Abstract
Toll-like receptors (TLR) classically recognize pathogen-associated danger signals but are also activated via endogenous ligands. For evaluation of their role in inflammatory kidney disease, the function of TLR was analyzed in two mouse models of cryoglobulinemic membranoproliferative glomerulonephritis (MPGN; mice transgenic for thymic stromal lymphopoietin [TSLP], with or without deletion of the Fcγ receptor IIb). Expression of TLR1 through 9 and TLR11 mRNA was detectable in whole kidneys and in isolated glomeruli of wild-type mice, with TLR3 and TLR4 having the highest absolute levels of expression. TLR1, 2, and 4 were increased in TSLP transgenic mice and even higher in TSLP transgenic FcγRIIb-deficient mice. TLR5 through 9 and 11 were upregulated to similar degrees in TSLP transgenic and TSLP transgenic FcγRIIb-deficient mice. Immunohistochemical studies of nephritic glomeruli localized TLR4 protein to podocytes. Cultured podocytes also expressed TLR4, and stimulation with TLR4-specific ligands resulted in a marked induction of chemokines; this was reduced by specific knockdown of TLR4 with siRNA. Fibrinogen, a potential endogenous TLR4 ligand, was shown to induce a similar profile of chemokines. In conclusion, it was demonstrated that TLR4 is constitutively expressed by podocytes and is upregulated in MPGN, where it may mediate glomerular injury by modulating expression of chemokines; therefore, TLR4 may link podocytes with the innate immune system to mediate MPGN triggered by the deposition of immune complexes.
Cryoglobulins are immunoglobulins or complexes of immunoglobulins that circulate in the serum and precipitate in the cold and redissolve after rewarming. Cryoglobulinemia is a systemic disease with a wide spectrum of manifestations resulting from deposition of cryoglobulins in various organs.1 Up to 50% of patients with mixed cryoglobulinemia develop renal involvement.2 The typical renal manifestation of mixed cryoglobulinemia is a membranoproliferative glomerulonephritis (MPGN) induced by deposits of immune complexes in glomerular capillary walls and mesangial areas, monocyte/macrophage infiltration, and sometimes intracapillary “thrombi” composed of cryoprecipitable immune complexes.3 The pathogenesis of this important form of glomerular injury is still incompletely understood.3
Toll-like receptors (TLR), sensors in the innate immune system, have been best characterized as a system to recognize danger signals to the host arising from pathogen-associated molecular patterns (e.g., microbial wall components and nucleic acids).4,5 Eleven members of the TLR family (TLR1 through TLR11) have been identified so far in mice and 10 in humans.6 Recent studies of autoimmunity suggest that activation of TLR also occurs with endogenous ligands, leading to activation of intrinsic renal cells7; hence, they are candidate mediators of inflammatory glomerular diseases. We therefore analyzed the expression pattern of TLR in a mouse model of chronic cryoglobulinemic glomerulonephritis (GN).
Mice overexpressing thymic stromal lymphopoietin (TSLP), an IL-7–like cytokine with B cell–promoting properties, form large amounts of circulating cryoglobulins of mixed IgG-IgM composition and develop a systemic inflammatory disease that involves the kidneys, lungs, liver, spleen, and skin, similar to what occurs in humans with cryoglobulinemia.8 Renal involvement in TSLP mice is characterized by a MPGN with monocyte/macrophage infiltration of glomeruli; marked mesangial extracellular matrix expansion; increased glomerular cellularity; and prominent accumulation of subendothelial, mesangial, and intracapillary immune complex deposits, closely resembling the morphologic features of human MPGN.9 Previously, this model was used to show the importance of regulation of effector leukocytes via Fcγ receptors (FcγR). One of these, FcγRIIb, protects from autoimmune injury, and FcγRIIb knockout mice transgenic for TSLP (TSLP/FcγRIIb− mice) show aggravation of MPGN compared with TSLP-transgenic mice.10 We used these animal models to demonstrate a possible and previously undescribed role for the innate immune system, via TLR4, in mediation of cryoglobulinemic MPGN.
RESULTS
Histopathology
The TSLP transgenic mice developed cryoglobulinemia, a systemic inflammatory disease involving kidneys, lungs, liver, spleen, and skin, and glomerular immune complex deposition with MPGN as described previously.9 The renal injury was uniformly present in TSLP mice at day 30 and showed a marked progression at day 120 in male mice. Glomerular lesions were further exacerbated uniformly in TSLP mice with deletion of normally inhibitory FcγRIIb (Figure 1, A through D). Electron microscopy revealed characteristic deposits of immune complexes in mesangial regions and between split glomerular capillary basement membranes, with relatively little damage to podocyte foot processes (Figure 1E).
Figure 1.
