Abstract
The epithelial sodium channel (ENaC) plays a major role in the regulation of sodium balance and BP by controlling Na+ reabsorption along the renal distal tubule and collecting duct (CD). ENaC activity is affected by extracellular nucleotides acting on P2 receptors (P2R); however, there remain uncertainties over the P2R subtype(s) involved, the molecular mechanism(s) responsible, and their physiologic role. This study investigated the relationship between apical P2R and ENaC activity by assessing the effects of P2R agonists on amiloride-sensitive current in the rat CD. Using whole-cell patch clamp of principal cells of split-open CD from Na+-restricted rats, in combination with immunohistochemistry and real-time PCR, we found that activation of metabotropic P2R (most likely the P2Y2 and/or 4 subtype), via phospholipase C, inhibited ENaC activity. In addition, activation of ionotropic P2R (most likely the P2X4 and/or 4/6 subtype), via phosphatidylinositol-3 kinase, either inhibited or potentiated ENaC activity, depending on the extracellular Na+ concentration; therefore, it is proposed that P2X4 and/or 4/6 receptors might function as apical Na+ sensors responsible for local regulation of ENaC activity in the CD and could thereby help to regulate Na+ balance and systemic BP.
ATP and other nucleotides are released from epithelial cells to activate, in an autocrine and/or paracrine manner, a variety of surface membrane receptors known as P2 receptors (P2R).1,2 According to their sequence identity and signal transduction, P2R are subdivided into two types: Ionotropic P2X (P2XR) and metabotropic P2Y (P2YR). There are seven P2XR subunits that form seven homomeric (P2X1 to 7), seven established heteromeric (P2X1/2, 1/4, 1/5, 2/3, 2/6, 4/6, and 4/7), and an additional six predicted heteromeric (P2X1/3, 1/6, 2/5, 3/5, 4/5, and 5/6) nonselective cation channel assemblies.3–5 Characterized P2XR have distinct biophysical and/or pharmacologic profiles. In addition, there are eight G protein–coupled P2YR (P2Y1, 2, 4, 6, 11, 12, 13, and 14), also with distinct pharmacologic profiles; they couple to either phospholipase C (PLC) to increase inositol triphosphate (IP3) levels or adenylyl cyclase to change cAMP levels.5
Activation of P2R can alter the activity of certain epithelial cell membrane transport proteins.1,2,6 A key transport protein shown to be affected by the activation of P2R is the amiloride-sensitive epithelial Na+ channel (ENaC).2,6–8 ENaC is expressed at the apical membrane of principal cells (PC) in the collecting duct (CD), and its activity is an important factor in the regulation of arterial BP, because it determines the extent of Na+ reabsorption from tubular fluid along the distal nephron.9,10
Given the importance of ENaC in controlling arterial BP, it is perhaps not surprising that there have been several studies on the modulation of its activity by P2R in the CD. To date, P2R-mediated modulation of ENaC activity has been demonstrated by means of electrophysiologic techniques in murine and amphibian immortalized renal CD/distal nephron cell lines7,8,11–15 and in the Xenopus oocyte heterologous expression system (using recombinant rat P2XR and ENaC proteins).16 It has also been reported in the CD of rat17 and mouse18,19 using in vivo and in vitro luminal perfusion studies, respectively. The prevailing view from these studies is that P2R inhibit ENaC activity; however, open issues remain with regard to the P2R subtype(s) involved, the membrane localization of P2R in the CD, and the intracellular mechanism(s) responsible for inhibition.
To address these issues, we investigated the relationship between the activation of apical P2R and ENaC activity in PC (in situ) in microdissected split-open rat CD, using whole-cell patch-clamp electrophysiology, in combination with real-time PCR and immunohistochemical techniques. Understanding the possible regulation of ENaC activity by P2R will extend our insight into the local and potentially “intracrine” control of renal tubular sodium transport and may also provide a novel therapeutic target for abnormal renal sodium transport in hypertension.
RESULTS
Urinary Na+ Levels
As anticipated, urinary Na+ concentrations in Na+-restricted rats were significantly less at day 10 (4.3 ± 0.9 mmol/L; n = 5) than those in Na+-replete rats (115 ± 11 mmol/L; n = 5; P < 0.01). This degree of Na+ restriction is known to enhance the expression of functional ENaC in the apical membrane of PC in the rat CD.20–22
Immunohistochemical Co-localization of P2R and ENaC Subunits
Positive immunofluorescence (localized to membrane domains and/or intracellular regions of PC and/or intercalated cells [IC]) was seen for P2X2, 4, 5, and 6 ion channel subunits and P2Y2, 4, 6, 11, 12, and 13 metabotropic receptors in the CD of Na+-replete rats (identified by positive aquaporin 2 [AQP2] staining). The same P2R subtypes were localized to the CD of Na+-restricted rats but with the addition of P2X1 and loss of P2X5 ion channel subunits (Table 1). For all P2R antibodies used, preincubation with their respective antigenic peptides abolished positive immunofluorescence (see Supplemental Data 1). Positive immunofluorescence was also seen for α-, β-, and γ-ENaC subunits in the CD of both Na+-replete and Na+-restricted rats; this immunofluorescence appeared stronger in the PC of Na+-restricted rats.
Table 1.
Comparison of immunohistochemical localization of P2R in the CD of rats maintained on a normal (0.5% NaCl) or low (0.01% NaCl) Na+ diet for 8 to 10 da
| P2R | Na+ Replete
|
Na+ Restricted
|
||||
|---|---|---|---|---|---|---|
| CCD | OMCD | IMCD | CCD | OMCD | IMCD | |
| P2X1 | − | − | − | IC | IC | a/IC |
| P2X2 | int | int | int/a | int | int | int/a |
| P2X3 | − | − | − | − | − | − |
| P2X4 | A/B | A/B | A/B | A/B | A/B | A/B |
| P2X5 | int | int | − | − | − | − |
| P2X6 | A/B | A/B | A/B | A/B | A/B | A/B |
| P2X7 | − | − | − | − | − | − |
| P2Y1 | − | − | − | − | − | − |
| P2Y2 | int | int/a/b | a/B | int | int/a/b | a/B |
| P2Y4 | A | A | A | A | A | A |
| P2Y6 | A/int | A/int | A/int | int | int | int |
| P2Y11 | A | A | A | A | A | A |
| P2Y12 | int | int | int | int | int | int |
| P2Y13 | int | int | int | int | int | int |
| P2Y14 | − | − | − | − | − | − |
AQP2 was used as a marker of CD. Kidneys were perfusion-fixed with paraformaldehyde (4%) and sliced to 8 μm thickness. Only cortical collecting duct (CCD) and outer medullary collecting duct (OMCD) tubules were used in patch-clamp and real-time PCR investigations. −, immunofluorescence (IF) not detected; A, positive IF (+IF) localized to apical membrane; a, weak +IF localized to apical membrane; B, +IF localized to basolateral membrane; b, weak +IF localized to basolateral membrane; int, +IF localized to intracellular loci; IC, +IF localized to intercalated cells and not PC; IMCD, inner medullary collecting duct.
With respect to PC only, in Na+-replete rats strong immunostaining for P2X4 and 6 and for P2Y4, 6, and 11 was consistently observed in the apical domain of PC throughout the CD, and weaker apical staining was also seen for P2X2 and P2Y2 (both localized to the medullary CD only) and α-ENaC. Strong basolateral immunostaining for P2X4 and 6 was seen in all PC; basolateral staining for P2Y2 was evident only in medullary CD PC. Punctate intracellular staining (i.e., staining that could not be confidently localized to the plasma membrane of PC) was seen for P2X2 and 5; P2Y2, 6, 12, and 13 (Table 1); and β- and γ-ENaC.
In PC from Na+-restricted rats, consistently positive β-ENaC and γ-ENaC staining became evident in the apical domain throughout the CD, and intracellular immunostaining for P2X5 disappeared. Localization and staining of all other P2X subunits was unchanged, except for the appearance of weak expression of P2X1 subunits in the inner medullary CD (Figure 1, A and B). With respect to P2YR, P2Y2, 4, 11, 12, and 13 staining was unchanged (Figure 1C) and P2Y6 showed restricted intracellular staining (see Table 1). As an incidental finding, positive P2X1 staining became evident in IC, but this was not investigated further.
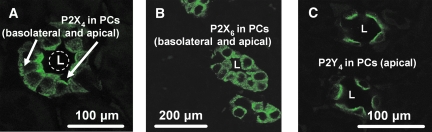
Figure 1.
