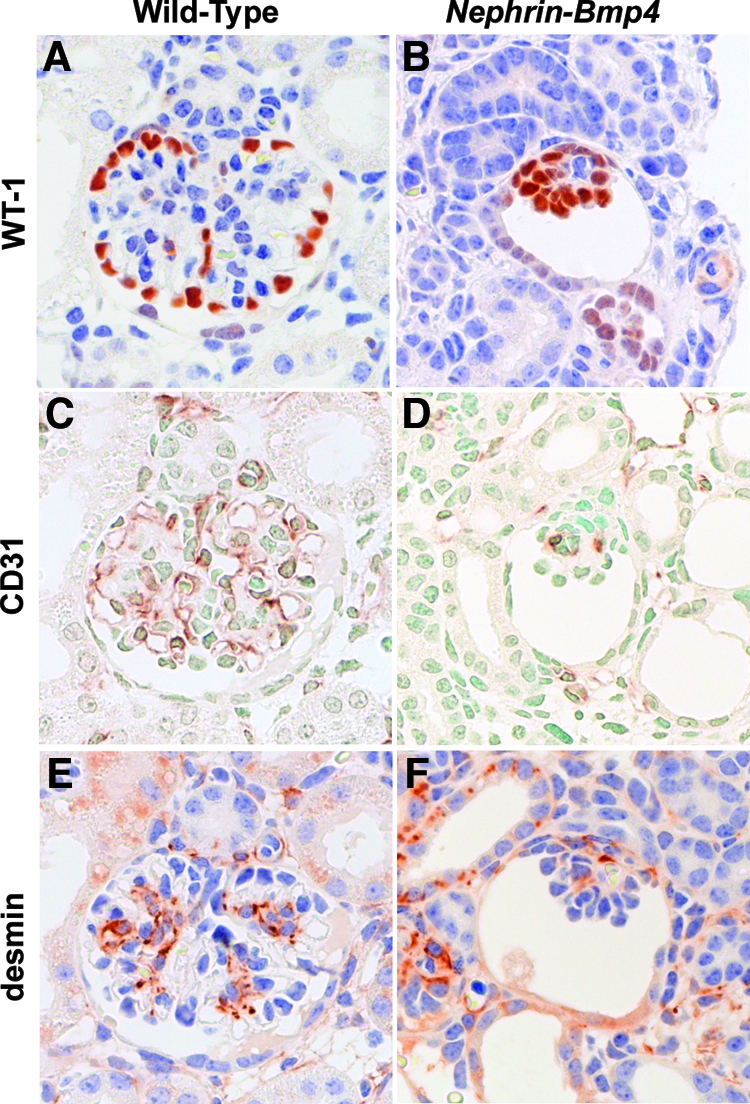
Immunohistologic analysis of Nephrin-Bmp4 mice. Immunostaining for WT-1 (A and B), CD31 (C and D), or desmin (E and F) in glomeruli from wild-type (A, C, and E) and Nephrin-Bmp4 (B, D, and F) mice. Most cells in the abnormal glomeruli of Nephrin-Bmp4 mice were WT-1–positive cells (B). Only a few CD31-positive cells (D) or desmin-positive cells (F) were present in glomeruli from Nephrin-Bmp4 mice. Magnification, 400.