TSLP transgenic mice as a model of MPGN. (A and B) WT mice with normal renal histology at the age of 120 d (A, silver stain; B, periodic acid-Schiff [PAS] stain). (C through E) TSLP transgenic mice at the age of 120 d show typical signs of MPGN as deposition of PAS-positive material (immune deposits including the containing cryoglobulins) in peripheral capillaries and the mesangium (D, PAS stain). The glomerular tuft shows prominent increase of silver staining mesangial matrix (C, silver stain). (E) Electron micrograph of a glomerulus depicting widespread deposition of electron-dense immune complexes in mesangial areas and in subendothelial portions of glomerular capillary walls. Podocytes show focal effacement of foot processes, but many of these are preserved (arrows). There are red blood cells in the capillary lumen. (M, mesangium; D, deposits; CL, capillary lumen). Magnification, ×100.
TLR Expression in the Kidney of TSLP-Transgenic Mice and TSLP-Transgenic FcγRIIb− Mice
Quantitative reverse transcriptase–PCR (RT-PCR) to assess mRNA expression of TLR in total kidney and sieved glomeruli of male wild-type (WT) control mice (aged 30 and 120 d), TSLP mice (30 and 120 d), and TSLP/FcγRIIb− mice (120 d) revealed expression of TLR1 through TLR9 and TLR11 mRNA, in both whole kidneys and isolated glomeruli of WT mice. Absolute expression in WT kidneys was highest for TLR3 and TLR4. Other receptors, notably TLR8 and TLR9, showed only low absolute expression levels in WT mice. Comparison of TLR mRNA WT with diseased mice relative to a standard (expression of cyclophilin) revealed prominent differences in the glomerular compartment (Figure 2, Table 1). TLR 1, 2, and 4 were upregulated in mice with MPGN (three-fold increase for TLR4 in TSLP mice compared with WT mice at 30 d; a seven-fold increase was seen in TSLP/RIIb− mice; Table 1) and showed further upregulated expression in TSLP/FcγRIIb- mice. TLR5, 6, 7, 8, 9, and 11 also were upregulated in the TSLP transgenic mice, but expression was not further induced in TSLP/FcγRIIb− mice. Only TLR3 showed no significant differences between the study groups. These differences were apparent only in the isolated glomerular compartment; such changes were not apparent when mRNA from whole kidneys of WT and diseased mice was examined. Some TLR exhibited a very prominent fold change in expression relative to controls as a result of the very low absolute levels of expression at baseline to which disease mice were compared. Whereas the majority of regulated TLR showed a higher expression early during the disease (day 30 versus day 120), the expression of TLR1 and TLR11 remained high at the later time point.
Figure 2.
Glomerular TLR4 expression and regulation during MPGN in vivo. Glomeruli were isolated by sieving from (WT), TSLP, and TSLP/FcγRIIb−, respectively, at the given time points. Expression of TLR4 mRNA was analyzed by real-time PCR. At least eight mice were investigated per experimental group and time point. Relative expression values are depicted as means ± SEM.
Table 1.
Glomerular TLR mRNA expression during murine MPGN
| TLR | WT
|
TSLP
|
TSLP/FcγRIIb−
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30 D
|
120 D
|
30 D
|
120 D
|
|||||||
| Relative to Cyclophilin (%) | Relative to WT Mice at 30 D | Relative to Cyclophilin (%) | Relative to WT Mice at 30 D | Relative to Cyclophilin (%) | Relative to WT Mice at 30 D | Relative to Cyclophilin (%) | Relative to WT Mice at 30 D | Relative to Cyclophilin (%) | Relative to WT Mice at 30 D | |
| TLR1 | 0.49 (±0.30) | 1 | 0.39 (±0.04) | 0.80 | 3.30 (±1.50) | 6.70 | 3.01 (±1.12) | 6.11 | 4.77 (±1.43) | 9.70 |
| TLR2 | 0.54 (±0.04) | 1 | 0.58 (±0.04) | 1.07 | 3.13 (±1.28) | 5.80 | 2.07 (±0.51) | 3.83 | 4.44 (±1.24) | 8.23 |
| TLR3 | 2.92 (±0.25) | 1 | 2.66 (±0.11) | 0.91 | 3.68 (±0.61) | 1.26 | 3.68 (±0.45) | 3.47 | 4.24 (±0.55) | 1.45 |
| TLR4 | 1.25 (±0.19) | 1 | 0.94 (±0.28) | 0.75 | 3.82 (±0.71) | 3.07 | 1.36 (±0.46) | 1.09 | 8.50 (±1.52) | 6.83 |
| TLR5 | 0.20 (±0.07) | 1 | 0.18 (±0.04) | 0.90 | 4.28 (±2.30) | 21.40 | 1.81 (±0.91) | 9.05 | 1.32 (±0.55) | 6.60 |
| TLR6 | 0.22 (±0.12) | 1 | 0.19 (±0.02) | 0.86 | 3.73 (±1.67) | 16.95 | 1.67 (±0.67) | 7.59 | 2.07 (±0.79) | 9.40 |
| TLR7 | 0.87 (±0.15) | 1 | 0.75 (±0.28) | 0.86 | 3.83 (±1.98) | 4.40 | 1.79 (±0.30) | 2.06 | 2.72 (±0.45) | 3.13 |
| TLR8 | 0.03 (±0.001) | 1 | 0.10 (±0.02) | 3.33 | 2.27 (±1.44) | 75.67 | 0.81 (±0.25) | 27.00 | 0.67 (±0.22) | 22.33 |
| TLR9 | 0.06 (±0.008) | 1 | 0.23 (±0.045) | 3.83 | 4.88 (±2.93) | 81.33 | 2.56 (±0.94) | 42.67 | 0.58 (±0.16) | 9.67 |
| TLR11 | 0.07 (±0.008) | 1 | 0.28 (±0.04) | 4.00 | 2.43 (±0.04) | 34.71 | 2.32 (±0.80) | 33.14 | 0.40 (±0.09) | 5.71 |
In Vivo Expression of TLR4 by Podocytes and Tubular Epithelial Cells in Murine Kidneys
Because literature data for activation of TLR by endogenous ligands were available for TLR4 (e.g., engagement by heat-shock proteins, fibrinogen, heparan sulfate, hyaluronan, lung surfactant protein), we further focused on TLR4. Immunohistochemical studies of paraffin-embedded kidneys obtained from WT mice using specific antibodies from three different sources revealed expression of TLR4 in glomeruli (Figure 3, A and B), in a distribution most consistent with a podocyte distribution. The most discrete glomerular staining was obtained with the Zymed antibody. Distal but not proximal tubular epithelial cells also showed a specific staining pattern for TLR4, confirming a previously published in vitro study11 (data not shown).
