Abstract
Intraglomerular hypertension and glomerular hyperfiltration likely contribute to the pathogenesis of diabetic nephropathy, and tubuloglomerular feedback (TGF) has been suggested to play a role in diabetic hyperfiltration. A1 adenosine receptor (A1AR) null mice lack a TGF response, so this model was used to investigate the contribution of TGF to hyperfiltration in diabetic Ins2+/− Akita mice. TGF responses in Ins2+/− A1AR−/− double mutants were abolished, whereas they were attenuated in Ins2+/− mice. GFR, assessed at 14, 24, and 33 wk, was approximately 30% higher in Ins2+/− than in wild-type (WT) mice and increased further in Ins2+/− A1AR−/− mutants (P < 0.01 versus both WT and Ins2+/− mice at all ages). Histologic evidence of glomerular injury and urinary albumin excretion were more pronounced in double-mutant than single-mutant or WT mice. In summary, the marked elevation of GFR in diabetic mice that lack a TGF response indicates that TGF is not required to cause hyperfiltration in the Akita model of diabetes. Rather, an A1AR-dependent mechanism, possibly TGF, limits the degree of diabetic hyperfiltration and nephropathy.
Intraglomerular hypertension and glomerular hyperfiltration are suspected to play a major role in the pathogenesis of diabetic nephropathy.1 A variety of vasoactive systems have been found to be altered in diabetes, but no single vascular factor that fully accounts for the hyperfiltration of early diabetes has been identified.2 In this study, we investigated the role of adenosine 1 receptors (A1AR) in the chronic hemodynamic alterations associated with diabetes by comparing filtration rates in diabetic mice during a 5- to 6-mo period in the presence and absence of A1AR. A major incentive for these studies was our previous finding that A1AR-deficient mice lack a tubuloglomerular feedback (TGF) response. Because TGF has been implicated as a causal mechanism in diabetic hyperfiltration,3,4 we performed these studies with the expectation that GFR in diabetic mice lacking TGF would be normal and that the histopathology would be less pronounced.
The diabetic model used in this study was the Akita heterozygote strain (Ins2+/−). We demonstrate that double-mutant mice carrying the A1AR deficiency in an Ins2+/− background maintain the phenotype of absence of TGF. Contrary to expectations, double mutants with both diabetes and TGF deficiency have an exaggerated increase of GFR, augmented albuminuria, and worsened glomerular injury compared with single-mutant Akita mice, suggesting that A1AR exert a restraining effect on GFR. It is possible that the increased injury is a consequence of absence of TGF, but other actions of A1AR have not been excluded.
RESULTS
Glycemia and Glycosuria
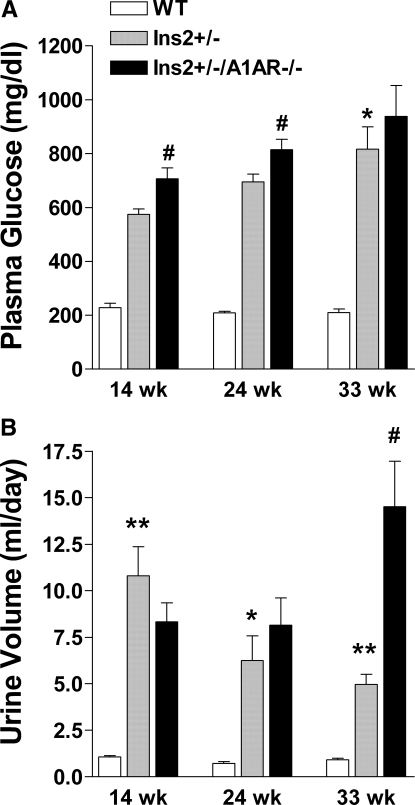
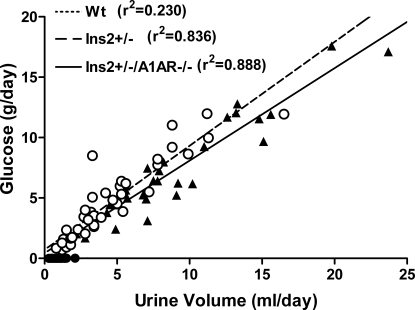
Plasma glucose was significantly higher in Ins2+/− and Ins2+/−/A1AR−/− mice than WT at all time points (Figure 1). Furthermore, glucose was slightly higher in double than single mutants at 14 and 24 wk but not at 33 wk. Both diabetic strains had markedly increased urine flows, although urine volume increased with age in the double mutants, whereas it fell in the single-mutant mice (Figure 1). Urinary glucose excretion and urine flow were highly correlated in the diabetic mice (Figure 2). There was a significant increase of plasma aldosterone in the double mutants compared with WT, whereas the difference in the single-mutant mice did not reach significance (Table 1). Plasma renin concentrations were not significantly different between groups.