Dysregulated Vegf Expression on Podocytes in Nephrin-Bmp4 Mice
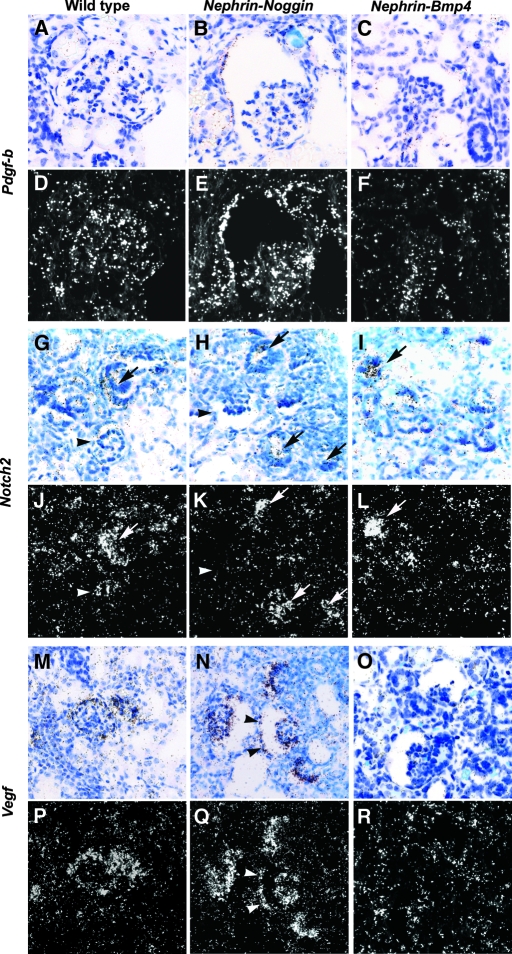
Results from the two types of transgenic mice suggest that a tightly regulated BMP dosage in glomeruli is essential for normal development of glomerular capillary tufts. Other molecules have been reported to be important in glomerular capillary tuft formation. Thus, Pdgf-b or Notch2 null mutants were shown to have microaneurysms similar to Nephrin-Noggin mice.12–15 In our study, in situ hybridization at birth revealed that Pdgf-b (Figure 10, B and E) and Notch2 (Figure 10, H and K) mRNA in glomeruli of Nephrin-Noggin mice were comparable to those in wild-type mice (Figure 10, A, D, G, and J). Nephrin-Bmp4 mice seemed to show decreased Pdgf-b mRNA as a result of a lack of endothelial cells (Figure 10, C and F) and comparable Notch2 mRNA (Figure 10, I and L). In this connection, transgenic mice overexpressing Vegf on podocytes also showed collapsed glomerular capillary tufts similar to those seen in Nephrin-Noggin.11 Furthermore, mice deficient of Vegf in podocytes showed glomeruli that lacked endothelial and mesangial cells similar to those seen in Nephrin-Bmp4 mice.11 In situ hybridization in this study revealed that, whereas Vegf mRNA was not appreciably changed in the podocytes of Nephrin-Noggin mice (Figure 10, N and Q), Nephrin-Bmp4 mice seemed to have decreased expression (Figure 10, O and R), when compared with wild-type mice (Figure 10, M and P).
Figure 10.
In situ hybridization study for Pdgf-b, Notch2, and Vegf in wild-type, Nephrin-Noggin, and Nephrin-Bmp4 mice. Renal mRNA for Pdgf-b (A through F), Notch2 (G through L), and Vegf (M through R) were analyzed in wild-type (A, D, G, J, M, and P), Nephrin-Noggin (B, E, H, K, N, and Q), and Nephrin-Bmp4 (C, F, I, L, O, and R) mice at birth. Bright field (A through C, G through I, and M through O) and dark field (D through F, J through L, and P through R) images are shown. The level of Pdgf-b mRNA in glomerulus was comparable between wild-type (A and D) and Nephrin-Noggin (B and E) mice. In Nephrin-Bmp4 (C and F), Pdgf-b mRNA was decreased. In wild-type mice, comma-shaped bodies (arrows in G and J) but not glomeruli in later stages (arrowheads in G and J) intensely expressed Notch2 mRNA. In both Nephrin-Noggin (H and K) and Nephrin-Bmp4 (I and L) mice, the Notch2 expression pattern was comparable to that in wild-type mice (G and J). Intense Vegf mRNA was observed in podocytes of wild-type mice (M and P). Similar or somewhat more intense Vegf signal was observed in both visceral and parietal (arrowheads) epithelial cells of Nephrin-Noggin mice (N and Q). In Nephrin-Bmp4, Vegf mRNA was markedly suppressed (O and R). Magnification, ×200.
