Abstract
Increased aortic stiffness is a major factor responsible for the high cardiovascular mortality in patients with end-stage renal disease, but the impact of kidney transplantation on recipient aortic stiffness remains poorly defined. The use of expanded-criteria kidney donors is associated with decreased recipient survival compared with the use of standard-criteria donors, although the underlying mechanisms are incompletely understood. It was hypothesized that donor characteristics may affect recipient aortic stiffness, which may contribute to cardiovascular mortality in these patients. Aortic stiffness was evaluated by measurement of carotid-femoral pulse wave velocity in 74 cadaveric kidney recipients at 3 and 12 mo after transplantation. At 3 mo, aortic stiffness was associated exclusively with recipient-related factors: Age, gender, and mean BP. At 12 mo, age of the donor kidney emerged as an additional determinant. The change in aortic stiffness between 3 and 12 mo strongly correlated with donor age; stiffness improved in recipients of young kidneys (first tertile of donor age) and worsened in recipients of older kidneys (upper tertile of donor age). At 12 mo, the carotid-femoral pulse wave velocity was >1 m/s higher in recipients of the oldest kidneys than in the recipients of younger kidneys. The association between donor age and aortic stiffness was independent of recipient age, gender, mean BP, pretransplantation dialysis duration, conventional cardiovascular risk factors, medication, posttransplantation events, and GFR. These results demonstrate that the impact of kidney transplantation on recipient aortic stiffness is dependent on donor age and suggest that ongoing damage to large arteries might contribute to the mechanism underlying the association of old-donor kidneys and increased cardiovascular mortality.
Successful renal transplantation confers significant survival advantage compared with dialysis.1 An important component of this benefit is long-term reduction of cardiovascular (CV) progression and mortality.2 Nonetheless, the annual risk for CV death in transplant recipients remains 50-fold higher than in the general population.3 Premature CV death with a functioning graft is one of the leading factors in reducing long-term graft survival overall. Thus, reduction in CV mortality would dramatically improve long-term results of kidney transplantation.
Large-artery damage is one of the most important factors responsible for the high prevalence of CV disease in renal patients.4 Carotid-femoral pulse wave velocity (PWV), a direct, noninvasive, and reproducible method for estimating aortic stiffness,5 offers a means of investigating these large-artery changes. PWV has shown an independent predictive value for total and CV mortality, in populations both with high6–8 and with low CV risk.9–13 In patients with ESRD, PWV provides discriminative prognostic power above and beyond conventional CV risk factors.6,7 In transplant patients, increased stiffness of the common carotid artery predicts the occurrence of CV events.14 More recently, recipient PWV was associated with the combined end point of doubling plasma creatinine and CV events.15
The impact of kidney transplantation on recipient aortic stiffness remains poorly defined. Short-term improvement after living-donor kidney transplantation16 and no change at 1 yr after cadaveric-kidney transplantation17 have been described. With the growing shortage of organs, expanded-criteria-donor (ECD) kidneys are increasingly used for transplantation. ECD transplantation has been associated with decreased patient survival when compared with standard-criteria donors.18 The underlying mechanisms are incompletely understood. Taking into account the current large heterogeneity of donor characteristics, we set out to reevaluate the impact of kidney transplantation on recipient aortic stiffness. In particular, we hypothesized that certain donor characteristics could independently affect recipient aortic stiffness. To investigate this hypothesis, we measured PWV in the short-term, at 3 mo after transplantation, and in the stable state at 1 yr in a contemporary cohort of recipients of a first cadaveric kidney transplant.
RESULTS
Study Population Characteristics and Main Posttransplantation Events
Baseline characteristics of the study population according to tertiles of donor age are presented in Table 1. Donor age range was 17 to 70 yr with a mean of 45.5 ± 15.9 yr. Fifteen (20.3%) donors fulfilled the ECD definition criteria (donor age ≥60 yr, or 50 to 59 yr with at least two of the following conditions: Cerebrovascular cause of death, history of hypertension, and serum creatinine >130 μmol/L). All of them fell in tertile 3, which was thus composed of 65% ECD. Other demographic characteristics, pretransplantation dialysis duration, and CV risk factors did not differ significantly among groups. The occurrence of biopsy-proven acute rejection and new-onset diabetes within the first year were similar in the three groups.
Table 1.