Confocal images demonstrating positive immunofluorescence staining for P2R in the cortical CD of Na+-restricted rats. (A) Apical and basolateral staining (FITC) for P2X4 subunits in PC, similar to that seen for P2X6 (B). (C) Apical staining (FITC) for P2Y4 receptors in PC. In all experiments, CD were identified by positive AQP2 co-staining. L, lumen.
Expression of P2R mRNA in CD
To quantify the relative abundance of P2R mRNA in microdissected CD (i.e., PC and IC) of Na+-replete and Na+-restricted rats, we calculated a ratio of the P2R gene of interest to a constitutively expressed housekeeping gene (hypoxanthine phosphoribosyl transferase [HPRT]). No suitable primers were available for P2Y11, 12, 13, and 14 receptors, but all other P2R were assessed.
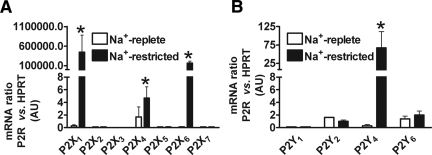
We failed to detect significant levels (i.e., >1 arbitrary unit; corresponding to <10 P2R transcripts per cell; for calculation, see Supplemental Data 2) of P2X2, 3, 5, and 7 and P2Y1 mRNA in rats maintained on either of the Na+ diets (n = 6), which broadly agrees with the immunohistochemical findings. In contrast, significant amounts of mRNA (all of a similar value [i.e., approximately 2 arbitrary units]; for calculation of number of transcripts per cell, see Supplemental Data 2) were detected for P2X4 and P2Y2 and 6 in CD of Na+-replete rats (Figure 2). CD from Na+-restricted rats showed a significant increase in abundance of P2X4 mRNA (by two-fold; n = 6; P < 0.01) but no change in P2Y2 or P2Y6 mRNA levels. Whereas P2X1 and 6 and P2Y4 mRNA levels were deemed insignificant in Na+-replete rats, Na+-restricted rats showed marked abundance of mRNA for each of these subtypes (Figure 2).
Figure 2.
Comparison of P2R mRNA levels in microdissected CD from Na+-replete and Na+-restricted rats. Data presented as a ratio between the P2R gene of interest and the constitutively expressed housekeeping gene HPRT. (A) mRNA levels for P2X1, 4, and 6 subunits were significantly increased in Na+-restricted rats (n = 6; P < 0.01). (B) mRNA levels for P2Y4 receptors were significantly increased in Na+-restricted rats (n = 6; P < 0.01).
Whole-Cell P2R-Mediated Currents
Whole-cell perforated patch-clamp recording was possible from individual PC in the rat CD because electrical coupling is very low between these cells, as reported by Frindt et al.22 As also reported by Frindt et al., the whole-cell capacitance, an electrical index of membrane area, was found to be significantly greater in PC from Na+-restricted rats (32 ± 6 pf; n = 40) than from Na+-replete rats (15 ± 4 pf; n = 40). A low-Na+ diet approximately doubles the combined surface area of a PC's apical and basolateral membranes.22
Figure 3 shows ATP- and other P2R agonist-evoked (all at 10 μM) whole-cell inward currents in voltage-clamped (holding potential [Vh] = −60 mV) PC in situ from rats maintained on a Na+-replete diet. Agonists were selected according to the P2R identified in our immunohistochemical and real-time PCR studies (see Table 2).
Figure 3.
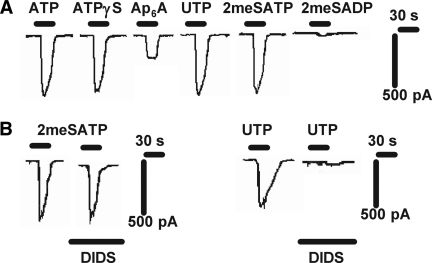
P2R agonists evoke whole-cell inward currents in voltage-clamped rat CD PC in situ. (A) Typical trace showing a series of P2R agonist-evoked (10 μM) currents. The PC was voltage-clamped at −60 mV, and 3 min was left between agonist applications. (B) Currents evoked by 2meSATP were insensitive to DIDS (100 μM; preincubated for 3 min) and shown to be cationic, consistent with P2X channels. Currents evoked by Ap6A, UTP (see B), and 2meSADP were abolished by DIDS (100 μM; preincubated for 3 min), consistent with P2Y activation of Ca2+-dependent Cl− currents.
Table 2.
Pharmacologic properties corresponding to P2R subtypes identified in rat CD PCa
| Parameter | P2X4 | P2X6 | P2X4/6 | P2Y2 | P2Y4 | P2Y6 | P2Y11 |
|---|---|---|---|---|---|---|---|
| ATP | Agonist | − | Agonist | Agonist | Agonist | i.a. | Agonist |
| ATPγS | Agonist | − | Agonist | Agonist | Agonist (partial) | i.a. | Agonist |
| Ap6A | i.a. | − | i.a. | Agonist | i.a. | i.a. | i.a. |
| UTP | i.a. | − | i.a. | Agonist | Agonist | Agonist (partial) | Agonist |
| 2meSATP | Agonist | − | Agonist | Agonist (partial) | Agonist (partial) | Agonist (partial) | Agonist (weak) |
| 2meSADP | i.a. | − | i.a. | i.a. | i.a. | Agonist | i.a. |
| BzATP | i.a. | − | i.a. | Agonist | Antagonist (weak) | i.a. | Agonist |
P2R were identified in our immunohistochemistry and real time-PCR studies. Information taken from Wildman et al.41,42,59 and King and Townsend-Nicholson.5 Agonist, potent agonist at rat P2R orthologue; Agonist (partial), partial agonist at rat P2R orthologue; Agonist (weak), weak agonist at rat P2R orthologue where EC100 activity would be expected ≥1 mM; i.a., inactive agonist at 10 μM; −, where the P2R subtype functions poorly as a homomeric assembly and therefore agonist activity cannot be determined.
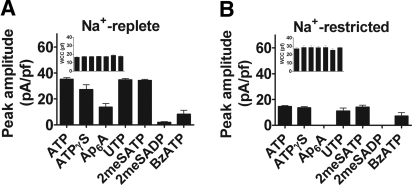
In PC from Na+-replete rats, the amplitudes of 10 μM agonist-evoked currents were 581 ± 19 pA (for ATP; n = 10), 468 ± 65 pA (ATPγS; n = 5), 232 ± 45 pA (Ap6A; n = 5), 600 ± 123 pA (UTP; n = 8), 594 ± 21 pA (2meSATP; n = 5), 28 ± 11 (2meSADP; n = 5), and 114 ± 39 pA (BzATP; n = 3; Figure 4A, data normalized for cell size). Currents evoked by the application of Ap6A, UTP, BzATP, and 2meSADP were completely abolished after preincubation (3 min) and co-application of DIDS (4,4′-Di-isothiocyanatostilbene-2,2′-disulfonic acid; a Ca2+-activated Cl− channel blocker; 100 μM; n = 3 in each case; example in Figure 3B), suggesting that their action was mediated through G protein–coupled P2YR and the involvement of Ca2+-dependent chloride “reporter” currents. Currents evoked by 2meSATP were unaffected after DIDS treatment (Figure 3B).
Figure 4.
Whole-cell P2R-mediated currents in PC in situ. (A) Histogram comparing the peak amplitude of 10 μM agonist-evoked currents (normalized for cell size; n = 5 to 10 for each agonist) in Na+-replete rats (data from 23 rats). Inset shows corresponding whole-cell capacitance (WCC) measurements. (B) As in A, histogram showing mean peak amplitude for agonist-evoked currents (n = 5 to 10 for each agonist), this time in Na+-restricted rats (data from 30 rats). Inset shows corresponding WCC measurements. All data are means ± SEM.
In PC of Na+-restricted rats, the amplitudes of 10 μM agonist-evoked currents were 386 ± 20 pA (ATP; n = 10), 380 ± 25 pA (ATPγS; n = 5), 273 ± 48 pA (UTP; n = 6), 388 ± 21 pA (2meSATP; n = 5), and 224 ± 38 (BzATP; n = 3; Figure 4B). Ap6A and 2meSADP (both 10 μM) failed to evoke significant inward currents (i.e., currents >20 pA; n = 5). Currents evoked by the application of UTP and BzATP were abolished after preincubation (3 min) and co-application of DIDS (100 μM; n = 3 in each case; data not shown); currents evoked by 2meSATP were unaffected after DIDS treatment.