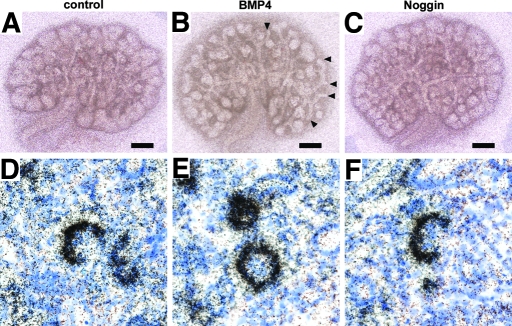
The findings in transgenic mice raised the possibility that BMP4's acting on developing podocytes may directly regulate the expression of Vegf in these cells. To test this hypothesis, we cultured E13.5 wild-type metanephroi and incubated them with BMP4 or Noggin for 48 h. In the control explants, rudimentary glomeruli were formed with podocytes expressing Vegf (Figure 11D). Treatment with BMP4 or Noggin revealed that there was no appreciable difference in the intensity of Vegf mRNA signal in podocytes among control, BMP4-treated, and Noggin-treated explants, as revealed by in situ hybridization (Figure 11, E and F). Of note, under this experimental condition, exogenous BMP4 inhibited the condensation of metanephric mesenchyme around the tip of ureteric buds (Figure 11B) as reported previously,6,16 whereas exogenous noggin caused no appreciable change in the gross morphology of explants (Figure 11, A and C).
Figure 11.
Gross morphology and Vegf expression in podocytes of E13.5 metanephric explants treated with BMP4 or Noggin. Metanephroi harvested from E13.5 wild-type mice were treated with vehicle (A and D), BMP4 (B and E), or Noggin (C and F) for 48 h. (B) The metanephros treated with BMP4 developed poor expansion of condensed mesenchyme, leading to lack of mesenchyme around some tips of ureteric buds (arrowheads). (C) The explant treated with Noggin was morphologically indistinguishable from the control explant (A). (D through F) Bright field images of Vegf in situ hybridization are shown. The expression level of Vegf was comparable among the control (D), BMP4-treated (E), and Noggin- treated explants (F). Bar = 300 μm in A through C. Magnification, ×200 in D through F.
Localization of Phosphorylated Smad in the Kidney at Birth
Two Bmp type I receptor genes, Alk3 and Alk6, which encode proteins that can bind BMP4 in vitro, are also expressed during kidney and urinary tract development.8 We then performed in situ hybridization in newborn kidney tissues with probes for Alk3 and Alk6. In that, we confirmed that Alk3 is expressed within developing glomeruli (Figure 12A). The signal for Alk6 was at near-background level (Figure 12B); however, we could not determine which cells within developing glomeruli expressed Alk3 because of poor resolution.
Figure 12.
Expression of Alk3, Alk6, and phosphorylated Smad in a developing kidney at birth. In situ hybridization for Alk3 (A) and Alk6 (B) and immunostaining with phospho-Smad1/Smad5/Smad8 antibody in a kidney tissue from a wild-type mouse at birth (C and D). Alk3 (arrowheads in A) mRNA was detected in developing glomeruli. The signal for Alk6 was at near-background level (arrowheads in B). Phospho-Smad–positive cells were found in proximal tubules (C) and the developing glomeruli (arrowheads in C). High-power field image of glomeruli revealed that positive cells were located in the center (D) but not in the peripheral area of the glomerular capillary tuft. Magnifications: ×200 in A through C; ×400 in D.
It is widely known that BMP activates the Smad pathway, following its binding to the receptors. Thus, BMP4 and BMP7 phosphorylate Smad1, 5, and 8, which, in turn, move into the nucleus to regulate the transcription of target genes.17 We therefore performed immunohistochemistry for phosphorylated Smad1/Smad5/Smad8 to examine in which cells BMP signaling is activated.