Baseline characteristics of the study population and main posttransplantation events, according to tertiles of donor agea
| Characteristic | Donor Age (yr; n = 74)
|
P | ||
|---|---|---|---|---|
| <41(n = 24) | 41 to 53(n = 27) | >53(n = 23) | ||
| Donor age (yr; mean ± SD) | 28.3 ± 7.9 | 48.1 ± 7.4 | 59.6 ± 5.3 | 0.000 |
| ECD (%) | 0 | 0 | 65.2 | |
| Demographics | ||||
| recipient age (yr; mean ± SD) | 46 ± 12 | 47 ± 12 | 50 ± 8 | 0.431 |
| recipient gender (M/F) | 13/11 | 13/14 | 13/10 | 0.827 |
| pretransplantation dialysis time (mo; mean ± SD) | 29 ± 20 | 37 ± 32 | 33 ± 28 | 0.536 |
| CV risk factors | ||||
| previous diabetes (%) | 4.2 | 11.1 | 8.7 | 0.658 |
| hypertension (%) | 87.5 | 88.9 | 82.6 | 0.798 |
| current smoking (%) | 20.8 | 11.1 | 21.7 | 0.595 |
| dyslipidemia (%) | 50.0 | 48.1 | 65.2 | 0.429 |
| previous CVD (%) | 4.2 | 3.7 | 13.0 | 0.350 |
| BMI (kg/m2; mean ± SD) | 23 ± 3 | 22 ± 4 | 22 ± 4 | 0.394 |
| Main posttransplantation events | ||||
| BPAR (%) | 16.6 | 22.2 | 13.0 | 0.690 |
| NOD (%) | 21.7 | 22.2 | 21.7 | 0.982 |
BMI, body mass index; BPAR, biopsy-proven acute rejection; CVD, CV disease; NOD, new-onset diabetes.
Medication
The immunosuppressive regimen consisted of a calcineurin inhibitor (CNI), mycophenolate mofetil, and steroids in all patients. The type of CNI, the different classes of antihypertensive medications, and the use of statins were similar in the various tertiles, at 3 and 12 mo (Table 2).
Table 2.
Medication according to tertiles of donor agea
| Medication | Donor Age (yr; %; n = 74)
|
P | ||
|---|---|---|---|---|
| <41(n = 24) | 41 to 53(n = 27) | >53(n = 23) | ||
| Immunosuppressive agents | ||||
| cyclosporin A | 25.0 | 14.8 | 26.1 | 0.197 |
| tacrolimus | 75.0 | 85.2 | 73.9 | 0.557 |
| MMF | 100.0 | 100.0 | 100.0 | NS |
| steroids | 100.0 | 100.0 | 100.0 | NS |
| BP- and lipid-lowering agents | ||||
| ACEI/ARB | ||||
| 3 mo | 26.1 | 17.9 | 17.4 | 0.705 |
| 12 mo | 54.2 | 51.9 | 69.6 | 0.401 |
| CCB | ||||
| 3 mo | 45.8 | 51.9 | 65.2 | 0.428 |
| 12 mo | 50.0 | 51.9 | 65.2 | 0.517 |
| BB | ||||
| 3 mo | 45.8 | 55.6 | 60.9 | 0.673 |
| 12 mo | 54.2 | 33.3 | 56.5 | 0.187 |
| diuretics | ||||
| 3 mo | 4.2 | 3.7 | 4.3 | 0.991 |
| 12 mo | 16.7 | 18.5 | 13.0 | 0.870 |
| statins | ||||
| 3 mo | 37.5 | 25.9 | 26.1 | 0.492 |
| 12 mo | 41.7 | 29.6 | 52.2 | 0.367 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BB, β blockers; CCB, calcium channel blockers; MMF, mycophenolate mofetil.
Renal Function Estimates and Hemodynamic Parameters
At 3 mo, all patients could be classified as having stage 1 (n = 34) or stage 2 (n = 40) chronic kidney disease (CKD) with a mean estimated GFR (eGFR) of 65 ± 18 ml/min per 1.73 m2. At 12 mo, all patients but one could be classified as having stage 1 (n = 44) or stage 2 (n = 29) with a mean eGFR of 68 ± 22 ml/min per 1.73 m2.
Not surprising, eGFR tended to be lower and proteinuria higher in recipients of the oldest kidneys (upper tertile). Over the entire cohort, we observed a negative correlation between donor age and eGFR, both at 3 mo (R = −0.323, P = 0.005) and at 12 mo (R = −0.393, P = 0.001).