Figure 3.
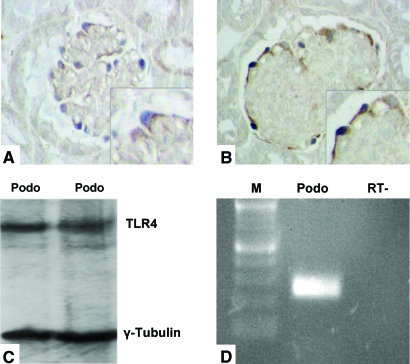
Podocytes express TLR4 in vivo and in vitro. Immunohistochemical localization of TLR4 protein in glomeruli of WT mice (A) and TSLP/FcγRIIb− mice (B). (A and B) Double staining for TLR4 (brown signal) and podocytes (identified by staining for p27kip1; blue signal). TLR4 knockout mice served as control and showed no staining for TLR4 (data not shown). (C and D) Cultured primary murine podocytes show a constitutive expression of TLR4 by Western blot analysis (C) and RT-PCR (D). Size marker (M), lane 1; podocyte total RNA (Podo), lane 2; RT− control, lane 3. Magnification, ×100.
A critical control used kidney tissue from TLR4-deficient mice, which showed no staining with these antibodies (data not shown). To localize TLR4 expression within the glomeruli to a specific cell population, we performed double immunolabeling with glomerular cell–specific markers. To identify podocytes, we used p27kip1, a cell-cycle inhibitor protein that is expressed in mature podocytes and is not detectable in mesangial or glomerular endothelial cells.12 Double immunolabeling with TLR4 and p27kip1 confirmed expression of TLR4 by podocytes (Figure 3A) and not by other glomerular cell types. Visual estimation of TLR4 expression in podocytes as a gauge of relative differences between WT and diseased mice demonstrated higher expression in TSLP mice compared with WT mice and still further increase in TSLP/FcγRIIb− mice (Figure 3B). Unfortunately, because of the lack of appropriate antibodies, convincing and reproducible staining patterns for murine TLR other than TLR4 could not be established in the tissue prepared from our mouse model.
Expression of TLR4 Protein and mRNA by Podocytes In Vitro
The immunohistochemical staining for TLR4 in normal and diseased kidney tissue in a podocyte-specific pattern led us to examine cultured mouse podocytes for expression of TLR4. To confirm expression of TLR4 by mouse podocytes, we conducted Western blot analysis with a TLR4-specific antibody and RT-PCR. Western blot analysis revealed a single band at the known size of TLR4 (approximately 95 kD),13 (Figure 3C). RT-PCR confirmed concurrent expression of TLR4 mRNA (Figure 3D).
Activation of Cultured Podocytes with the TLR4 Ligands LPS and Lipid A Induces Chemokine Expression
To demonstrate potential functionality of TLR4, we incubated cultured podocytes with LPS, a well-established exogenous TLR4 ligand. To test the specificity of TLR4 engagement, we performed parallel experiments in which podocytes were stimulated with Lipid A, which acts as the hydrophobic anchor of LPS14 and is the only part of LPS that signals through TLR4.15 RNA was prepared from cells growing under standard conditions, as well as from cells that had been stimulated with TLR4 ligands for different time intervals. By real-time RT-PCR, expression of specific chemokines was determined from both unstimulated and stimulated cells under these conditions. A strong induction of the chemokines CCL2, CCL7, CXCL1, and CXCL5 and a moderate induction of CCL3, CCL5, CXCL7, CXCL9, CXCL11, and CXCL13 could be demonstrated by stimulation of cells with both LPS and Lipid A (Figure 4).
Figure 4.