Figure 1.
Glycemia and urine flow in WT, Ins2+/−, and Ins2+/−/A1AR−/− mice. (A) Plasma glucose concentration at 14, 24, and 33 wk of age (WT n = 6, Ins2+/− n = 10, Ins2+/−/A1AR−/− n = 8). (B) Urine volume (ml/d) at the three ages (WT n = 38, Ins2+/− n = 40, Ins2+/−/A1AR−/− n = 31). *P < 0.05, **P < 0.01 versus WT, and #P < 0.05 versus Ins2+/−.
Figure 2.
Relationship between urine flow and urinary glucose excretion of individual Ins2+/− (○; n = 38), Ins2+/−/A1AR−/− (▴; n = 40), and WT mice (dots on x axis; n = 31)
Table 1.
Plasma concentrations of renin and aldosterone in WT, Ins2+/−, and Ins2+/−/A1AR−/− micea
| Parameter | Renin (ng AngI/ml per h) | Aldosterone (pg/ml) |
|---|---|---|
| WT | 679 ± 124 (7) | 658 ± 70 (24) |
| Ins2+/− | 436 ± 75 (7) | 1072 ± 142 (16) |
| Ins2+/−/A1AR−/− | 534 ± 87 (13) | 1055 ± 135 (25)b |
Data are means ± SE; numbers of observations are given in parentheses. Data are pooled for all age groups. AngI, angiotensin I.
P < 0.05 versus WT.
GFR and Kidney and Body Weights
As shown in Figure 3 for individual animals, GFR of Ins2+/−/A1AR−/− mice (n = 8) was significantly higher than that of both WT (n = 6) and Ins2+/− mice (n = 10) at all ages. GFR of Ins2+/− mice tended to be higher than WT, but this difference achieved significance only at 24 wk. When factored by body weight, GFR was significantly higher in Ins2+/− than WT in all age groups (Figure 4), as a result of the significantly lower body weights in the diabetic mice (Figure 5A). Body weight differences between WT and diabetic mice were especially pronounced at 33 and 38 wk. Kidney wet weights in diabetic mice were significantly higher than in WT already at 12 to 20 wk (Figure 5B). Kidney weight increased further in the double-mutant mice but not in the single-mutant mice. Mean GFR in C57Bl6 A1AR−/− single-mutant mice, not included in the longitudinal study, averaged 309 ± 94 μl/min (n = 7; age 20.4 ± 11 wk) compared with a GFR of 337 ± 89 μl/min in C57Bl6 WT mice (NS; n = 12; age 20.5 ± 4 wk).
Figure 3.
GFR of a cohort of WT, Ins2+/−, and Ins2+/−/A1AR−/− mice at 14, 24, and 33 wk of age (smaller number of mice at 33 wk reflects loss of individual mice). Statistical comparisons between groups by ANOVA (GFR of double-mutant Akita mice was significantly higher than WT in all age groups).
Figure 4.