At 3 mo, PWV values differed only slightly among groups (P = 0.039). At 12 mo, intergroup comparison was highly significant (P = 0.007), with PWV being >1 m/s higher in the upper tertile of donor age compared with other recipients (Table 3).
Table 3.
Renal and hemodynamic parameters according to tertiles of donor age
| Parameter | Donor Age (yr; Mean ± SD; n = 74)
|
P | ||
|---|---|---|---|---|
| <41(n = 24) | 41 to 53(n = 27) | >53(n = 23) | ||
| Renal function estimates | ||||
| 3-mo eGFR (ml/min per 1.73 m2) | 70 ± 19 | 68 ± 11 | 60 ± 19 | 0.126 |
| 3-mo proteinuria (g/d) | 0.17 ± 0.19 | 0.18 ± 0.16 | 0.34 ± 0.61 | 0.261 |
| 12-mo eGFR (ml/min per 1.73 m2) | 76 ± 22 | 68 ± 25 | 61 ± 14 | 0.063 |
| 12-mo proteinuria (g/d) | 0.14 ± 0.09 | 0.23 ± 0.22 | 0.34 ± 0.47 | 0.102 |
| Hemodynamic parameters | ||||
| 3-mo PWV (m/s) | 9.43 ± 1.48 | 8.79 ± 1.37 | 9.97 ± 1.70 | 0.039 |
| 3-mo MBP (mmHg) | 93 ± 10 | 95 ± 10 | 94 ± 11 | 0.837 |
| 12-mo PWV (m/s) | 9.00 ± 1.62 | 8.86 ± 1.26 | 10.25 ± 1.98 | 0.007 |
| 12-mo MBP (mmHg) | 92 ± 9 | 93 ± 9 | 93 ± 11 | 0.897 |
Multiple Linear Regression Analysis
PWV According to Donor Age.
At 3 mo, PWV was associated exclusively with recipient-related factors: Age, mean BP (MBP), and gender. The association with previous diabetes and current smoking did not reach statistical significance (P = 0.057 and P = 0.115, respectively). PWV was not associated with donor age, in either univariate (P = 0.235) or multivariate analysis (P = 0.566; data not shown).
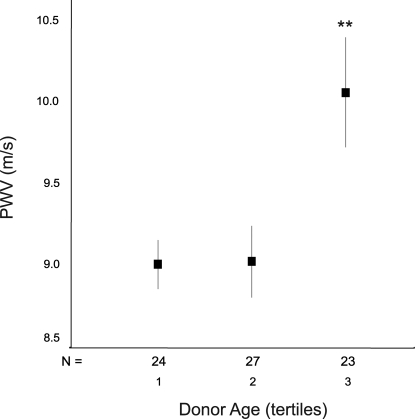
At 12 mo, PWV was associated with the same three recipient characteristics. In addition, donor age emerged as a significant determinant of PWV, both in univariate analysis (R = 0.336, P = 0.004) and after adjustment for recipient age, gender, and MBP (P = 0.011). The association between 12-mo PWV and 12-mo eGFR did not reach statistical significance (R = −0.205, P = 0.080). The strength of the association between donor age and 12-mo PWV was slightly lowered after further adjustment for eGFR and current smoking but remained significant (P = 0.03 and P = 0.025, respectively). The association was not affected after further adjustment for pretransplantation dialysis duration, acute rejection, new-onset diabetes, other CV risk factors, or medications (Table 4). Figure 1 displays the relationship between donor age and 12-mo PWV after adjustment for recipient-related parameters and provides an estimate of the independent quantitative impact of donor age on recipient aortic stiffness. Adjusted PWV differed >1 m/s between the two extreme tertiles of donor age (P = 0.001).
Table 4.