Time course of chemokine induction in podocytes upon activation of TLR4. Cultured podocytes were stimulated with LPS or Lipid A during the shown time range. Relative expression values were analyzed by real-time RT-PCR and normalized to the housekeeping gene cyclophilin. Significant differences are indicated for P < 0.05 (*) or P < 0.01 (**). At least three independent experiments were performed.
Effects of TLR4-Specific Small Interfering RNA
For demonstration that the podocyte response to LPS was TLR4 dependent, TLR4-specific small interfering RNA (siRNA) was generated to inhibit experimentally the expression of this receptor protein in cultured podocytes. Transient transfection of cultured cells with TLR4-specific siRNA resulted in a highly significant reduction of both TLR4 mRNA (Figure 5A) and TLR4 surface protein (Figure 5B). Controls were performed by using TLR9-specific siRNA, a nonsilencing siRNA, and mock transfections, each showing no significant changes in TLR4 expression (Figure 5A). Furthermore, TLR4-specific siRNA-pretreated podocytes were markedly less responsive to Lipid A as compared with control podocytes (Figure 5C). Stimulation with LPS was much less affected by siRNA pretreatment (Figure 5C), suggesting that additional TLR4-independent pathways exist for activation of podocytes by other components of LPS. Control experiments using a TLR9-specific siRNA or nonsilencing siRNA had no influence on the effects of LPS and Lipid A (data not shown).
Figure 5.
Effects of TLR4-specific siRNA on cultured podocytes. (A) Reduction of TLR4 mRNA in podocytes after transfection with TLR4-specific siRNA for 12 or 24 h (real-time RT-PCR); TLR9-specific siRNA and a nonsilencing siRNA served as controls. (B) Presence of TLR4 protein on the surface of podocytes as detected by FACS analysis. (Left) Naive cells. (Right) Cells incubated with TLR4-specific siRNA for 48 h. (C) Effect of pretreatment with TLR4-specific siRNA on chemokine induction by LPS and Lipid A (real-time RT-PCR). In A and C, significant differences are indicated for P < 0.05 (*) or P < 0.01 (**).
Fibrinogen as Potential Endogenous Ligand for Podocyte TLR4 Activation during MPGN
Because typical exogenous (i.e., infection-associated) ligands were unlikely to be relevant in this model, in which mice were kept under pathogen-free conditions, we approached identification of potential pathophysiologically relevant endogenous ligands of TLR4 in MPGN by microarray analysis of gene expression in the sieved glomeruli. This revealed the presence and/or induction of heat-shock proteins, hyaluronic acid, and fibrinogen as candidate endogenous TLR ligands (data not shown). Of several candidate molecules tested for their ability to activate cultured podocytes (e.g., heat-shock proteins, hyaluronic acid), only fibrinogen was found to activate several chemokines in a pattern similar to that of Lipid A. Pretreatment with TLR4-specific siRNA significantly reduced the fibrinogen-dependent induction of these chemokines (Figure 6A). Staining of kidney sections prepared from diseased TSLP mice revealed significant glomerular deposition of fibrinogen during the course of MPGN in vivo (Figure 6B). In control experiments, TLR9-specific siRNA had no influence on the effects of fibrinogen (data not shown).
Figure 6.
Fibrinogen activates podocytes via TLR4. (A) Fibrinogen stimulation of cultured podocytes led to an induction of chemokines that was inhibitable by transfection with TLR4-specific siRNA. Significant differences are indicated for P < 0.05 (*) or P < 0.01 (**). (B) Deposition of fibrinogen (brown signal) in glomeruli of MPGN mice shown by immunohistochemistry. Magnification, ×1000.
DISCUSSION
In this study, we introduced a potential role for the innate immune system through engagement of TLR4 in the amplification phase of cryoglobulinemic MPGN. We demonstrated that podocytes constitutively expressed TLR4, that podocyte TLR4 was upregulated in cryoglobulinemic MPGN, and that engagement of podocyte TLR4 led to release of chemokines, which may promote the local recruitment of leukocytes and amplification of glomerular injury characteristic of this model.
Podocytes are not usually thought to have a role in the mediation of inflammatory GN. Podocyte peptides may be an antigenic target in cases of membranous glomerulopathy and may be injured in a variety of noninflammatory glomerulopathies but are not recognized as having active roles in mediation of acute or chronic GN of immune complex origin; however, data are emerging that podocytes may have immunomodulatory activities that contribute to renal injury.16 Our studies showed that podocytes could have a novel proinflammatory function during the course of GN. We showed that podocytes react to immune complex–mediated damage to the glomerular filtration barrier and upregulate innate immune receptors, especially TLR4. Activation of podocyte TLR4 led to the local release of chemokines, which likely enabled recruitment of inflammatory leukocytes, which may exacerbate or ameliorate the glomerulonephritic injury. This scenario is supported by our in vitro studies showing that podocytes responded to TLR4 agonists by release of specific chemokines and that such a response could be abolished by treatment with TLR4-specific siRNA.