In developing glomeruli, positive cells were located in the center but not in the peripheral area of the glomerular capillary tuft (Figure 12D), suggesting that the phosphorylated Smad are present in mesangial and/or endothelial cells but not in podocytes during the development of glomerulus. Many positive cells were also observed in developing tubules (Figure 12C).
DISCUSSION
Dysregulation of Bmp4 in developing podocytes resulted in abnormal glomerular capillary tuft formation. Thus, Nephrin-Noggin mice deficient in BMP in podocytes developed glomerular microaneurysms, lacked mesangial but not endothelial cells, and, later, developed collapse of glomerular capillary tufts. Nephrin-Bmp4 mice with overexpression of podocyte BMP4 developed glomeruli lacking both endothelial and mesangial cells. In this study, the possibility remains that lack of BMP7 is also involved in the formation of the phenotypes seen in the Nephrin-Noggin mice. Whereas Bmp2 is not expressed, Bmp7 is expressed in podocytes in a manner similar to Bmp4. Of note, it was reported that there is distinct functional redundancy between Bmp4 and Bmp7 during kidney development.18 It is conceivable, therefore, that BMP4 and BMP7 share common functions during glomerular development.
In Nephrin-Noggin mice, formation of microaneurysms in glomerular capillary tufts was observed as the earliest detectable phenotype, which was morphologically similar to that found in Pdgf-b or Notch2 mutant mice.12,13 Notably, however, we found that the expression pattern of Pdgf-b or Notch2 was comparable between the wild-type and Nephrin-Noggin mice. In Nephrin-Bmp4 mice, the expression level of Pdgf-b in glomeruli seemed to be decreased, most likely reflecting the loss of endothelial cells in these mice because Pdgf-b is normally expressed in endothelial cells.19
Among the various molecules implicated in the regulation of glomerular capillary formation, Vegf mRNA seemed to be decreased in Nephrin-Bmp4 mice and intense Vegf expression remained in Nephrin-Noggin mice, although in situ hybridization, by nature, was not quantitative. Moreover, a striking phenotypic similarity was observed between Nephrin-Noggin–and Vegf-overexpressing mice and between Nephrin-Bmp4 and Vegf null mutant mice. Thus, podocyte-specific Vegf null mutant mice developed glomeruli lacking endothelial and mesangial cells similarly to Nephrin-Bmp4 mice.11 Podocyte-specific overexpression of Vegf led to collapsed glomerular capillary tufts with decreased number of mesangial cells, similar to Nephrin-Noggin mice. These findings seemed to suggest that the abnormal phenotypes observed in Nephrin-Noggin and Nephrin-Bmp4 mice may be caused by Vegf upregulation and downregulation, respectively; however, there were some differences between Bmp transgenic mice and Vegf transgenic mice. Some Nephrin-Bmp4 mice were alive at birth, unlike Vegf null mutant mice. It is interesting that a report described that BMP4 regulates Vegf transcription in angioblasts of zebrafish.20 This report, together with our results, intrigued us to speculate that BMP4 may directly inhibit transcription of Vegf in developing podocytes; however, our studies using cultured mouse metanephroi indicate that BMP4 does not significantly affect Vegf mRNA in podocytes. It is possible that the in vitro experimental systems, lacking physiologic blood flow and oxygen supply, may not represent in vivo circumstances. In addition, this study did not verify that the podocytes used in this experiment express the receptor for BMP4 or that BMP4 indeed activates the signaling pathway (i.e., phosphorylation of Smad). Nonetheless, these results do not support the notion that BMP4 is a direct transcriptional regulator of Vegf in podocytes. In this regard, immunostaining for phosphorylated Smad revealed that the BMP signaling can be activated in endothelial and/or mesangial cells but not in podocytes (Figure 12). Taken together, we postulate that BMP4 acts as a paracrine factor acting on the precursor cells of the glomerular capillary tuft. Another striking phenotype of the Nephrin-Noggin mice was that podocytes, identified by expressions of WT-1 and synaptopodin and formation of foot processes, were present on Bowman's capsule (i.e., in parietal podocytes).9,10 Similar to normal podocytes, these parietal podocytes expressed intense Vegf. Moreover, an abnormal capillary network was formed in proximity to the parietal podocytes across the basement membrane of Bowman's capsule. The precise mechanism for the development of parietal podocytes is not clear but raises an intriguing possibility that BMP4 determines the location of the boundary between podocytes and parietal epithelial cells.