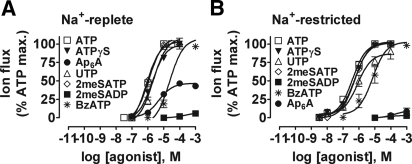
Concentration-response (C/R) curves were constructed for the range of nucleotide agonists used. As shown in Figure 5A, in Na+-replete rats, agonist C/R relationships demonstrated that ATPγS, UTP, 2meSATP, and BzATP all are full agonists, with all but the last having similar potency to that of ATP. EC50 values were as follows: ATP 1.3 ± 0.8 μM (n = 5); ATPγS 2.8 ± 1.1 μM (n = 5); UTP 2.8 ± 0.9 μM (n = 5); 2meSATP 1.3 ± 0.9 μM (n = 4); BzATP 18 ± 3 μM (n = 3). In contrast, Ap6A was only a partial agonist and 2meSADP was inactive at concentrations up to 1 mM.
Figure 5.
C/R curves for P2R agonist-evoked whole-cell inward currents in voltage-clamped rat CD PC in situ. (A) Results from Na+-replete rats. (B) Results from Na+-restricted rats. In all cases, data are normalized to maximal response to ATP; n = 3 for all C/R relationships.
In Na+-restricted rats, C/R curves were shifted approximately 0.5 log units to the left for all full agonists, with the exception of BzATP, for which there was no significant change (Figure 5B). The other difference was that Ap6A activity was virtually abolished. EC50 values were as follows: ATP 0.3 ± 0.5 μM (n = 5); ATPγS 0.4 ± 0.3 μM (n = 5); UTP 0.9 ± 0.4 μM (n = 5); 2meSATP 0.4 ± 0.4 μM (n = 5); and BzATP 8 ± 2 μM (n = 3).
Current/Voltage Relationships
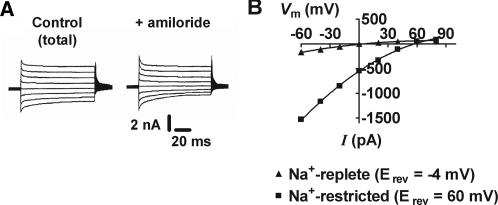
Steady-state current/voltage (I/V) relationships for whole-cell currents from PC were investigated for amiloride-sensitive currents (ENaC-mediated currents [Iam-s]). Figure 6 shows typical amiloride-sensitive I/V relationships recorded from Na+-replete and Na+-restricted rats. Data for I/V curves were calculated as the difference between total current amplitude and current amplitude in the presence of 10 μM amiloride (blocker of ENaC activity; as shown in Figure 6A from a Na+-restricted rat). I/V curves for Iam-s showed inward rectification, with mean reversal potentials (Erev) of −4 ± 4 mV (n = 6) and 58 ± 7 mV (n = 46) in PC from Na+-replete and Na+-restricted rats, respectively (Figure 6B). In Na+-replete rats, Iam-s was close to zero (n = 6), indicating a paucity of apical ENaC. The effect of amiloride (in Na+-restricted rats) was found to be reversible, but, with time, Iam-s decreased and often became undetectable after 20 min.
Figure 6.
Whole-cell ENaC-mediated currents in PC in situ. (A) Typical currents from a PC in situ, from Na+-restricted rats, under whole-cell voltage-clamp conditions. Holding potential was 0 mV, and test voltages were from −60 to 80 mV in 20-mV intervals. Current traces were obtained before and after addition of 10 μM amiloride to allow calculations of the amiloride-sensitive current (Iam-s). Iam-s was calculated as the difference between total current amplitude and current amplitude in the presence of amiloride. (B) Typical amiloride-sensitive I/V relationships recorded from Na+-replete and Na+-restricted rats. In Na+-replete rats, Iam-s is close to zero.
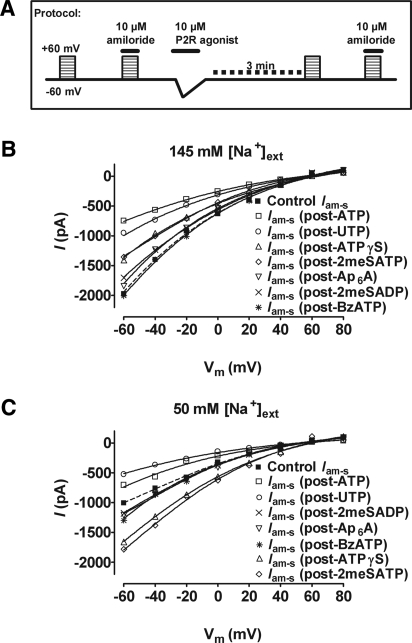
In PC from Na+-restricted rats, amiloride-sensitive I/V relationships were recorded after the activation of apical P2R by agonists (all 10 μM; representing a maximal or near-maximal active agonist concentration; 3 min was allowed to elapse before the I/V protocol was run; see Figure 7A for a schematic of the protocol; only one measurement was made per cell). After challenging with ATP, UTP, ATPγS, or 2meSATP, Iam-s was found to be significantly reduced in amplitude (by 62 ± 6, 52 ± 6, 29 ± 7, and 31 ± 4%, respectively, on the basis of calculations using values at −60 mV; P < 0.01; n = 12 in all cases), yet the Erev value and inward rectification did not change significantly (Figure 7B). The inward currents evoked by Ap6A, 2meSADP, and BzATP failed to alter significantly the amplitude or Erev of subsequent amiloride-sensitive I/V relations (n = 12). Erev values for Iam-s were as follows: 58 ± 7 mV (control), 57 ± 6 mV (post-ATP), 56 ± 8 mV (post-UTP), 62 ± 4 mV (post-ATPγS), 58 ± 9 mV (post-2meSATP), 57 ± 6 mV (Ap6A), 58 ± 5 mV (2meSADP), and 60 ± 12 mV (BzATP).
Figure 7.
Perfusion of the apical membrane of whole-cell patch-clamped PC from Na+-restricted rats with P2R agonists (all 10 μM with 30-s application duration, resulting in the activation of apically expressed P2R) affects the amplitude of subsequent amiloride-sensitive I/V relations (performed 3 min after P2R inactivation). Data are mean values; error bars are not shown for ease of viewing/interpretation. (A) Experimental protocol. (B) When the concentration of Na+ in the perfusate ([Na+]ext) was 145 mM, ATP, UTP, 2meSATP, and ATPγS significantly reduced the amplitude of subsequent amiloride-sensitive currents (Iam-s) without altering rectification or the reversal potential (P < 0.01; n = 12 in each case) . (C) When Na+ concentration in the perfusate was decreased to 50 mM, the inhibitory effects of UTP were similar to those seen in A (n = 5), but the inhibitory effects of ATP were decreased (n = 4). In contrast, 2meSATP and ATPγS significantly increased the amplitude of subsequent Iam-s (P < 0.01) without altering rectification or the reversal potential (n = 5 in both cases).
It was reported previously that extracellular Na+ ([Na+]ext) inhibits the activity of P2X receptors.23,24 Given that we found P2X4 subunits to be the predominant apically expressed P2XR in CD PC and our [Na+]ext levels were relatively high compared with in vivo micropuncture studies of the rat distal nephron,17 we next investigated the effect of decreasing [Na+]ext concentration from 145 to 50 mM (the concentration of Na+ expected in the luminal fluid of the distal tubule) on nucleotide-evoked inhibition of Iam-s in Na+-restricted rats (Figure 7C). When [Na+]ext was decreased, control values for amiloride-sensitive I/V relationships were found to be significantly smaller in amplitude (decreased by 49 ± 6%, on the basis of calculations using values at −60 mV normalized to the amiloride-insensitive current; P < 0.05; n = 5), with no significant change in inward rectification or mean reversal potential. Furthermore, at this decreased [Na+]ext, 2meSATP and ATPγS significantly increased the amplitude of amiloride-sensitive I/V relationships by 77 ± 4 and 64 ± 7% (P < 0.01) without altering inward rectification or mean reversal potential. Decreasing [Na+]ext had no significant effect on the UTP-mediated decreases in Iam-s amplitude but decreased the inhibitory effect of ATP from 62 ± 6% inhibition to 30 ± 9% inhibition (P < 0.05; n = 5), without altering the Erev or inward rectification. Erev values for Iam-s were as follows: 53 ± 6 mV (control), 56 ± 6 mV (post-ATP), 49 ± 10 mV (post-UTP), 59 ± 6 mV (post-ATPγS), 58.5 ± 9 mV (post-2meSATP), 52 ± 5 mV (Ap6A), 53 ± 5 mV (2meSADP), and 52 ± 7 mV (BzATP).