Mean GFR in absolute terms (μl/min; top) and normalized for body weight (μl/min × 100 g; bottom) in WT and single- and double-mutant Akita mice at 14, 24, and 33 wk. *P < 0.05 and **P < 0.01 versus WT; #P < 0.05 and ##P < 0.01 versus single-mutant mice (ANOVA and Bonferroni post hoc test).
Figure 5.
Mean body weights (top) and mean kidney weights (bottom) of WT and single- and double-mutant Akita mice at 14, 24, and 33 wk of age. *P < 0.05 and **P < 0.01 versus WT; #P < 0.05 versus same genotype in 14-wk age group (ANOVA and Bonferroni post hoc test).
TGF
A flow increase from 0 to 30 nl/min reduced stop flow pressure (PSF) from 42.5 ± 1.2 to 34.9 ± 2 mmHg in WT (mice/tubules 5/12; P < 0.001) and from 45.2 ± 1.2 to 43 ± 1.4 mmHg in Ins2+/− (mice/tubules 3/14; P < 0.01) and caused no change in double-mutant mice (37.7 ± 1.7 versus 38.2 ± 1.7 mmHg; mice/tubules 3/14). Representative PSF recordings are shown in Figure 6, and individual results are summarized in Figure 7. Mean arterial BP in these studies averaged 90 ± 3 mmHg in WT, 94 ± 1.5 mmHg in Ins2+/−, and 87 ± 2.7 mmHg in double-mutant mice. Early proximal flow rate (EPFR), a close correlate of single-nephron GFR, fell from 9.7 ± 0.5 to 5 ± 0.5 nl/min in WT (mice/tubules 3/12; P < 0.001) and from 9.7 ± 0.55 to 6.9 ± 0.6 in Ins2+/− (mice/tubules 3/10; P < 0.001) and did not change in double mutants (11 ± 0.5 versus 11.1 ± 0.5 nl/min; mice/tubules 3/11). The relative change of EPFR was greater in WT than in Ins2+/− mice (48.4 ± 4.7 versus 29.4 ± 4.1%; P = 0.008). Whereas EPFR at 0 nl/min was not different between strains, EPFR at 30 nl/min was significantly higher than in WT in both Ins2+/− (P < 0.05) and Ins2+/−/A1AR−/− (P < 0.001). Average response magnitudes of PSF and EPFR in the three genotypes are shown in Figure 8. It is to be noted that addition of glucose to the perfusate reduced the TGF response.5,6 The use of glucose-free Ringer solutions in this study should therefore have facilitated the detection of any residual capacity for TGF regulation
Figure 6.
Representative examples of mean arterial BP (MAP) and PSF recordings without and with (gray areas) perfusion of the loop of Henle at 30 nl/min in WT (A), Ins2+/− (B), and Ins2+/−/A1AR−/− mice (C)
Figure 7.
(Top) Individual PSF measurements at loop of Henle perfusion rates of 0 and 30 nl/min in WT (left, circles), Ins2+/− (middle, triangles), and Ins2+/−/A1AR−/− mice (right, dots). (Bottom) Individual measurements of EPFR at 0 and 30 nl/min in the three groups of mice. Lines connect data from the same tubule. Broken lines connect mean values ± S.E.
Figure 8.
Mean PSF (top) and EPFR responses (bottom) in WT, Ins2+/−, and Ins2+/−/A1AR−/− mice. TGF responses are PSF or EPFR at 0 nl/min to PSF or EPFR at 30 nl/min). **P < 0.01 significance of change.
Urine Albumin Excretion and Glomerulosclerosis
Albumin excretion of Ins2+/−/A1AR−/− double mutants was significantly elevated compared with either WT or Ins2+/− (Figure 9), whereas the albuminuria of Ins2+/− mice was less pronounced (Figure 9). The histologic evidence for sclerosis, as examined by staining with Masson trichrome (Figure 10A), for mesangial expansion with periodic acid-Schiff (Figure 10B), and for interstitial fibronectin with immunolabeling (Figure 10C) was more pronounced in the double-mutant mice than in the single-mutant Ins2+/−. The fraction of the glomerular tuft occupied by stained material was significantly higher in double-mutant compared with single-mutant Ins2+/− (P < 0.01) and WT mice (P < 0.001). In contrast, there was no significant difference in the degree of sclerosis in diabetic Ins2+/− mice with functioning A1AR compared with WT mice (Figure 11).