The Nephrin-Noggin mice showed glomerular cystic change and decreased tubular population. The cystic glomeruli had only rudimentary tubules. This indicates that glomerular cystic changes were secondary to loss of the proximal tubules; however, it remains unclear how forced expression of Noggin in podocytes leads to the loss of proximal tubules. The possibility includes that the abnormal periglomerular capillary network hinders formation or perfusion of peritubular capillaries in Nephrin-Noggin mice. Alternatively, Noggin released from podocytes reaches the neighboring proximal tubule cells, where it inhibits survival of proximal tubular cells supported by BMP. Indeed, immunohistochemistry for phosphorylated Smad revealed that the BMP signaling is also activated during the development of proximal tubules (Figure 12).
Finally, whereas a striking phenotypic similarity exists as mentioned already, it is noteworthy that there are some obvious differences between Nephrin-Bmp4 and podocyte-specific Vegf null mutant mice and also between Nephrin-Noggin–and Vegf-overexpressing mice. For example, in contrast to Vegf knockouts, Nephrin-Bmp4 mice are alive and fertile. The difference is likely due to the less severe kidney phenotype in Nephrin-Bmp4 mice, where a portion of glomeruli seemed normal. In regard to Nephrin-Noggin mice, dilation of Bowman's space and parietal podocytes were present, which were not reported in Vegf-overexpressing mice.11
In summary, studies of two types of transgenic mouse carrying gain or loss of BMP function selectively in podocytes revealed that BMP expressed in developing podocytes play an essential role in the formation of normal glomeruli.
Concise Methods
Generation of Nephrin-Noggin and Nephrin-Bmp4 Transgenic Mice
The transgene construct for Nephrin-Noggin was reported previously.8 An IMAGE clone containing 1.6 kb of mouse Bmp4 cDNA (GenBank accession no. BC034053) was obtained from Invitrogen (Carlsbad, CA). The Bmp4 cDNA fragment was combined with a 5.4-kb Nephrin promoter fragment (Cite), internal ribosomal entry site, enhanced green fluorescence protein, and the polyadenylation signal from SV40. More than 400 and 200 fertilized eggs obtained from mating between C57BL/6 and DBF1 mice were injected with Nephrin-Noggin and Nephrin-Bmp4 transgene, respectively. The transgenic mice were identified by PCR for the enhanced green fluorescence protein gene on tail DNA using the following primers: 5′-GAAGCAGCACGACTTCTTCAAGTC-3′ and 5′-TGGCGGATCTTGAAGTTCACCTTG-3′.
Bmp4 +/− Mice
Bmp4 +/− mice were maintained by backcrossing to wild-type C57BL/6 mice.3 The mutant gene was identified by PCR using the following primers: Neo1, 5′-TCCTGCCGAGAAAGTATCCATCAT-3′ and neo2 5′-GTAAAGCACGAGGAAGCGGTCAGC-3′.
SDS-PAGE Analysis of Urine
Twenty-four-hour urine was collected from wild-type and Nephrin-Bmp4 mice at 9 wk of age using metabolic cages. Two microliters of urine sample was separated by 5 to 10% gradient SDS-PAGE gel.