Our findings add to an emerging body of evidence that TLR play a role in renal inflammation and GN, usually in settings that may be triggered or augmented by infections. As examples, an aggravation of horse apoferritin–induced immune complex GN by bacterial CpG-DNA via TLR9 was previously reported.17 Studies in other models have characterized pathophysiologic roles for TLR7 and TLR9 expressed by infiltrating immune cells in the setting of infection-associated exacerbation GN and for several TLR on glomerular mesangial cells in murine lupus nephritis.18–20 TLR9-binding CpG-DNA was found to trigger the onset of diffuse proliferative lupus nephritis in MRL(lpr/lpr) mice.21 A role for renal TLR expression was also described in models of infective murine pyelonephritis.22,23 Particularly relevant to our studies in cryoglobulinemic mice is the recently described induction of TLR3 on glomerular mesangial cells in human hepatitis C–associated MPGN accompanied by locally increased mRNA expression of the chemokines CCL2 and CCL5.24 Unfortunately, suitable reagents to visualize directly TLR3 in the mouse for direct comparisons between the murine and human forms of MPGN are lacking; however, the absence of upregulated mRNA for TLR3 in nephritic mouse glomeruli of TSLP mice as detectable by RT-PCR analysis points to a potentially important difference between hepatitis C–associated human MPGN and the murine model, which does not require an infectious stimulus. Expression of TLR4 or TLR other than TLR3 has not yet been reported in human MPGN. In aggregate, these studies and our own point to multiple potential roles for TLR in the induction and evolution of GN but are too preliminary at present to permit unification of these observations into a sharply defined mechanistic process.
The physiologic function of TLR4 in podocytes is unknown, but we speculate that it may enable podocytes, by virtue of their unique location in the urinary space, to perform surveillance functions and respond to the presence of pathogens or proteins normally foreign to this space by recruitment of leukocytes. Such a function would support and extend a previous report by Reiser et al.,16 who described an induced synthesis of the immune co-stimulatory molecule B7–1 in podocytes after stimulation with LPS. Here we explicitly demonstrated expression and activation of podocyte TLR4 by both LPS and Lipid A. Whereas the effects of Lipid A could be blocked nearly completely after prestimulation of cells with TLR4-specific siRNA, LPS-induced changes were affected less prominently. These findings point to the presence of other LPS-binding receptors, such as TLR2, on podocytes. We further showed that in addition to these exogenous ligands, fibrinogen, a candidate glomerular endogenous ligand for TLR4 and known to contribute to some pathologic manifestations of acute GN, induced the TLR4-dependent expression of a variety of chemokines known to be important in immune-mediated glomerular disease. Fibrinogen was previously identified as an endogenous ligand for TLR425 and is known to contribute to pathologic manifestations of acute GN and is present in some severe types of GN at the time of TLR4 induction.26,27 Our results suggest that in glomerulonephritides in which fibrinogen is present, it may have immunomodulatory effects that are mediated through TLR4. In contrast, in the podocyte model system used here, other potential endogenous TLR4 ligands (i.e. heat-shock proteins, hyaluronic acid) did not significantly activate TLR4; however, until tested further, we remain cautious that these negative studies with endogenous TLR4 ligands other than fibrinogen may be a species-specific effect or may depend on the experimental conditions tested.
In summary, we demonstrated a novel finding that podocytes are a component of the innate immune system and that engagement of podocyte TLR4 likely leads to recruitment of inflammatory cells in glomerular injury. We recognize some caveats in assessing of the importance of TLR4 engagement in cryoglobulinemic MPGN. Critical details about the postulated series of events remain to be elucidated, such as more specific characterization of TLR4 ligands, in addition to fibrinogen, that may initiate or propagate glomerulonephritic injury and specification of downstream mediators (e.g., chemokines) of TLR4 engagement that are induced in this model in vivo. The relative contribution of TLR4 engagement versus that of other TLR that we have shown to be upregulated in diseased glomeruli also needs definition. This last characterization is well suited to future testing by the use of mouse strains deficient in various TLR and components of their downstream signaling pathways.
CONCISE METHODS
Animal Study and Experimental Design
The experimental protocol was approved by the Animal Care Committee of the University of Washington. Mice were housed under specific pathogen-free conditions. We investigated three groups of male mice: C57BL/6 WT, TSLP transgenic (TSLP) mice (in a C57/BL6 background as described previously9), and combined TSLP transgenic FcγIIb receptor knockout mice (TSLP/FcγRIIb−, on the same C57/BL6 background as described previously10). Eighteen mice per experimental group were killed at 30 d of age (i.e., early during the disease [only the WT and TSLP transgenic mice group] and at 120 d of age [all three groups]). At the end of the study, mice were anesthetized, blood was drawn by cardiac puncture, and organs were collected. Portions of renal tissue from six mice of each group were snap-frozen in liquid nitrogen for further RNA isolation, and the remaining tissue was fixed in 10% neutral buffered formalin for histology and immunohistochemistry. Renal tissue from 12 mice of each group was used for sieving of glomeruli following standard protocols.28 Paraffin-embedded kidneys obtained from TLR4-deficient mice were obtained from Dr. Kelly Smith, University of Washington, and used as a control tissue for the TLR4 antibodies.29
Tissue Preparation and Histologic Stains
Fixed tissues were processed and embedded in paraffin, then sectioned and stained with hematoxylin and eosin, periodic acid-Schiff reagent, and periodic acid silver methenamine following standard protocols.