Figure 9.
Urinary albumin excretion (μg/d) in WT, Ins2+/−, and Ins2+/−/A1AR−/− mice in three age groups (>10 to 20, 20 to 30, and 30 to 40 wk); numbers in bars indicate the number of mice in each group. **P < 0.01 compared to WT; #P < 0.05 and ##P < 0.01 compared to Ins2+/− (ANOVA and Bonferroni post hoc test).
Figure 10.
Renal tissue sections (4 μm) from WT (left), Ins2+/− (middle), and Ins2+/−/A1AR−/− mice (right) at 26 to 28 wk of age. (A) Masson trichrome staining. (B) Periodic acid-Schiff staining. (C) Immunostaining with an anti-fibronectin antibody (1:50 to 1:100). Magnifications: ×100 in A; ×200 in B and C.
Figure 11.
Glomerular score based on light microscopic analysis of trichrome stained tissue sections. Glomerular score was quantified from the fraction of the glomerular tuft occupied by stained material and scored as 1 = 0 to 25%, 2 = 25 to 50%, 3 = 50 to 75%, and 4 = 75 to 100%. Mean values represent the average of 20 individual glomerular scores per section and are derived from 15 sections of WT mice, 14 sections of Ins2+/− mice, and 11 sections of Ins2+/−/A1AR−/− mice. Mean age of WT was 34 ± 7 wk, of Ins2+/− was 32 ± 9 wk, and of Ins2+/−/A1AR−/− was 27 ± 10 wk.
Mesangial Area Fraction
The mesangial area fraction assessed by electron microscopy was significantly higher in Ins2+/−/A1AR−/− mice than in WT mice (P < 0.05 by ANOVA), whereas the difference between WT and Ins2+/− mice was NS (Figure 12). In addition, compression of luminal space and diffuse deposition of collagen fibers was found to be more pronounced in Ins2+/−/A1AR−/− kidneys compared with WT mice. Only moderate lesions were noted in Ins2+/− kidneys. There was no consistent evidence for glomerular basement membrane thickening in either strain.
Figure 12.
Fractional mesangial area in WT (open circles), Ins2+/− (closed circles), and Ins2+/−/A1AR−/− (closed triangles) mice. ANOVA results given for comparisons with WT.
DISCUSSION
The main finding of this study is the demonstration of markedly augmented hyperfiltration in type 1 diabetic Akita mice that lack A1 adenosine receptors (Ins2+/−/A1AR−/−). In contrast, the increase of GFR in single-mutant Akita mice was less pronounced. Hyperfiltration in Ins2+/−/A1AR−/− mice was accompanied by increased albumin excretion and worsened glomerulosclerosis compared with single-mutant Akita mice. Our results are compatible with the notion that A1AR reduce the impact of factors that increase GFR and thereby limit the degree of diabetic hyperfiltration and possibly of diabetic nephropathy.