Histologic Analysis
Kidneys were dissected from each of the three types of mice at E16.5 and at birth, fixed in 4% buffered paraformaldehyde (PFA), embedded in paraffin, and sectioned. The sections were stained with hematoxylin and eosin and/or periodic acid-Schiff and were used for in situ hybridization and immunostaining.
Transmission Electron Microscopy
For electron microscopy, renal tissues were fixed in 2.5% glutaraldehyde/0.05 M phosphate buffer, further fixed in 1% OsO4/0.05 M phosphate buffer, and dehydrated in serial graded ethanol and acetone.
β-Galactosidase Staining for Bmp4 +/− Mice
Whole Bmp4 +/− embryos at E15.5 or kidneys dissected from Bmp4 +/− mice at P0 were fixed in 4% buffered PFA at 4°C for 15 min; rinsed in PBS (pH 7.4) containing 0.02% Nonidet P-40 and 0.01% Na-deoxycholate three times for 15 min; and then stained in PBS (pH 7.4) containing 2 mM MgCl2, 0.02% Nonidet P-40, 0.01% Na-deoxycholate, 20 mM Tris-HCl (pH 7.4), 5 mM K3Fe[CN]6, 5 mM K4Fe[CN]6, and 1 mg/ml Bluo-Gal (Invitrogen) at 37°C for 24 h. After staining, tissues were fixed again in 4% buffered PFA for 12 h at 4°C, embedded in paraffin, sectioned at 3-μm, and counterstained with eosin.
Immunohistochemistry
The following primary antibodies were used: Mouse monoclonal anti-rat synaptopodin antibody (Progen, Heidelberg, Germany), rabbit anti-human WT-1 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-human Desmin (1:50; DAKO, Glostrup, Denmark), rat monoclonal anti-mouse CD31 (1:50; BD PharMingen, San Diego, CA), and rabbit anti–phospho-Smad1/Smad5/Smad8 antibody (1:200; Cell Signaling Technology, Danvers, MA). For synaptopodin, WT-1, desmin, and phospho-Smad1/Smad5/Smad8 staining, tissue sections were microwaved. For CD31 staining, sections were digested with protease XXV (NeoMarkers, Fremont, CA) at 37°C for 10 min.
Morphometric Analysis
Maximum longitudinal sections of wild-type and transgenic kidneys were photographed, and their maximum areas were measured. Kidney volume was estimated by the product of the maximum longitudinal measurement and the dorsiventral diameter, the latter measured on a micrometric grid. Glomerular density was determined in three nonadjacent transverse sections at the hilum of each kidney, and a mean value was obtained for each kidney. Tubular area was measured in the outermost 500 μm of cortical margin on the maximum longitudinal sections for each kidney, and data were expressed as tubule area/outermost cortex area. The number of mesangial cells identified by desmin-positive staining was determined in all glomeruli in three nonadjacent transverse sections at the hilum of each kidney. The mean value was calculated for each kidney.
In Situ Hybridization
The partial cDNA for mouse Vegf (GenBank, M95200) that contained 196 bp of the 3′ coding region of the VEGF-188, Pdgf-b (MN011057) that contained almost full-length coding region of the PDGF-B, and Notch2 (D32210) were cloned by reverse transcription–PCR from mouse embryonic kidney RNA using the following oligonucleotides: 5′-GATGTGAATGCAGACCAAAG-3′ and 5′-GAAACAACCCTAATCTTCCCG-3′ for Vegf, 5′-CTGGGCGCTCTTCCTTCCTCT-3′ and 5′-AATAACCCTGCCCACACTCTTG-3′ for Pdgf-b, and 5′-CAATGGGTAAGAAGGCTAGGCG-3′ and 5′-GGAAAGGATGATAGGTTGGCCA-3′ for Notch2. The amplified cDNA fragments were subcloned into PCR4-TOPO or PCRII-TOPO (Invitrogen), and riboprobes were synthesized with T3, Sp6, and T7 polymerase, respectively. Riboprobes for Noggin, Bmp4, Alk3, and Alk6 were synthesized as described previously.5,8
Metanephric Organ Culture
Kidney rudiments were dissected from wild-type embryos at E13.5, placed on a 12-mm diameter 0.4-μm Nucleopore filter (Corning-Costar, Cambridge, MA), and cultured in DMEM supplemented with 20% FBS (Invitrogen) and 0.5× streptomycin/penicillin (Invitrogen) with or without 100 ng/ml recombinant human BMP4 (R&D Systems, Minneapolis, MN) or 1.0 μg/ml recombinant mouse Noggin (R&D Systems) at 37°C for 48 h. The tissues were fixed in 4% buffered PFA and subjected to in situ hybridization.