Immunohistochemistry
Immunohistochemistry was performed as described previously.30,31 For TLR4 staining, antigen retrieval was done by steam -heating using a decloaking chamber pro (Biocare Medical, Concord, CA). For all samples, concurrent negative controls consisted of substitution of the primary antibody with irrelevant rabbit or guinea pig mAb. Three different specific TLR4 antibodies from Zymed Laboratories (Carlsbad, CA), Santa Cruz Biotechnology (Santa Cruz, CA), and H.-J. Gröne (German Cancer Research Center, Heidelberg, Germany) were used to detect TLR4 in the mouse kidney sections. A p27kip1 antibody from Labvision (Fremont, CA) was used to stain podocytes, as described previously.12 A polyclonal rabbit antibody (Dako, Carpinteria, CA) was used to stain fibrinogen.
RNA Isolation and Real-Time PCR
Sieved glomeruli were used for mRNA analysis. Extraction of total RNA was performed using the RNeasy Midi Kit (Qiagen, Valencia, CA). For cDNA synthesis, the Ambion RETROscriptTM kit (Ambion, Austin, TX) was used. Parallel to each probe, 2 μg of isolated total RNA was processed without reverse transcription to control for contaminating genomic DNA (RT− control).
Real-time RT-PCR was performed on a TaqMan ABI 7700 sequence detection system (PE Applied Biosystems, Darmstadt, Germany) as described previously.32 Cyclophilin was used as a reference gene. All water controls were negative for target and housekeeper. Sequences of TLR primers are shown in Table 2.
Table 2.
Sequences of PCR primers
| TLR | Forward Primer | Reverse Primer |
|---|---|---|
| mTLR1 | 5′-GGACCTACCCTTGCAACCAA | 5′-GGTGGCACAAGATCACCTTT |
| mTLR2 | 5′-CGCCCTTTAAGCTGTGTCTC | 5′-CGATGGAATCGATGATGTTG |
| mTLR3 | 5′-AGCATCAAAAGAAGCCGAAA | 5′-CTTGCTGAACTGCGTGATGT |
| mTLR4 | 5′-CCTGATGACATTCCTTCT | 5′-AGCCACCAGATTCTCTAA |
| mTLR5 | 5′-CTGGGGACCCAGTATGCTAA | 5′-ACAGCCGAAGTTCCAAGAGA |
| mTLR6 | 5′-ACACAATCGGTTGCAAAACA | 5′-GGAAAGTCAGCTTCGTCAGG |
| mTLR7 | 5′-ATTCCTTGCCTCCTGAGGTT | 5′-GCTGAGGTCCAAAATTTCCA |
| mTLR8 | 5′-AGTTTGCACATTCCCTGGAG | 5′-AGAGGAAGCCAGAGGGTAGG |
| mTLR9 | 5′-ACTGAGCACCCCTGCTTCTA | 5′-GGCTCAGGCTAAGACACTGG |
| mTLR11 | 5′-GGGACTTTGGGATTGGAAAT | 5′-CTAAGGCCTGTCCTGTGAGC |
Values relative to basal controls are provided as means ± SEM. Statistical analyses were performed by t test or ANOVA test, respectively. Significant differences are indicated for P < 0.05 or P < 0.01.
Protein Preparation and Western Blotting
Protein obtained frozen kidney tissue was minced and washed thoroughly in PBS, and Western blotting was performed using the Zymed anti-TLR4 antibody. The Western blotting procedures were described previously.30
Cell Culture Experiments
Immortalized mouse podocytes in culture were obtained from transgenic H-2Kb-tsA58 mice (ImmortoMouse; Jackson Laboratory, Bar Harbor, ME) as described previously and characterized.33 Experiments were performed using early passage growth-restricted, conditionally immortalized mouse podocytes previously designated as clone B6.34,35 Under growth-restricted conditions (absence of IFN-γ, culture at 37°C), proliferation was markedly reduced and cells underwent cytoskeletal rearrangement with the formation of arborizing cellular processes and expressed podocyte-specific proteins, resembling the morphologic appearance of mature differentiated podocytes in vivo. To test for functional responses, cultured podocytes were stimulated with LPS extracted from Escherichia coli serotype O55:B5 (specific activity at least 500,000 endotoxin units/mg; Sigma, Hamburg, Germany), with Lipid A prepared from Salmonella minnesota (Sigma, Hamburg, Germany), or with fibrinogen from bovine plasma (LPS-free; Sigma, Hamburg, Germany). Potential contamination of fibrinogen with LPS was also experimentally excluded by incubation with polymyxin B (which binds and inactivates any potential LPS contamination) and by addition of heat denaturation, which inactivates fibrinogen but not LPS and abolishes the observed fibrinogen effect, as reported by others25 (data not shown).