When extracellular Na+ concentrations were changed from 145 to 50 mM, maximal amplitudes of DIDS-insensitive currents evoked by ATP, 2meSATP, and ATPγS (100 μM, Vh = −60 mV) were significantly increased, by 11 ± 4, 32 ± 9, and 36 ± 5%, respectively (P < 0.05; n = 5). In contrast, the amplitude of UTP-evoked currents did not change significantly. The C/R curves for ATP, 2meSATP, and ATPγS were not significantly shifted when [Na+]ext was decreased (data not shown).
Mechanisms of Iam-s Inhibition and Potentiation
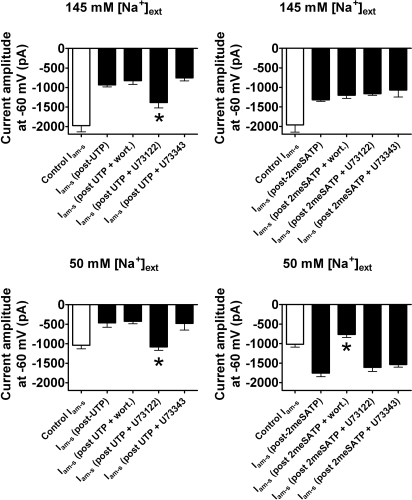
Previous studies demonstrated that activation of phospholipase C β (PLCβ), which can be blocked by U73122, resulting in the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) and/or activation of phosphatidylinositol-3 kinase (PI3K; which can be blocked by wortmannin or LY294002), may be involved in P2R-mediated inhibition of ENaC activity.14,16,25,26 With these observations in mind, the molecular mechanisms of P2R-mediated inhibition and potentiation of Iam-s amplitude were investigated pharmacologically in Na+-restricted rats. Two P2R agonists were selected for investigation, on the basis of their modulation of Iam-s: 2meSATP (predicted in this system to target apical P2X4 and/or 4/6 receptor) and UTP (predicted to target P2Y2 and/or 4 receptor).
When P2R were activated by UTP (10 μM) and [Na+]ext was 145 mM, preincubation (3 min) and co-application with U73122 (100 nM) led to a significant decrease in the percentage inhibition of Iam-s amplitude from 53 ± 8 to 30 ± 8% (Vh = −60 mV; P < 0.01; n = 4; Figure 8A), with no change in the amplitude of the UTP-evoked current. Preincubation/co-application with wortmannin (100 nM), U73343 (100 nM; the inactive form of U73122), or LY294002 (50 μM; data not shown) had no significant effect on the degree of UTP-mediated inhibition of Iam-s (n = 3 in all cases; Figure 8A). When P2R were activated by 2meSATP (10 μM), preincubation and co-application of wortmannin (100 nM), U73122 (100 nM), U73343 (100 nM), or LY294002 (50 μM; data not shown) did not significantly affect Iam-s inhibition (n = 3; Figure 8B).
Figure 8.
P2YR-mediated inhibition and P2XR-mediated stimulation of ENaC activity in whole-cell patch-clamped PC from Na+-restricted rats are mediated via PLC and PI3K activation, respectively. (A) When ENaC activity is inhibited after activation of P2Y2 and/or 4 receptor by UTP ([Na+]ext = 145 mM), P2R-mediated inhibition is significantly inhibited (denoted by *) by co-application and incubation (3 min) of U73122 (100 nM; n = 4; P < 0.01). (B) When ENaC activity is inhibited by 2meSATP-evoked currents (i.e., P2X4 and/or 4/6 mediated), P2R-mediated inhibition is unaffected by co-application and incubation (3 min) of wortmannin or U73122 (both 100 nM; P < 0.01; n = 4). (C) As in A but where the concentration of Na+ in the perfusate is decreased to 50 mM (n = 3; significance denoted by *). (D) When the concentration of Na+ in the perfusate is decreased to 50 mM, 2meSATP-evoked stimulation of ENaC activity is abolished by co-application and incubation (3 min) of wortmannin (100 nM; n = 4; significance denoted by *). Data are means ± SEM.
When [Na+]ext was lowered to 50 mM, U73122 (100 nM) treatment abolished UTP-mediated inhibition of Iam-s (n = 3), whereas wortmannin (100 nM), U73343 (100 nM), or LY294002 (50 μM; data not shown) had no significant effect (n = 3 in all cases; Figure 8C). When P2R were activated by 2meSATP (10 μM), treatment with wortmannin (100 nM) or LY294002 (50 μM; data not shown) abolished P2R-mediated increases in Iam-s (n = 3), whereas U73122 (100 nM) or U73343 (100 nM) had no significant effect (n = 4; Figure 8D).
DISCUSSION
The main findings of this investigation revealed that activation of apically expressed P2R can differentially regulate ENaC activity in PC of the rat CD. More specifically, we present functional evidence that (1) activation of metabotropic P2YR (probably the P2Y2 and/or 4 subtype) decreases Iam-s amplitude (i.e., inhibits ENaC activity) at any [Na+]ext, and (2) activation of ionotropic P2XR (probably P2X4 and/or 4/6 subtype) can either decrease or increase the amplitude of Iam-s (i.e., inhibit or potentiate ENaC activity), depending on [Na+]ext. The latter finding leads to the proposal that P2X4 and/or 4/6 receptor ion channels function as apically expressed Na+ sensors responsible for rapid (within 3 min) and local regulation of ENaC activity in the rat CD in response to changes in luminal [Na+].
P2R Expression in the CD
We characterized pharmacologically and immunohistochemically P2R expressed in PC of the cortical and outer medullary CD, in both Na+-replete and Na+-restricted rats. We consistently demonstrated apical expression of P2X4 and 6 subunits and P2Y2, 4, 6, and 11 receptors in Na+-replete rats and apical expression of P2X4 and 6 subunits and P2Y2, 4, and 11 receptors in Na+-restricted rats in which functional ENaC was expressed (see Table 1). Furthermore, using real-time PCR, we demonstrated that dietary Na+ restriction increases mRNA levels of apically expressed P2X4 and 6 subunits and P2Y4 receptors but not of P2Y2 receptors (see Figure 2).
Our immunohistochemical findings demonstrating apical localization of P2R in rat PC are broadly in accordance with previous studies that collectively reported P2X4 and 6 and P2Y2, 4, 6, and 11 localization/expression in native epithelia of rats, mice, and rabbits and in epithelial cell lines.6,27–39 They also extend the findings of an earlier study in which, using Na+-replete rats and a limited number of commercially available P2R antibodies, we demonstrated a variety of P2R expressed throughout the nephron.40
P2 Receptor-Mediated Inhibition of Iam-s Amplitude
It has already been established that the activation of apically expressed P2R by extracellular nucleotides can decrease Iam-s amplitude in the CD,11–14,17–19 but argument remains over the P2R subtype(s) responsible. Previous pharmacologic profiling studies using mouse isolated CD reported that apical G protein–coupled P2Y2-like receptors mediated ATP-evoked inhibition of ENaC activity.18,19 Furthermore, distal nephron cell line studies using mouse M1 and Xenopus A6 cells suggested the exclusive involvement of an apical P2Y2-like subtype.11,12,14 In contrast, in a more recent in vivo microperfusion study in the rat, we proposed an apical ionotropic ATPγS-sensitive P2XR-mediated effect,17 likely to be via a P2X4/6 receptor ion channel.41 In this context, we have demonstrated that certain recombinant rat P2XR (including P2X4 and 4/6) can inhibit ENaC activity in Xenopus oocytes.16 Finally, a study using mouse mIMCD-K2 cells suggested the involvement of both P2X (including P2X4) and P2Y (including P2Y2) subtypes.13
By using a variety of P2R agonists (and taking account of our immunohistochemistry and real-time PCR findings), this study provides evidence for both DIDS-insensitive P2XR-mediated (P2X4 and/or 4/6) and DIDS-sensitive P2YR-mediated (P2Y2 and/or 4) decreases in Iam-s amplitude in PC from rat CD. Thus, our findings are consistent with previous reports that suggested P2Y2 and/or ATPγS-sensitive P2XR (including P2X4) involvement.11–14,17–19 In addition, we suggest that additional P2R subtypes/subunits are involved (i.e., rat P2Y4, which is pharmacologically similar to rat P2Y2,42 and P2X6). We have been unable to distinguish between P2X4 and P2X4/6 subtypes and between P2Y2 and P2Y4 receptors because of their similar pharmacologic profiles and the lack of any truly selective P2R agonists and antagonists3,5,42; however, our real-time PCR studies suggested a greater P2X4/6 and P2Y4 involvement, because mRNA levels for these subunits/receptors were significantly increased after dietary Na+ restriction and consequent ENaC upregulation (see Figure 2).