Statistical Analysis
Data were expressed as means ± SD. Statistical significance between two groups was determined using the t test. P < 0.05 was considered to be significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by a Grant-in Aid for Scientific Research of Japan Society for the Promotion of Science.
Parts of this study were presented in abstract form at the annual meetings of the American Society of Nephrology; October 29 through November 1, 2004; St. Louis, MO; and November 8 through 13, 2005; Philadelphia, PA.
We thank Dr. Valentina Kon for comments on this manuscript and Ms. Suguri Niwa, Ms. Naoko Sasaoka, Ms. Chie Sakurai, and Ms. Shiho Imai for excellent technical support.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Hogan BL: Bone morphogenetic proteins: Multifunctional regulators of vertebrate development. Genes Dev 10: 1580–1594, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Winnier G, Blessing M, Labosky PA, Hogan BL: Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9: 2105–2116, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Lawson KA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL: Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13: 424–436, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL: Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol 188: 235–247, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki Y, Oshima K, Fogo AB, HoganBL, Ichikawa I: Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105: 863–873, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyazaki Y, Oshima K, Fogo AB, Ichikawa I: Evidence that bone morphogenetic protein 4 has multiple biological functions during kidney and urinary tract development. Kidney Int 63: 835–844, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Dudley AT, Robertson EJ: Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn 208: 349–362, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki Y, Ueda H, Yokoo T, Utsunomiya Y, Kawamura T, Matsusaka T, Ichikawa I, Hosoya T: Inhibition of endogenous BMP in the glomerulus leads to mesangial matrix expansion. Biochem Biophys Res Commun 340: 681–688, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Gibson IW, Downie I, Downie TT, Han SW, More IAR, Lindop GB: A study of the vascular pole of the human glomerulus. Kidney Int 41: 211–214, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Bariety J, Mandet C, Hill GS, Bruneval P: Parietal podocytes in normal human glomeruli. J Am Soc Nephrol 17: 2770–2780, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C: Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8: 1875–1887, 1994 [DOI] [PubMed] [Google Scholar]
- 13.McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T: Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development 128: 491–502, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C: Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125: 3313–3322, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, Takemoto M, Gustafsson E, Fassler R, Betsholtz C: Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development 131: 1847–1857, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun T, Herzlinger D: Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development 134: 1967–1975, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Shi Y, Massague J: Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Oxburgh L, Dudley AT, Godin RE, Koonce CH, Islam A, Anderson DC, Bikoff EK, Robertson EJ: BMP4 substitutes for loss of BMP7 during kidney development. Dev Biol 286: 637–646, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Seifert RA, Alpers CE, Bowen-Pope DF: Expression of platelet-derived growth factor and its receptors in the developing and adult mouse kidney. Kidney Int 54: 731–746, 1998 [DOI] [PubMed] [Google Scholar]
- 20.He C, Chen X: Transcription regulation of the vegf gene by the BMP/Smad pathway in the angioblast of zebrafish embryos. Biochem Biophys Res Commun 329: 324–330, 2005 [DOI] [PubMed] [Google Scholar]