12-Month carotid-femoral PWV according to donor agea
| Model | R2 (%) | B | 95% CI | P |
|---|---|---|---|---|
| Univariate analysis | 11.3 | 0.0416 | 0.014 to 0.069 | 0.004 |
| Multivariate analysis | ||||
| M = recipient age + MBP + gender | 47.3 | 0.0289 | 0.007 to 0.051 | 0.011 |
| Renal parameters | ||||
| M + eGFR | 47.5 | 0.0263 | 0.002 to 0.050 | 0.030 |
| M + proteinuria | 47.1 | 0.0353 | 0.011 to 0.060 | 0.005 |
| M + acute rejection | 47.5 | 0.0247 | 0.007 to 0.052 | 0.010 |
| M + pretransplantation dialysis time | 47.5 | 0.0296 | 0.007 to 0.052 | 0.010 |
| CV risk factors | ||||
| M + current smoking | 49.8 | 0.0261 | 0.003 to 0.049 | 0.025 |
| M + diabetes | 51.3 | 0.0354 | 0.013 to 0.058 | 0.002 |
| M + previous CVD | 48.1 | 0.0324 | 0.009 to 0.056 | 0.008 |
| M + dyslipidemia | 47.3 | 0.0356 | 0.012 to 0.059 | 0.012 |
| M + NOD | 49.8 | 0.0244 | 0.007 to 0.042 | 0.008 |
| Medications | ||||
| M + ACEI/ARB | 47.3 | 0.0285 | 0.006 to 0.051 | 0.013 |
| M + CCB | 49.7 | 0.0372 | 0.013 to 0.062 | 0.004 |
| M + BB | 50.0 | 0.0337 | 0.011 to 0.057 | 0.005 |
| M + diuretics | 47.6 | 0.0347 | 0.012 to 0.057 | 0.003 |
| M + statins | 48.3 | 0.0292 | 0.007 to 0.052 | 0.011 |
Data are standardized regression coefficients (B) and their 95% confidence intervals (CI) for the association of donor age and PWV.
Figure 1.
Carotid-femoral PWV at 12 mo according to tertiles of donor age. PWV is adjusted for recipient age, gender, and MBP; data are means ± SEM. ANOVA: P = 0.005; post hoc analysis: Tertile 3 versus 2, P = 0.014; tertile 3 versus 1, P = 0.011. **Tertile 3 versus collapsed tertiles 1 + 2, P = 0.001. Tertiles of donor age: 1 = 17 to 41 yr; 2 = 41 to 53 yr; 3 = 53 to 70 yr.
PWV Change between 3 and 12 Mo According to Donor Age.
PWV change between 3 and 12 mo (ΔPWV) was highly variable from patient to patient: PWV increased >.5 m/s in 17 (22%) patients and decreased >0.5 m/s in 17 (22%) patients. Over the entire population, mean PWV did not change significantly (data not shown).
In linear regression analysis, individual ΔPWV was associated with concurrent MBP change and recipient age. In addition, it was strongly associated with donor age. This association was highly significant, both in univariate analysis (P = 0.007) and after adjustment for recipient age and concurrent MBP change (P = 0.003). After adjustment for MBP, donor age contributed to most of the variance of ΔPWV. This association was not affected after further adjustment for eGFR and other potential confounders (Table 5).
Table 5.
Δ PWV according to donor agea
| Model | R2 (%) | B | 95% CI | P |
|---|---|---|---|---|
| Univariate analysis | 5.9 | 0.0258 | 0.007 to 0.044 | 0.007 |
| Multivariate analysis | ||||
| M = recipient age+ ΔMBP | 57.1 | 0.0195 | 0.007 to 0.032 | 0.003 |
| Renal parameters | ||||
| M + ΔeGFR | 57.4 | 0.0259 | 0.009 to 0.036 | 0.002 |
| M + proteinuria | 62.3 | 0.0239 | 0.011 to 0.037 | 0.000 |
| M + acute rejection | 57.1 | 0.0197 | 0.007 to 0.033 | 0.003 |
| M + pretransplantation dialysis time | 57.1 | 0.0240 | 0.011 to 0.037 | 0.000 |
| CV risk factors | ||||
| M + current smoking | 57.7 | 0.0189 | 0.006 to 0.032 | 0.005 |
| M + diabetes | 59.2 | 0.0216 | 0.009 to 0.035 | 0.001 |
| M + previous CVD | 57.3 | 0.0212 | 0.008 to 0.035 | 0.002 |
| M + dyslipidemia | 59.8 | 0.0213 | 0.008 to 0.034 | 0.001 |
| M + NOD | 59.6 | 0.0239 | 0.011 to 0.037 | 0.000 |
| Medications | ||||
| M + ACEI/ARB | 57.3 | 0.0200 | 0.007 to 0.033 | 0.003 |
| M + CCB | 62.9 | 0.0192 | 0.006 to 0.032 | 0.005 |
| M + BB | 64.3 | 0.0273 | 0.015 to 0.040 | 0.000 |
| M + diuretics | 63.9 | 0.0299 | 0.014 to 0.039 | 0.000 |
| M + statins | 58.0 | 0.0186 | 0.006 to 0.031 | 0.005 |
Data are standardized regression coefficients (B) and their 95% CI for the association of donor age and Δ PWV.