Male Akita mice develop marked hyperglycemia and glycosuria soon after weaning, with plasma glucose levels reaching 30 mM by 9 wk of age.7 Insulin deficiency is the consequence of a severe pancreatic β cell decline resulting from a mutation of the insulin 2 gene and the proteotoxic effect of misfolded insulin.8,9 GFR of Akita mice tended to increase as early at 14 wk of age, and it was significantly elevated at age 24 wk. In a previous study in Akita mice that did not include comparison with WT controls, GFR was reported to be 520 ± 120 μl/min, similar to our value of 457 ± 29 μl/min and consistent with mild hyperfiltration.10 Overall, the degree of hyperfiltration in various diabetic mouse models has been modest and seems to depend on both duration and strain. In this regard, hyperfiltration has been found at 3 mo of age in both OVE26 mice, a type 1 diabetic model, and in db/db mice, a model of type 2 diabetes11–13; no hyperfiltration was observed in either strain when mice were older than 5 mo.12,14 In an extensive series of studies in streptozotocin-induced diabetic mice, hyperfiltration was found in only two of the six strains examined when GFR was expressed in absolute terms; C57Bl/6 mice, the genetic background of all mice used in this study, did not show GFR differences between controls and diabetics.15 GFR was elevated, however, when values were normalized for body weight, reflecting the consistently reduced body weight of the diabetic animals.15
Akita mice have a markedly increased kidney/body weight ratio, reflecting an increase of kidney weight as well as a reduction of body weight. This is in agreement with previous studies16,17 and with the earlier observation that glomerular volumes of 16-wk-old Akita mice are 38% larger than those of control glomeruli.17 Because the size of glomeruli seems to increase in rough proportion to overall kidney size, an increase in the filtering surface area may be in part responsible for the increased GFR.18 Another factor contributing to hyperfiltration in early diabetes is glomerular hyperperfusion and glomerular capillary hypertension.19 Afferent arteriolar relaxation has been suggested to result from a primary deactivation of TGF.20 In fact, the integrated function of the TGF mechanism in Akita mice, as determined by microperfusion experiments, shows a reduction in maximum responsiveness. Nevertheless, hyperfiltration can occur in Akita mice even in the complete absence of TGF, indicating that the TGF alteration may be a secondary consequence of afferent arteriolar dilation rather than its cause. An increase of renal nitric oxide production may contribute to diabetic hyperperfusion, hyperfiltration, and TGF attenuation, because renal hemodynamics seem to be more sensitive to nitric oxide synthase blockade in a number of diabetic models,13,21,22 although this has not been studied in Akita mice.
Diabetic mice without A1AR were generated by crossing Akita mice with A1AR-deficient mice that lack TGF.23 Double mutants homozygous for the A1AR deletion and heterozygous for the Ins2 mutation (Ins2+/−/A1AR−/−) were viable and not grossly variant from respective parental single mutants; however, absence of A1AR markedly enhanced the extent of hyperfiltration in Ins2+/− diabetic mice. The causes of this are not entirely clear. It was noted previously that the A1AR-mediated vasoconstrictor response to adenosine was augmented in diabetic compared with control rats and that the expression of A1AR as well as A2AR was upregulated in diabetes.24,25 Deletion of A1AR in the double-mutant mice could therefore result in vasodilation of renal resistance vessels above that seen in single-mutant diabetic mice. Nevertheless, we did not detect significant differences in GFR and renal blood flow between WT and A1AR-deficient mice, suggesting that A1AR do not exert major tonic constrictor effects under resting conditions.26 Our data also show that the hyperglycemia of Ins2+/− mice is modestly but significantly worsened by ablation of A1AR. This elevation of nonfasting glucose may be related to our recent observation that A1AR-deficient mice have reduced glucose tolerance and insulin sensitivity.27 It is unclear whether this increase in plasma glucose could be directly responsible for the augmentation of GFR in the double-mutant mice. Complete absence of a response of PSF and EPFR to changes in loop of Henle perfusion rate was observed in Ins2+/−/A1AR−/− mice. Hyperfiltration in the absence of TGF regulation is not compatible with the notion that the increase of GFR in diabetic mice may be caused by the TGF mechanism. Hyperfiltration was recently also observed in A1AR−/− mice with alloxan-induced diabetes.28 Furthermore, previous studies in rats using the streptozotocin model of diabetes showed that blocking TGF acutely by complete or partial proximal fluid removal was associated with a consistently higher single-nephron GFR.19,29–31 Thus, rather than causing diabetic hyperfiltration, TGF seems to restrict it by providing a tonic vasoconstrictor input to afferent arterioles.