Extraction of total RNA was performed using the RNeasy Mini Kit (Qiagen, Hilden, Germany) with additional DNase digestion. Subsequent real-time PCR was performed as described previously.32
Treatment of Podocytes with siRNA
Podocytes (300,000 cells per/well, cultured in six-well plates) were incubated with 25 nM siRNA for 24 to 48 h using HiPerfect Transfection Reagent (Qiagen, Hilden, Germany) for transient transfection. Specific siRNA to silence selectively murine TLR4 or murine TLR9 as well as appropriate control siRNA (Allstars negative control) was designed and produced by Qiagen (Hilden, Germany). Fluorescence-labeled siRNA was used in pilot experiments to determine optimal transfection rates according to the manufacturer's protocol.
FACS Analysis of Cultured Cells
For FACS analysis cultured podocytes were detached with Accutase (PAA, Colbe, Germany) to preserve surface molecules. Flow cytometry was performed as described previously36 using a monoclonal anti-TLR4 antibody (clone MTS510; eBioscience, San Diego, CA) to specifically detect murine TLR4.
Gene Expression Profiling with Affymetrix U133A Arrays
Hybridization of RNA obtained from sieved glomeruli to mouse genome U133A arrays containing approximately 14,500 defined genes was carried out according to the manufacturer's instructions (Affymetrix, Santa Clara, CA). The GeneChips were scanned by a Hewlett Packard confocal laser scanner and visualized using the Affymetrix GeneChip 5.0 software.
Data Collection and Analysis
Data normalization, log transformation, statistical analysis, and pattern study were performed with the GeneSpring software. Raw data were normalized using per-chip and per-gene two-step global normalization methods to scale the expression levels around 1. We also performed logarithmic transformation of the normalized data, yielding more symmetric data for further parametric statistical analysis. Welch t test was applied for statistical comparison among TSLP, TSLP/FcγRIIb−, and control mice. P value was set to <0.01. Finally, through the NetAffx analysis center online, biologic roles of the significantly varied genes in the glomerular gene expression profiles were annotated.
DISCLOSURES
None.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (BA 2137) and the University of Regensburg (ReForM program) to B.B., the Else-Kröner-Fresenius Foundation to M.C.B., and the National Institutes of Health (DK 68802 and DK 44757) to C.E.A.
We thank Gabriele Spatar, Antje Böttinger, Lydia Wang, and Lee Wang for expert technical work.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Are Podocytes Passive or Provocative in Proteinuric Glomerular Pathology?” on pages 651–653.
M.C.B. and B.B. contributed equally to this work.
REFERENCES
- 1.Dispenzieri A, Gorevic PD: Cryoglobulinemia. Hematol Oncol Clin North Am 13: 1315–1349, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Fabrizi F, Colucci P, Ponticelli C, Locatelli F: Kidney and liver involvement in cryoglobulinemia. Semin Nephrol 22: 309–318, 2002 [PubMed] [Google Scholar]
- 3.Smith KD, Alpers CE: Pathogenic mechanisms in membranoproliferative glomerulonephritis. Curr Opin Nephrol Hypertens 14: 396–403, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Aderem A, Ulevitch RJ: Toll-like receptors in the induction of the innate immune response. Nature 406: 782–787, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Janeway C Jr: Innate immunity. N Engl J Med 343: 338–344, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Uematsu S, Takeuchi O: Pathogen recognition and innate immunity. Cell 124: 783–801, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Wagner H: Endogenous TLR ligands and autoimmunity. Adv Immunol 91: 159–173, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Levin SD, Koelling RM, Friend SL, Isaksen DE, Ziegler SF, Perlmutter RM, Farr AG: Thymic stromal lymphopoietin: A cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol 162: 677–683, 1999 [PubMed] [Google Scholar]
- 9.Taneda S, Segerer S, Hudkins KL, Cui Y, Wen M, Segerer M, Wener MH, Khairallah CG, Farr AG, Alpers CE: Cryoglobulinemic glomerulonephritis in thymic stromal lymphopoietin transgenic mice. Am J Pathol 159: 2355–2369, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mühlfeld AS, Segerer S, Hudkins K, Carling MD, Wen M, Farr AG, Ravetch JV, Alpers CE: Deletion of the fcgamma receptor IIb in thymic stromal lymphopoietin transgenic mice aggravates membranoproliferative glomerulonephritis. Am J Pathol 163: 1127–1136, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, Nagai Y, Takeuchi O, Akira S, Matsuguchi T: Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol 169: 2026–2033, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Combs HL, Shankland SJ, Setzer SV, Hudkins KL, Alpers CE: Expression of the cyclin kinase inhibitor, p27kip1, in developing and mature human kidney. Kidney Int 53: 892–896, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van ’t Veer C: In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol 168: 1286–1293, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Raetz CR, Whitfield C: Lipopolysaccharide endotoxins. Annu Rev Biochem 71: 635–700, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller SI, Ernst