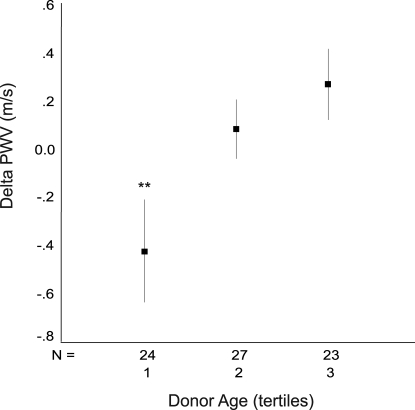
The graded impact of donor age on the MBP-independent component of ΔPWV is shown in Figure 2. MBP-adjusted PWV decreased 0.43 m/s between 3 and 12 mo in recipients of young-donor kidneys (lower tertile of donor age; P = 0.028). In contrast, PWV increased in recipients of old-donor kidneys (upper tertile of donor age; P = 0.022).
Figure 2.
ΔPWV between 3 and 12 mo according to tertiles of donor age. ΔPWV is adjusted for concurrent MBP change; data are means ± SEM. ANOVA: P = 0.014; post hoc analysis: Tertile 3 versus 1, P = 0.015; tertile 2 versus 1, P = 0.093. Tertiles of donor age: 1 = 17 to 41 yr; 2 = 41 to 53 yr; 3 = 53 to 70 yr. **Tertile 3 versus Tertile 1.
DISCUSSION
In this longitudinal study, we found an independent association between donor age and kidney recipient aortic stiffness at 1 yr after transplantation. Whereas recipient PWV was associated exclusively with classical recipient-related parameters in the short term, 3 mo after transplantation, donor age emerged as an independent determinant of aortic stiffness at 1 yr. Aortic stiffness change within the first year was strongly and independently related to donor age. Aortic stiffness improved in recipients of young-donor kidneys, whereas it worsened in recipients of old-donor kidneys. As a result, after adjustment for recipient characteristics, PWV at 1 yr was >1 m/s higher in recipients of old-donor kidneys compared with other patients.
Few studies have evaluated the determinants of aortic stiffness in stable-state kidney recipients. In a recent cross-sectional study, Kneifel et al.19 found that kidney-recipient arterial stiffness, as evaluated by means of applanation tonometry at a mean of 17 mo after transplantation, was independently associated with allograft function and donor age. Our study not only confirms this result but also provides a direct estimate of central arterial stiffness through the generally accepted “gold standard” PWV5 and comprehensively adjusts for potential confounders within the framework of a longitudinal design.
We found that donor age was a significant determinant of individual PWV change within the first year after transplantation. To the best of our knowledge, there are only two published studies with serial assessment of PWV after kidney transplantation. One study showed a short-term improvement at 3 mo16 in living-donor kidney recipients and the second study showed no change at 1 yr in deceased-donor kidney recipients17 when PWV was adjusted for MBP. The two studies each had only a small number of patients and did not assess the determinants of individual arterial stiffness change. PWV change with time must always be interpreted in light of concurrent MBP change, because, in addition to structural elements within the arterial wall and vascular smooth muscle tone, aortic stiffness is related to distending pressure.20 In our study, after adjustment for MBP, ΔPWV was strongly and primarily associated with donor age. This association was not affected after further adjustment for potential confounders such as recipient age, pretransplantation dialysis duration, conventional CV risk factors, allograft function estimates, and medications; therefore, this result is a strong indication of an influence of donor age on the arterial wall. In this respect, the contrasting isobaric change in PWV in the two extreme tertiles of donor age is striking. It shows for the first time that the mechanical properties of large arteries can improve after cadaveric-kidney transplantation. Furthermore, this result indicates that, at least within the first year, the benefit of kidney transplantation on the arterial wall seems to be mainly determined by donor age, excluding recipients of oldest donor kidneys.