It is clear from our experiments that P2X-mediated inhibition (i.e., evoked by ATPγS or 2meSATP) is approximately 50% less than that mediated by P2Y receptors (i.e., evoked by UTP; see Figure 7A), which may explain why several studies have failed to implicate clearly an inhibitory P2X effect (i.e., the P2Y effect may mask the P2X effect).
P2X Receptor–Mediated Potentiation of Iam-s Amplitude
The most striking finding of this study is that when [Na+]ext was reduced (from 145 to 50 mM; one where [Na+]ext-mediated inhibition of P2X4 and/or 4/6 activity is reduced),23,24 P2X4 and/or 4/6 activation did not inhibit ENaC activity but potentiated it. To our knowledge this is the first report of apical P2XR potentiating ENaC activity in the CD and of a P2R differentially regulating an ion channel. A possible reason for this is that previous electrophysiologic and perfusion studies investigating the effect of P2R activation on ENaC activity used extracellular solutions containing 145 mM Na+ (to optimize Na+ absorption).11–13,18,19 Our novel observation when using 50 mM [Na+]ext is important because this intraluminal Na+ concentration is much more physiologic (with respect to the distal nephron) than the previously used 145 mM.
The only other investigation to have used a physiologic Na+ concentration is a microperfusion study of the rat CD in vivo, in which a series of P2R agonists were tested intraluminally.17 In that study, a small but consistent inhibitory influence of ATPγS on CD Na+ reabsorption was found. It is difficult to reconcile this in vivo observation with our finding in patch-clamped rat PC that activation of P2X4 and/or 4/6 receptors (by the application of ATPγS) caused potentiation of ENaC activity at a luminal Na+ concentration of 50 mM. It is conceivable that subtle differences between the experimental conditions (e.g., in the in vivo study, P2R agonists were applied for approximately 5 min and at a dosage of 1 mM) might account for the discrepancy and that in vivo inhibitory effects of P2YR activation were able to override stimulatory effects of P2XR activation, which conceivably could have been desensitized by the high concentration of agonist, although it should be noted that Shirley et al.17 found that relatively selective agonists of P2Y2 and 4 receptors had no detectable effect on Na+ reabsorption.
Mechanism of P2R-Mediated Regulation of ENaC
As with the P2R subtype(s) responsible for inhibition of ENaC in the CD, there is also controversy over the proposed mechanism(s). Proposals have included activation of PLC14,25; decreased levels of PIP2 in the plasma membrane as a result of PLC- and/or protein kinase C–induced hydrolysis26; increases in intracellular Ca2+ after inositol IP3 and diacylglycerol formation26; activation of protein kinase C after IP3 and diacylglycerol formation26; influxes of extracellular Ca2+ ions16; increases in intracellular Na+ and downstream involvement of Go proteins16,43; activation of PI3K16; and changes in intracellular Cl− levels, which in some cases involved Gi proteins.11,16,25
In this study, we investigated the mechanism(s) responsible for both P2R-mediated inhibition and potentiation of ENaC in PC of the CD in situ. Our data suggest the involvement of PLC activation in the inhibition of Iam-s by P2YR activation, irrespective of [Na+]ext concentration (given that P2Y-mediated decreases in Iam-s amplitude can be inhibited by U73122), which agrees with mechanisms already proposed.14,25,26 It is interesting that P2Y-mediated inhibition was completely abolished by U73122 when [Na+]ext was low but only reduced when [Na+]ext was high, which suggests that more than one mechanism is responsible for P2Y-mediated inhibition of ENaC when [Na+]ext is high (but not involving PI3K activation, because wortmannin and LY294002 have no effect; see Figure 8A).
The results with wortmannin (and LY294002) suggest the involvement of PI3K activation in the potentiation of Iam-s by P2X4 and/or 4/6 activation when [Na+]ext was low (50 mM). In this respect, activation of PI3K by P2X4 receptor assemblies was previously described in embryonic stem cells and microglia.44,45 When [Na+]ext was high (145 mM), P2X4 and/or 4/6 activation resulted in an inhibition of Iam-s, yet this was not altered by U73122, wortmannin, or LY294002. In contrast, when P2X4 and/or 4/6 receptors were activated in the absence of extracellular Na+ (i.e., 2meSATP was prepared in a Na+-free Ringer solution), Iam-s was not inhibited (data not shown), indicating a requirement for Na+ influx in P2X-mediated inhibition of ENaC. Furthermore, we showed previously that activation of P2X4 and P2X4/6 receptors when [Na+]ext was relatively high (100 mM) inhibited ENaC activity through an increase in intracellular Na+ and Ca2+,16 which may also be the case here.
Is the P2X4 and/or 4/6 Receptor a Na+ Sensor?
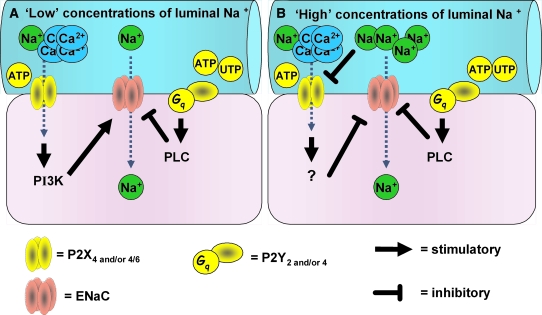
The results of this study indicate that whereas apical P2Y2 and/or 4 receptors are consistently inhibitory with respect to ENaC activity, P2X4 and/or 4/6 receptors have the ability to inhibit or potentiate ENaC, depending on the prevailing luminal Na+ concentration. Under normal circumstances, where functional ENaC is sparse, the luminal Na+ concentration is approximately 50 mM throughout the distal tubule46 but may rise to approximately 100 mM along the CD. In conditions of Na+ restriction, although the Na+ concentration in the distal tubule is little affected, it falls progressively in the CD and may be as low as 3 mM in the final urine.47 Under the latter condition, it would be expected that stimulation of apical P2X4 and/or 4/6 receptors should increase apical ENaC activity and facilitate Na+ reabsorption in the CD (see Figure 9).
Figure 9.
Regulation of ENaC activity by P2R in PC of Na+-restricted rats. ENaC activity is differentially regulated by P2X4 and/or 4/6 receptors, depending on the concentration of luminal Na+. Real-time PCR and immunohistochemistry suggest that levels of P2Y4 and P2X4 and/or 4/6 are increased when ENaC is expressed. Nucleotides have been proposed to be released from tubular cells. (A) When the concentration of luminal Na+ is low (i.e., at 50 mM in our experiments), activation of apically expressed P2X4 and/or 4/6 receptors (which are highly permeable to Ca2+ and, to a lesser extent, Na+ [ratio 4:1]; and in our experiments selectively targeted by 2meSATP) increases ENaC activity through the activation of PI3K. In contrast, activation (by either ATP or UTP) of apically expressed P2Y2 and/or 4 receptors inhibits ENaC activity through the activation of PLC. The overall effect of P2R activation (i.e., both P2X and P2Y, by using ATP) is a small degree of ENaC inhibition. (B) When the concentration of luminal Na+ is high (i.e., at 145 mM in our experiments), apically expressed P2X4 and/or 4/6 receptor channel activity is inhibited. In this situation, activation of P2X4 and/or 4/6 receptors results in inhibition of ENaC activity by an unidentified mechanism, possibly involving an influx of Na+. As before, activation of apically expressed P2Y2 and/or 4 receptor inhibits ENaC activity through the activation of PLC. The overall effect of P2R activation (i.e., both P2X and P2Y, by using ATP) is a larger degree of ENaC inhibition. We propose that P2X4 and/or 4/6 receptors act as extracellular Na+ sensors involved in the regulation of ENaC activity in PC of the rat CD.