RK, Bader MW: LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol 3: 36–46, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P: Induction of B7–1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113: 1390–1397, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anders HJ, Banas B, Linde Y, Weller L, Cohen CD, Kretzler M, Martin S, Vielhauer V, Schlöndorff D, Gröne HJ: Bacterial CpG-DNA aggravates immune complex glomerulonephritis: Role of TLR9-mediated expression of chemokines and chemokine receptors. J Am Soc Nephrol 14: 317–326, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Anders HJ, Vielhauer V, Eis V, Linde Y, Kretzler M, Perez de Lema G, Strutz F, Bauer S, Rutz M, Wagner H, Gröne HJ, Schlöndorff D: Activation of toll-like receptor-9 induces progression of renal disease in MRL-Fas(lpr) mice. FASEB J 18: 534–536, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Patole PS, Pawar RD, Lech M, Zecher D, Schmidt H, Segerer S, Ellwart A, Henger A, Kretzler M, Anders HJ: Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Fas(lpr) mice. Nephrol Dial Transplant 21: 3062–3073, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Pawar RD, Patole PS, Zecher D, Segerer S, Kretzler M, Schlöndorff D, Anders HJ: Toll-like receptor-7 modulates immune complex glomerulonephritis. J Am Soc Nephrol 17: 141–149, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Pawar RD, Patole PS, Ellwart A, Lech M, Segerer S, Schlöndorff D, Anders HJ: Ligands to nucleic Acid-specific toll-like receptors and the onset of lupus nephritis. J Am Soc Nephrol 17: 3365–3373, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Chassin C, Goujon JM, Darche S, du Merle L, Bens M, Cluzeaud F, Werts C, Ogier-Denis E, Le Bouguenec C, Buzoni-Gatel D, Vandewalle A: Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J Immunol 177: 4773–4784, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Patole PS, Schubert S, Hildinger K, Khandoga S, Khandoga A, Segerer S, Henger A, Kretzler M, Werner M, Krombach F, Schlöndorff D, Anders HJ: Toll-like receptor-4: Renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int 68: 2582–2587, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Wörnle M, Schmid H, Banas B, Merkle M, Henger A, Roeder M, Blattner S, Bock E, Kretzler M, Gröne HJ, Schlöndorff D: Novel role of toll-like receptor 3 in hepatitis C-associated glomerulonephritis. Am J Pathol 168: 370–385, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smiley ST, King JA, Hancock WW: Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 167: 2887–2894, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Drew AF, Tucker HL, Liu H, Witte DP, Degen JL, Tipping PG: Crescentic glomerulonephritis is diminished in fibrinogen-deficient mice. Am J Physiol Renal Physiol 281: F1157–F1163, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Helfrich MH, Horton MA, Feigen LP, Lefkowith JB: Fibrinogen mediates platelet-polymorphonuclear leukocyte cooperation during immune-complex glomerulonephritis in rats. J Clin Invest 94: 928–936, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlöndorff D: Preparation and study of isolated glomeruli. Methods Enzymol 191: 130–140, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S: Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide—Evidence for TLR4 as the Lps gene product. J Immunol 162: 3749–3752, 1999 [PubMed] [Google Scholar]
- 30.Banas MC, Parks WT, Hudkins KL, Banas B, Holdren M, Iyoda M, Wietecha TA, Kowalewska J, Liu G, Alpers CE: Localization of TGF-beta signaling intermediates Smad2, 3, 4, and 7 in developing and mature human and mouse kidney. J Histochem Cytochem 55: 275–285, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Hudkins KL, Giachelli CM, Cui Y, Couser WG, Johnson RJ, Alpers CE: Osteopontin expression in fetal and mature human kidney. J Am Soc Nephrol 10: 444–457, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Banas B, Wörnle M, Berger T, Nelson PJ, Cohen CD, Kretzler M, Pfirstinger J, Mack M, Lipp M, Gröne HJ, Schlöndorff D: Roles of SLC/CCL21 and CCR7 in human kidney for mesangial proliferation, migration, apoptosis, and tissue homeostasis. J Immunol 168: 4301–4307, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Wada T, Pippin JW, Terada Y, Shankland SJ: The cyclin-dependent kinase inhibitor p21 is required for TGF-beta1-induced podocyte apoptosis. Kidney Int 68: 1618–1629, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Hiromura K, Haseley LA, Zhang P, Monkawa T, Durvasula R, Petermann AT, Alpers CE, Mundel P, Shankland SJ: Podocyte expression of the CDK-inhibitor p57 during development and disease. Kidney Int 60: 2235–2246, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Petermann A, Hiromura K, Pippin J, Blonski M, Couser WG, Kopp J, Mundel P, Shankland SJ: Differential expression of d-type cyclins in podocytes in vitro and in vivo. Am J Pathol 164: 1417–1424, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banas B, Wörnle M, Merkle M, Gonzalez-Rubio M, Schmid H, Kretzler M, Pietrzyk MC, Fink M, Perez de Lema G, Schlöndorff D: Binding of the chemokine SLC/CCL21 to its receptor CCR7 increases adhesive properties of human mesangial cells. Kidney Int 66: 2256–2263, 2004 [DOI] [PubMed] [Google Scholar]