One-year adjusted PWV was >1 m/s higher in recipients of old-donor kidneys compared with other patients. A 1-m/s increase in PWV is expected to increase CV mortality by 5% in patients with low CV risk12,13 and total mortality as much as 39% in patients with high CV risk and ESRD.6 Although ECD transplantation confers survival advantage compared with remaining on dialysis,21,22 it has been associated with decreased patient survival when compared with standard-criteria donors.18 The underlying mechanisms of this association are unknown. With stiffening of large arteries, the reflected pressure wave reaches the heart in systole instead of diastole, potentially causing both increased cardiac afterload and reduced coronary filling.20 We propose that ongoing damage to large arteries or lack of improvement of arterial mechanical properties in recipients of old-donor kidneys could be a significant mechanism linking ECD transplantation, CV events, and lower recipient survival.
The clinical relevance of this hypothesis is emphasized by the recent demonstration that certain treatment options, such as high dosages of angiotensin-converting enzyme inhibitors, can improve arterial stiffness independent of concurrent MBP change.23,24 It would seem that both arterial stiffness and MBP must be reduced in renal patients: A previous study, in which a reduction in BP without any concomitant reduction in arterial stiffness, was associated with an adverse outcome.25 In the context of organ shortage, ECD kidneys currently account for 15 to 20% of deceased-donor kidneys transplanted.18 We suggest that the simple assessment of aortic stiffness could be a precious tool for identifying patients with increasing CV risk within the first year, enabling targeted interventions. Data to confirm the benefit of such strategy are required.
Despite a negative correlation between donor age and eGFR, the association between allograft function and PWV did not reach statistical significance. Allograft function was good in our cohort. Although the relationship between severe reduction of GFR and aortic stiffness is well documented,26 there is a lack of concordance in results in patients with mild to moderate renal insufficiency.27–29 Importantly, in this study the associations between donor age and 12-mo PWV, as well as between donor age and ΔPWV, were not affected after adjustment for allograft function.
This finding raises an intriguing question concerning GFR-independent mechanisms that possibly relate donor age to recipient aortic stiffness. In the context of transplantation, both immunologic and nonimmunologic characteristics of old-donor kidneys must be considered. Old-donor kidneys have been shown to intensify the early immune response.30 Immunologic changes induced by the presence of a graft might be observed, thickening the intima and obliterating the vasa vasorum of the recipient aorta, as previously reported in cardiac transplants.31 In this study, the upper tertile of donor age group was composed of 65% ECD. It is known that past hypertension and a cerebrovascular cause of death have been associated with vascular histologic alterations of the graft, which may affect renal renin-angiotensin system activity. Whether a significant correlation between vascular histologic alterations in the graft and recipient arterial stiffness can be found early after transplantation is the object of an ongoing study. Finally, it is tempting to speculate on the role of age-related alterations in the metabolic or endocrine function of the kidney, because, in addition to the renin-angiotensin system, other renal hormones may affect large-artery mechanical properties.32,33
The pathophysiologic relevance of our study is not restricted to kidney transplantation but should rather be placed within the general framework of interactions between kidney and the cardiovascular system. The cross-sectional design of previous studies showing a negative correlation between arterial stiffness and kidney function have not allowed investigators to establish a cause-and-effect relationship between kidney characteristics and arterial wall properties.26 Kidney transplantation provides an ideal model for assessing time-dependent interactions between the kidney and the CV system. Regarding this important issue, this study provides a strong indication that large-artery mechanical properties can be influenced by kidney characteristics. We showed that 1-yr aortic stiffness was significantly increased in recipients of old-donor kidneys (donor age >53 yr) compared with other patients. This result is reminiscent of the findings of large longitudinal studies showing that, in the general population, the 50- to 55-yr age bracket is the threshold when pulse pressure begins to increase steeply as a consequence of increased aortic stiffness.34 Whether age-related kidney alterations could be, at least in part, implicated in the age-dependent increase in aortic stiffness in the general population requires further studies.
Our study had several limitations. First, pretransplantation PWV was not available, and we cannot exclude a very short-term change in PWV within the first 3 mo; however, such a change would not have modified the interpretation of our main results, which was the emergence of donor age as an independent determinant of 1-yr stable-state recipient aortic stiffness. Second, the sample size was relatively small. A type II error could have precluded the identification of a selection bias for recipients of old-donor kidneys in the intertertile comparisons; however, in multiple linear regression analysis performed over the entire cohort, donor age remained strongly associated with PWV after adjustment for all potential confounders.