It is conceivable also that P2X4 and/or 4/6 receptor modulation of ENaC activity might be instrumental in allowing vasopressin-mediated changes in water excretion to take place without corresponding changes in sodium excretion. Vasopressin itself (in physiologic concentrations) tends to reduce sodium excretion in two ways: First, by a direct stimulatory effect on sodium reabsorption in the thick ascending loop of Henle and cortical CD48,49; and, second, as a result of enhanced water reabsorption in the CD and the consequent increase in intraluminal sodium concentration, which increases the driving force for sodium reabsorption. According to the findings in this study, any increase in intraluminal sodium concentration should suppress the potentiating effect of P2X4 and/or 4/6 receptors on ENaC activity and unmask the inhibitory effect of P2Y2 and/or 4 (and P2X4 and/or 4/6) receptors on ENaC activity. In this way, sodium excretion could be stabilized.
It is not possible to say at present whether this P2R-mediated modulation of ENaC activity occurs by altering the channel's apical membrane insertion or retrieval (as seen in the Xenopus oocyte expression system with regard to ENaC inhibition16) or by changing its open probability. What is clear is that through its action on functional ENaC activity, the apical P2R system has the potential to alter Na+ reabsorption significantly and in so doing may affect sodium balance and arterial BP. That hypertension is a feature of both P2X4 and P2Y2 knockout mice suggests an important interplay within the P2R regulatory system and sodium homeostasis in vivo.50,51
CONCISE METHODS
Animals
Adult male Sprague-Dawley rats (approximately 200 g; bred in-house) were maintained on rat chow pellets and tap water (ad libitum). Rat chow was manufactured by Special Diet Services (Witham, UK) to contain either a standard (“normal”) Na+ content (0.5% NaCl) or a low Na+ content (0.01% NaCl) intended to increase ENaC expression throughout the CD.22,52,53 Rats were maintained on either diet for 10 d and culled on day 11.
Antibodies
Polyclonal primary antibodies (Ab) recognizing intracellular domains of rat P2X subunit proteins were obtained from Prof. G. Burnstock (Autonomic Neuroscience Institute, UCL, London, UK; for epitope sequences, see Turner et al.40); P2Y1, 2, 4, 6, 11, 12, 13, and 14 receptor Ab were purchased from Alomone Laboratories (Jerusalem, Israel); α, β-, and γ-ENaC Ab were obtained from Prof. B. Rossier (University of Lausanne, Lausanne, Switzerland; for epitope sequences, see Lin et al.54); and AQP2 Ab was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Noncommercially available P2X Ab were previously demonstrated to show subunit specificity.55
Immunohistochemistry
Rats were terminally anesthetized by intraperitoneal injection of sodium pentobarbitone ([200 mg/ml] used at 60 mg/kg), and the kidneys were perfusion-fixed with paraformaldehyde (4%). Kidneys were removed, embedded in OCT compound (Agar Scientific, Stansted, UK), and snap-frozen. Tissues were sectioned at 8 μm using a cryostat (Reichert Jung CM 1800, Milton Keynes, UK). The slides were stored at −70°C and thawed at room temperature for 10 min before treatment.
Slide-mounted sections were washed in PBS, treated with 10% donkey serum PBS (1 h), incubated overnight at 4°C with either P2R or ENaC primary Ab, and then incubated for 2 h with AQP2 primary Ab. Binding was revealed with FITC-conjugated and Cy3-conjugated (Jackson ImmunoResearch Laboratories, West Grove, PA) secondary Ab. Positive AQP2 immunostaining was used to identify PC of the CD.
Slides were mounted using Citifluor mountant (Agar Scientific, Essex, UK) and examined using a Bio-Rad Radiance 2100 confocal laser scanning microscope linked to a Nikon E800 fluorescence microscope and using a ×40, 1.3 NA, oil immersion objective. Controls for nonspecific binding of primary and secondary Ab were performed by preincubating immune sera with their respective immunogenic peptide (overnight) or by omitting the primary Ab for histochemical procedures, respectively.
Isolation of Tubules
Rats were terminally anesthetized by intraperitoneal injection of sodium pentobarbitone ([200 mg/ml] used at 60 mg/kg). The kidneys were removed, decapsulated, sliced into thin corticomedullary pyramids, and placed in an ice-cold dissection solution containing (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 2 glucose, and 10 Hepes (pH 7.4, NaOH). CD from the cortical and outer medullary regions were isolated under a dissecting microscope, as described previously.56 Where necessary, collagenase (NB4G; 1 mg/ml for 20 min; SERVA, Heidelberg, Germany) was used on pyramids to aid tubule isolation. CD were identified by their anatomy and morphology, as described previously.57
Real-Time PCR
RNA was extracted from pools of microdissected CD (total length 15 to 20 mm) from the cortical and outer medullary regions using an adapted guanidium thiocyanate-phenol-chloroform method.58 One microgram of total RNA was reverse-transcribed with 0.5 μg oligo(-dt) 12 to 18 primer and a first-strand cDNA synthesis kit (Superscript II RNase H− reverse transcriptase; Life Technologies BRL, Paisley, UK). The resulting cDNA transcripts were used for PCR amplification using a Roche Lightcycler (Roche Diagnostics, Mannheim, Germany) and QuantiTect SYBR Green PCR kit (Qiagen, West Sussex UK). Gene-specific primers for P2R and the constitutively expressed gene HPRT were used (Table 3).
Table 3.
Primer sequences and expected product sizesa
| Gene | Accession No. | 5′ to 3′ Sequence | Size (bp) |
|---|---|---|---|
| Rat P2X1 | BC061742 | S: GAAGTGTGATCTGGACTGGCACGTAS: GCGTCAAGTCCGGATCTCGACTAA | 452 |
| Rat P2X2 | NM_053656 | S: CGACGACTGTATTGCCGAS: CTCCAATGACACCGCC | 374 |
| Rat P2X3 | NM_031075 | S: TGGCGTTCTGGGTATTAAGATCGGAS: CAGTGGCCTGGTCACTGGCGA | 440 |
| Rat P2X4 | NM_031594 | S: GAGGCATCATGGGTATCCAGATCAAGAS: GAGCGGGGTGGAAATGTAACTTTAG | 447 |
| Rat P2X5 | NM_080780 | S: TGTCATTCCATCTCAGGGGGAS: TTCGGCATCCTTTAGAAGGG | 286 |
| Rat P2X6 | X97376 | S: AAAGACTGGTCAGTGTGTGGCGTTCAS: TGCCTGCCCAGTGACAAGAATGTCAA | 520 |
| Rat P2X7 | NM_019256 | S: GTGCCATTCTGACCAGGGTTGTATAAAAS: GCCACCTCTGTAAAGTTCTCTCCGATT | 354 |
| Rat P2Y1 | NM_012800 | S: ACGTCAGATGAGTACCTGCGAS: CCCTGTCGTTGAAATCACAC | 289 |
| Rat P2Y2 | NM_017255 | S: ACTTTGTCACCACCAGCGTGAGAS: TGACGTGGAAAGGCAGGAAG | 279 |
| Rat P2Y4 | NM_031680 | S: TGTTCCACCTGGCATTGTCAGAS: AAAGATTGGGCACGAGGCAG | 294 |
| Rat P2Y6 | NM_057124 | S: TGCTTGGGTGGTATGTGGAGTCAS: TGGAAAGGCAGGAAGCTGATAAC | 339 |
| Rat HPRT | NM_012583 | S: CTGACCTGCTGGATTACATTAAS: TTTCGCTGATGACACAA | 410 |
For quantification mRNA expression, standard curves were generated with known amounts of each PCR product. PCR products for each gene were separated on 2% (wt/vol) agarose–Tris-acetate EDTA gel containing 0.5 μg/ml ethidium bromide (Sigma-Aldrich Co., Ltd., Poole, UK). PCR bands were observed under ultraviolet illumination, excised from the gel, and purified using a Geneclean kit (Qbiogene, Cambridge, UK). Purified DNA was serially diluted 10-fold covering a dynamic range of 6 logarithmic orders, and 1 μl of each standard was amplified by PCR using the relevant gene-specific primers. One set of P2R standards was amplified in duplicate with the HPRT standards to generate two standard curves. For each sample, a ratio of relative abundance of each gene to the housekeeping gene HPRT was calculated by the Lightcycler Relative Quantification software, Version 1.0 (Roche Diagnostics). Melting curve analysis was carried out to determine primer specificity. PCR products were also analyzed by gel electrophoresis and visualized using a Bio-Rad multi-imager (Bio-Rad, Hemel Hempstead, UK).
Flame Photometry
Upon removal of the kidneys, urine was collected from the bladder and the Na+ content was measured by flame photometry (model 543; Instrumentation Laboratory, Warrington, UK) to confirm that reduced dietary Na+ intake decreased urinary Na+ content.
Patch Clamp
Isolated tubules were immobilized on glass coverslips coated with poly-L-lysine (50 μg/ml in dissection solution) and transferred to a chamber (volume approximately 600 μl) mounted on the stage of an inverted microscope (Nikon Eclipse TE300). CD were opened with a sharpened micropipette to access the apical membrane. PC were identified by their flatness, abundance, and polygonal shape.
The whole-cell configuration of the patch-clamp technique was used; this was achieved through a nystatin perforated-patch method. For establishment of the perforated-patch, nystatin (50 to 100 μg/ml) was added to the pipette solution containing (in mM) 50 KCl, 90 K-gluconate, 3 MgCl2, 3 EGTA, and 10 Hepes (pH 7.2, KOH). The development of electrical access to the cell interior was monitored by following the nystatin-induced fall in access resistance (Ra). Experiments were initiated once Ra had fallen to a stable value of <35 MΩ (this typically took approximately 10 min) and subsequent series resistance compensation was applied. An Axopatch 200B patch-clamp amplifier (Axon Instruments, Sunnyvale, CA) and computer complete with pCLAMP 8.0 software (Axon Instruments) were used to store and analyze whole-cell currents. Patch pipettes were pulled from borosilicate glass capillaries (Harvard Apparatus, Edenbridge, UK) and had a resistance ranging between 4.0 and 6.0 MΩ. The reference electrode was an Ag/AgCl pellet. Current signals were filtered at 5 kHz and displayed on an oscilloscope (20 MHz digital storage; Gould, Eichstetten, Germany). Liquid junction potentials were calculated using pCLAMP software, and appropriate corrections were applied.
Split-open CD were slowly superfused (4 ml/min, low enough to avoid mechanical disturbances and associated endogenous ATP release; 21 to 23°C) with bathing solution that contained (in mM) 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, and 10 Hepes (pH 7.4, NaOH) by a gravity-fed, continuous-flow system that allowed drug addition and washout. In experiments to assess the effect of altering [Na+]ext, Ringer solution was altered to contain (in mM) 50 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, 90 TEA-Cl, ad 90 NMDG+ (pH 7.4, NaOH). P2R agonist-activated membrane currents were recorded at a Vh of −60 mV, sufficient to drive P2X-mediated inward cationic currents and P2Y-mediated Ca2+-dependent chloride currents. P2R agonists were applied for 30 s or until the current reached a peak, whichever was the longer, then washed out for 3 min to avoid rundown. Only cells that demonstrated an inward current amplitude (evoked by 10 μM ATP) >100 pA were used. Amiloride-sensitive whole-cell currents (Iam-s) were calculated as the difference between total currents and currents obtained in the presence of amiloride (10 μM). Steady-state I/V relations were determined for each whole-cell clamp by stepping the command voltage down to −60 mV and up to 80 mV from 0 mV in 20-mV increments for 100 ms.
Statistical Analyses
All data are presented as mean ± SEM; significance was evaluated by t test, paired or unpaired as appropriate (Instat 3.0; GraphPad Software, San Diego, CA), with P < 0.05 considered significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by the British Heart Foundation and St. Peter's Trust for Kidney, Bladder and Prostate Research (UK). S.S.P.W. is a recipient of a British Heart Foundation Research Fellowship, and W.H.W. is supported by National Institutes of Health grant NIHHL34100.
Part of this work was presented at the American Society of Nephrology Renal Week; November 11 through 14, 2006; San Diego, CA.38
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Schwiebert EM, Zsembery A: Extracellular ATP as a signalling molecule for epithelial cells. Biochim Biophys Acta 1615: 7–32, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Unwin RJ, Bailey MA, Burnstock G: Purinergic signalling along the renal tubule: current state of play. News Physiol Sci 18: 237–241, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Nicke A, King BF: Heteromerization of P2X receptors. In: Biological and Biophysical Aspects of Ligand-Gated Ion Channel Receptor Superfamilies: Research SignPost (Kerala, India), edited by Arias HR, 2006, pp 383–417
- 4.Roberts JA, Vial C, Digby HR, Agboh KC, Wen H, Atterbury-Thomas A, Evan RJ: Molecular properties of P2X receptors. Eur J Physiol 452: 486–500, 2006 [DOI] [PubMed] [Google Scholar]
- 5.King BF, Townsend-Nicholson A: Nucleotide and nucleoside receptors. Tocris Review 23: 1–11, 2003 [Google Scholar]
- 6.Leipziger J: Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284: F419–F432, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Gorelik J, Zhang Y, Sanchez D, Shevchuk A, Frolenkov G, Lab M, Klenerman D, Edwards C, Korchev Y: Aldosterone acts via an ATP autocrine/paracrine system: The Edelman ATP hypothesis revisited. Proc Natl Acad Sci U S A 102: 15000–15005, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Sanchez D, Gorelik J, Klenerman D, Lab M, Edwards C, Korchev Y: Basolateral P2X4-like receptors regulate extracellular ATP-stimulated epithelial Na+ channel activity in renal epithelia. Am J Physiol Renal Physiol 292: F1734–F1740, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Schild L, Kellenberger S: Structure function relationships of ENaC and its role in sodium handling. Adv Exp Med Biol 502: 305–314, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Kleta R, Bockenhauer D: Bartter syndromes and other salt-losing tubulopathies. Nephron Physiol 104: 73–80, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Cuffe JE, Bielfeld-Ackerman A, Thomas J, Leipziger J, Korbmacher C: ATP stimulates Cl- secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. J Physiol 524: 77–90, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas J, Deetjen P, Ko WH, Jacobi C, Leipziger J: P2Y2 receptor-mediated inhibition of amiloride-sensitive short circuit current in M-1 mouse cortical collecting duct cells. J Membr Biol 183: 115–124, 2001 [DOI] [PubMed] [Google Scholar]
- 13.McCoy DE, Taylor AL, Kudlow BA, Karlson K, Slattery MJ, Schwiebert LM, Schwiebert EM, Stanton BA: Nucleotides regulate NaCl transport in mIMCD-K2 cells via P2X and P2Y purinergic receptors. Am J Physiol Renal Physiol 277: F552–F559, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Ma HP, Li L, Zhou ZH, Eaton DC, Warnock DG: ATP masks stretch activation of epithelial sodium channels in A6 distal nephron cells. Am J Physiol Renal Physiol 282: F501–F505, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Olteanu D, Yoder BK, Liu W, Croyle MJ, Welty EA, Rosborough K, Wyss JM, Bell PD, Guay-Woodford LM, Bevensee MO, Satlin LM, Schwiebert EM: Heightened ENaC-mediated sodium absorption in a murine polycystic kidney disease model epithelium lacking apical monocilia. Am J Physiol Renal Physiol 290: C952–C963, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Wildman SS, Marks J, Churchill L, Peppiatt CM, Chraibi A, Shirley DG, Horisberger J-D, King BF, Unwin RJ: Regulatory interdependence of cloned epithelial Na+ channels and P2X receptors. J Am Soc Nephrol 16: 2585–2597, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Shirley DG, Bailey MA, Unwin RJ: In vivo stimulation of apical P2 receptors in collecting ducts: Evidence for inhibition of sodium reabsorption. Am J Physiol Renal Physiol 288: F1243–F1248, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Lehrmann H, Thomas J, Kim SJ, Leipziger J: Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol 13: 10–18, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Deetjen P, Thomas J, Lehrmann H, Sim SJ, Leipziger J: The luminal P2Y receptor in the isolated perfused mouse cortical collecting duct. J Am Soc Nephrol 11: 1798–1806, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Ono S, Kusano E, Muto S, Ando Y, Asano Y: A low Na+ diet enhances expression of mRNA for epithelial Na+ channel in rat renal inner medulla. Pflugers Arch 434: 756–763, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Frindt G, Palmer L: Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol 256: F143–F151, 1989 [DOI] [PubMed] [Google Scholar]