In conclusion, our longitudinal study showed that donor age independently affects recipient aortic stiffness change within the first year after kidney transplantation and emerges as an independent determinant of stable-state recipient aortic stiffness at 1 yr. Ongoing damage to recipient large arteries could be a significant pathophysiologic mechanism linking old-donor-kidney transplantation, increased CV risk, and decreased recipient survival. Additional studies are needed to identify the GFR-independent pathophysiologic link between donor age and recipient aortic stiffness and to evaluate the potential benefit of targeted therapeutic interventions.
CONCISE METHODS
Patients
Seventy-four (44%) of 168 consecutive recipients of a first cadaveric kidney transplantation performed in the Transplantation Unit of Foch Hospital between 2002 and 2005 were studied. Patients were eligible for inclusion when they were younger than 65 yr, had no recent history of CV disease or acute illness, and agreed to participate in the study, which was approved by our institutional review board. Patients with living-donor kidney transplantation, double-kidney transplantation, and multiorgan transplantation were not eligible for inclusion. Patients with graft failure (eGFR <15 ml/min per 1.73 m2 body surface area) before the end of the first year were excluded.
All patients were studied twice on an outpatient basis, 3 and 12 mo after renal transplantation. They underwent physical examination and standard laboratory and arterial measurements. Information on past and present CV risk factors, main posttransplantation events (biopsy-proven acute rejection and new-onset diabetes), and medication were collected.
Renal Function Estimates
The simplified Modification of Diet in Renal Disease (MDRD) equation was used for estimation of the GFR. Validation of the method in renal transplant recipients has been published in detail elsewhere.35 Values were expressed in ml/min per 1.73 m2. Proteinuria was determined on a 24-h urine collection.
Arterial Parameters
Patients were examined in a quiet, temperature-controlled room, and measurements were performed by a single operator (M.C.), who was blinded to donor characteristics. BP was measured after 15 min of rest in a supine position using a mercury sphygmomanometer and a cuff of appropriate size. Systolic BP (SBP) was defined as the onset of Korotkoff sounds (phase I). Diastolic BP (DBP) was defined as their disappearance (phase V). The average of three consecutive measurements was calculated. MBP was calculated as MBP = DBP + (SBP − DBP)/3.
PWV was measured along the descending thoraco-abdominal aorta using the foot-to-foot velocity method with an automatic device (Complior, Artech Medical, Pantin, France). This method enables online pulse wave recording and automatic calculation of PWV by dividing the distance between carotid and femoral measurement sites by the transit time of the wave. Validation of this method and its reproducibility have been previously reported, with intraobserver and interobserver repeatability coefficients of 0.935 and 0.890, respectively.36 PWV was defined as the mean of four determinations.
Statistical Analysis
Statistics were performed using SPSS 11.0 for Windows package software (SPSS, Chicago, IL). Descriptive statistics were used to evaluate recipient-, donor-, and transplantation-related characteristics. Quantitative data are expressed as means ± SD and as percentage for categorical variables. Data are presented according to tertiles of donor age. Differences between tertiles were tested using an F test for continuous variables and χ2 test for categorical variables. Bonferroni test was used for post hoc analysis of differences between continuous variables. Differences in hemodynamic parameters between 3 and 12 mo were tested using a paired t test. A two-sided P < 0.05 was considered to indicate statistical significance.
Simple and multiple linear regression analyses were used to investigate the associations between PWV and donor age. We examined donor age as a continuous variable. Associations were first analyzed without adjustments and then with adjustments for potential confounders. The main physiologic determinants of aortic stiffness are recipient age, MBP, and gender.20 These parameters contributed to 30 to 40% of variance in PWV in our cohort. For these reasons, they were considered first in the adjusted models. Other potential confounders tested included pretransplantation dialysis duration, traditional CV risk factors (current smoking, previous diabetes, previous CV disease, dyslipidemia, and previous hypertension), estimates of renal function (eGFR and proteinuria), transplantation-related parameters (acute rejection and new-onset diabetes), and medication with potential impact on aortic stiffness (CNI, antihypertensive medications, and statins). Two-sided P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Acknowledgments
We thank Eliane Rallet-Milly for excellent secretary assistance, Canan Kumas for excellent technical help, and Prof. Gary S. Hill for reviewing of the manuscript.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B: Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant 4: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ojo AO: Cardiovascular complications after renal transplantation and their prevention. Transplantation 82: 603–611, 2006 [DOI] [PubMed] [Google Scholar]
- 4.London GM, Druecke TB: Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int 51: 1678–1695, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H: Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99: 2434–2439, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Blacher J, Safer ME, Guérin AP, Pannier B, Marchais SJ, London GM: Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int 63: 1852–1860, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG: Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: An integrated index of vascular function? Circulation 106: 2085–2090, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A: Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37: 1236–1241, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S: Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 39: 10–15, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A: Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111: 3384–3390, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J: Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 113: 664–670, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC: Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam Study. Circulation 113: 657–663, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Barenbrock M, Kosch M, Joster E, Kisters K, Rahn KH, Hausberg M: Reduced arterial distensibility is a predictor of cardiovascular disease in patients after renal transplantation. J Hypertens 20: 79–84, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Bahous SA, Stephan A, Barakat W, Blacher J, Asmar R, Safar ME: Aortic pulse wave velocity in renal transplant patients. Kidney Int 66: 1486–1492, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Covic A, Goldsmith DJ, Gusbeth-Tatomir P, Buhaescu I, Covic M: Successful renal transplantation decreases aortic stiffness and increases vascular reactivity in dialysis patients. Transplantation 76: 1573–1577, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Zoungas S, Kerr PG, Chadban S, Muske C, Ristevski S, Atkins RC, McNeil JJ, McGrath BP: Arterial function after successful renal transplantation. Kidney Int 65: 1882–1889, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Ojo AO: Expanded criteria donors: Process and outcomes. Semin Dial 18: 463–468, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kneifel M, Scholze A, Burkert A, Offermann G, Rothermund L, Zidek W, Tepel M: Impaired renal allograft function is associated with increased arterial stiffness in renal transplant recipients. Am J Transplant 6: 1624–1630, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Safar ME: Arterial stiffness: A simplified overview in vascular medicine. Adv Cardiol 44: 1–18, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Ojo AO, Hanson JA, Meier-Kriesche H, Okechukwu CN, Wolfe RA, Leichtman AB, Agodoa LY, Kaplan B, Port FK: Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol 12: 589–597, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Laurent S, Boutouyrie P: Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension 49: 1202–1206, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Mitchell GF, Dunlap ME, Warnica W, Ducharme A, Arnold JM, Tardif JC, Solomon SD, Domanski MJ, Jablonski KA, Rice MM, Pfeffer MA: Long-term trandolapril treatment is associated with reduced aortic stiffness: The prevention of events with angiotensin-converting enzyme inhibition hemodynamic substudy. Hypertension 49: 1271–1277, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 103: 987–992, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Safar ME, London GM, Plante GE: Arterial stiffness and kidney function. Hypertension 43: 163–168, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Mourad JJ, Pannier B, Blacher J, Rudnichi A, Benetos A, London GM, Safar ME: Creatinine clearance, pulse wave velocity, carotid compliance and essential hypertension. Kidney Int 59: 1834–1841, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P, Boutouyrie P: Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int 69: 350–357, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Hermans MM, Henry R, Dekker JM, Kooman JP, Kostense PJ, Nijpels G, Heine RJ, Stehouwer CD: Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: The Hoorn study. J Am Soc Nephrol 23: 1942–1952, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Reutzel-Selke A, Jurisch A, Denecke C, Pascher A, Martins PN, Kessler H, Tamura A, Utku N, Pratschke J, Neuhaus P, Tullius SG: Donor age intensifies the early immune response after transplantation. Kidney Int 71: 629–636, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Fujita M, Russell ME, Masek MA, Rowan RA, Nagashima K, Billingham ME: Graft vascular disease in the great vessels and vasa vasorum. Hum Pathol 24: 1067–1072, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R: Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun 248: 324–329, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, Wu Y, Peixoto A, Crowley S, Desir GV: Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest 115: 1275–1280, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D: Hemodynamic patterns of age-related changes in blood pressure: The Framingham Heart Study. Circulation 96: 308–315, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Poggio ED, Wang X, Weinstein DM, Issa N, Dennis VW, Braun WE, Hall PM: Assessing glomerular filtration rate by estimation equations in kidney transplant recipients. Am J Transplant 6: 100–108, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI: Assessment of arterial distensibility by automatic pulse wave velocity measurement: Validation and clinical application studies. Hypertension 26: 485–490, 1995 [DOI] [PubMed] [Google Scholar]