- 22.Frindt G, Sackin H, Palmer LG: Whole-cell currents in rat cortical collecting tubule: Low-Na diet increases amiloride-sensitive conductance. Am J Physiol 258: F562–F567, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Ma W, Korngreen A, Uzlaner N, Priel Z, Silderberg SD: Extracellular sodium regulates airway ciliary motility by inhibiting a P2X receptor. Nature 400: 894–897, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Ma W, Korngreen A, Weil S, Cohen EB-T, Priel A, Kuzin L, Silberberg SD: Pore properties and pharmacological features of the P2X receptor channel in airway ciliated cells. J Physiol 571 3: 503–517, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook DI, O’Mullane LM, Lee IH, Dinudom A: The regulation of epithelial Na+ channels. Proc Phys Soc 2: SA19, 2006 [Google Scholar]
- 26.Ma HP, Eaton DC: Acute regulation of epithelial channel by anionic phospholipids. J Am Soc Nephrol 16: 3182–3187, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Taylor AL, Schwiebert LM, Smith JL, King C, Jones JR, Sorscher EJ, Schwiebert EM: Epithelial P2X receptor channel expression and function. J Clin Invest 104: 875–884, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim CH, Kim SS, Choi JY, Shin JH, Kim JY, Namkung W, Lee JG, Lee MG, Yoon JH: Membrane-specific expression of functional purinergic receptors in normal human nasal epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L835–L842, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Cha SH, Sekine T, Endou H: P2 purinoceptor localization along rat nephron and evidence suggesting existence of subtypes P2Y1 and P2Y2. Am J Physiol Renal Physiol 274: F1006–F1014, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Xia SL, Wang L, Cash MN, Teng X, Schwalbe RA, Wingo CS: Extracellular ATP-induced calcium signaling in mIMCD-3 cells requires both P2X and P2Y purinoceptors. Am J Physiol Renal Physiol 287: F204–F214, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Ecelbarger CA, Maeda Y, Gibson CC, Knepper MA: Extracellular ATP increases intracellular calcium in rat terminal collecting duct via a nucleotide receptor. Am J Physiol Renal Fluid Electrolyte Physiol 267: F998–F1006, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Kishore BK, Ginns SM, Krane CM, Nielsen S, Knepper MA: Cellular localization of P2Y2 purinoceptor in rat renal inner medulla and lung. Am J Physiol Renal Physiol 278: F43–F51, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Koster HPG, Hartog A, van Os CH, Bindels RJM: Inhibition of Na+ and Ca2+ reabsorption by P2U purinoceptors requires PKC but not Ca2+ signaling. Am J Physiol Renal Fluid Electrolyte Physiol 270: F53–F60, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Tschop J, Braun GS, Borscheid R, Horster MF, Huber SM: Ontogeny of purinergic receptor-regulated Ca2+ signaling in mouse cortical collecting duct epithelium. Cell Physiol Biochem 12: 75–82, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Wildman SS, Logeswaran T, Turner CM, Marks J, Churchill LJ, King BF, Unwin RJ: Immunohistochemical localisation of ATP-gated P2 receptors in the rat collecting duct: Effects of altering dietary Na+ intake. J Am Soc Nephrol 17: TH-PO906, 2006 [Google Scholar]
- 36.Wildman SS, Turner CM, Marks J, Shirley DG, King BF, Unwin RJ: Differential regulation of renal ENaC by apical P2X and P2Y receptors. FASEB 21: 937.3, 2007 [Google Scholar]
- 37.Wildman SS, Boone M, King BF, Deen PMT, Unwin RJ: Immunohistochemical labelling reveals dDAVP-dependent P2 receptor expression and P2 receptor-mediated inhibition of AQP2 trafficking in mpkCCD cultures. J Am Soc Nephrol 17: F-PO941, 2006 [Google Scholar]
- 38.Wildman SS, Turner CM, Marks J, King BF, Shirley DG, Wang W, Unwin RJ: Apical P2X receptors inhibit ENaC activity in microdissected collecting ducts from Na+-restricted rats. J Am Soc Nephrol 17: TH-PO905, 2006 [Google Scholar]
- 39.Li L, Lynch IJ, Zheng W, Cash MN, Teng X, Wingo CS, Verlander JW, Xia S-L: Apical P2XR contribute to [Ca2+]I signalling and Isc in mouse renal MCD. Biochem Biophys Res Commun 359: 438–444, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ: The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: An immunohistochemical study. Cells Tissues Organs 175: 105–117, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Wildman SS, Shirley DG, King BF, Unwin RJ: Potential regulation of Na+ reabsorption in Na+-restricted rats by an ATP-gated P2X4/6 receptor: Pharmacological evidence. J Am Soc Nephrol 15: F-PO091, 2004 [Google Scholar]
- 42.Wildman SS, Unwin RJ, King BF: Extended pharmacological profiles of rat P2Y2 and P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol 140: 1177–1186, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komwatana P, Dinudom A, Young JA, Cook DI: Activators of epithelial Na+ channels inhibit cytosolic feedback control: Evidence for the existence of a G protein-coupled receptor for cytosolic Na+. J Membr Biol 162: 225–232, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Heo JS, Han HJ: ATP stimulates mouse embryonic stem cell proliferation via protein kinase C, phosphatidylinositol 3-kinase/Akt, and mitogen-activated protein kinase signaling pathways. Stem Cells 24: 2637–2648, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S: Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia 55: 604–616, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Shirley DG, Walter SJ, Unwin RJ: Mechanism of the impaired natriuretic response to frusemide during sodium depletion: A micropuncture study in rats. Clin Sci (Lond) 91: 299–305, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Malnic G, Klose RM, Giebisch G: Micropuncture study of distal tubular potassium and sodium transport in rat nephron. Am J Physiol 206: 674–686, 1966 [DOI] [PubMed] [Google Scholar]
- 48.Greger R: Physiology of renal sodium transport. Am J Med Sci 319: 51–62, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Schafer JA, Hawk CT: Regulation of Na+ channels in the cortical collecting duct by AVP and mineralocorticoids. Kidney Int 41: 255–268, 1992 [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J: Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med 12: 133–137, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V: Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Schwartz GJ, Burg MB: Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol 235: F576–F585, 1978 [DOI] [PubMed] [Google Scholar]
- 53.O’Neil RG, Helman SI: Transport characteristics of renal collecting tubules: Influences of DOCA and diet. Am J Physiol 233: F544–F558, 1977 [DOI] [PubMed] [Google Scholar]
- 54.Lin W, Finger TE, Rossier BC, Kinnamon SC: Epithelial Na+ channel subunits in rat taste cells: Localisation and regulation by aldosterone. J Comp Neurol 405: 406–420, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Oglesby IB, Lachnit WG, Burnstock G, Ford AP: Subunit specificity of polyclonal antisera to the carboxy terminal regions of P2X receptors, P2X1 through P2X7. Drug Dev Res 47: 189–195, 1999 [Google Scholar]
- 56.Lu M, Giebisch G, Wang W: Nitric oxide-induced hyperpolarization stimulates low-conductance Na+ channel of rat CCD. Am J Physiol Renal Physiol 41: F498–F504, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Doucet A, Katz AI, Morel F: Determination of Na-K-ATPase activity in single segments of the mammalian nephron. Am J Physiol 237: F105–F113, 1979 [DOI] [PubMed] [Google Scholar]
- 58.Elalouf JM, Buhler JM, Tessiot C, Bellanger AC, Dublineau I, de Rouffignac C: Predominant expression of beta 1-adrenergic receptor in the thick ascending limb of rat kidney: Absolute mRNA quantitation by reverse transcription and polymerase chain reaction. J Clin Invest 91: 264–272, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wildman SS, Brown SG, King BF, Burnstock G: Selectivity of diadenosine polyphosphates for rat P2X receptor subunits. Eur J Physiol 367: 119–123, 1999 [DOI] [PubMed] [Google Scholar]
- 60.Turner CM, Ramesh B, Srai SKS, Burnstock G, Unwin RJ: Altered ATP-sensitive P2 receptor subtype expression in the Han:SPRD cy/+ rat, a model of autosomal dominant polycystic kidney disease. Cells Tissues Organs 178: 168–179, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Katugampola H, Burnstock G: Purinergic signalling to rat ovarian smooth muscle: Changes in P2X receptor expression during pregnancy. Cells Tissues Organs 178: 33–47, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Shibuya I, Tanaka K, Hattori Y, Uezono Y, Harayama N, Noguchi J, Ueta Y, Izumi F, Yamashita H: Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. J Physiol 514: 351–367, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.