Our observations confirm the presence of some of the histopathologic markers of diabetic nephropathy in Ins2+/− mice. Furthermore, as described by others in previous studies, we observed mesangial expansion as sign of early diabetic glomerular alterations by both light and electron microscopic examination.10,17,32,33 In addition, an increase of urinary albumin excretion was noted, indicative of some abnormality of glomerular permselectivity, but as in other studies in Ins2+/− mice, the increase in albumin excretion was mild.16,17,32,34 Our study shows that the glomerular abnormalities observed in Ins2+/− mice are enhanced in these mice when they lack A1AR. Thus, the effect of the A1AR null mutation on diabetes-associated glomerular structure and function correlates with the degree of hyperfiltration, but a contribution of the greater hyperglycemia in the double mutants to the renal pathology cannot be excluded. Recent studies in Ins2+/− mice showed that the bradykinin B2 receptor is another factor whose absence markedly aggravates diabetic nephropathy.16 Evidence has been accumulated to suggest that diabetes and especially diabetes in a bradykinin B2 receptor–deficient background represent states of accelerated senescence.35 It remains to be shown whether Ins2+/−/A1AR−/− mice are also characterized by increased expression of senescence markers.
In summary, our studies indicate that the modest hyperfiltration observed in mice with insulin-dependent diabetes is augmented when the animals lack A1AR. Because A1AR-deficient diabetic mice lack TGF regulation, the glomerular relaxation leading to hyperfiltration does not seem to be the result of inactivation of the TGF pathway. It is more likely that TGF acts as a system that prevents currently undefined vasodilatory factors from causing excessive hyperfiltration.
CONCISE METHODS
Animals
Male Akita mice (Ins2+/−; C57Bl/6 background) from Jackson Laboratories (Bar Harbor, ME) were crossed with female C57Bl/6 WT mice. For generation of Ins2+/−/A1AR−/− double mutants, female A1AR−/− mice (C57Bl/6 background) were crossed with male F2 mice that were heterozygous for both the Ins2 (Akita) and A1AR mutations. All experiments were performed in male mice. Animal experimentation was approved and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Genotyping for A1AR was done on tail DNA using PCR as described previously.23 Genotyping of Ins2 was done by standard PCR (primers TGCTGATGCCCTGGCCTGCT and TGGTCCCACATATGCACATG) using AmpliTaq DNA Polymerase (Applied Biosystems, Foster City, CA). The PCR product was then digested for 2 h with Fnu4H I restriction enzyme (cat. no. R01785; New England Biolabs, Ipswich, MA) and separated on Agarose/EtBr (3% [wt/vol]) gel.
GFR
GFR was measured by single-injection FITC inulin clearance as described by Qi et al.,36 modified to minimize plasma volume. FITC-inulin (5%), dialyzed overnight against 0.9% NaCl (final concentration approximately 3%), was injected at 3.7 μl/g body wt into the retro-orbital plexus during brief isoflurane anesthesia (recovery within approximately 20 s). At 3, 7, 10, 15, 35, 55, and 75 min, the mice were placed in a restrainer, the tail vein was punctured with a 30-G atraumatic needle, and approximately 2 μl blood was collected by capillarity into heparinized 5-μl microcaps (Drummond Scientific, Broomall, PA). A total of 500 nl of plasma was diluted 1:10 in 500 mmol of HEPES (pH 7.4) and measured against a standard curve (1 μl of approximately 3% FITC-inulin diluted 1:50, 1:100, and 1:500 in 500 mmol of HEPES). Fluorescence was determined in 1.7 μl in a Nanodrop-ND-3300 spectrometer (Nanodrop Technologies, Wilmington, DE). GFR was calculated using a two-compartment model of two-phase exponential decay.36
Micropuncture Studies
The preparation of the kidney for micropuncture and the techniques used to determine the responses of PSF or EPFR to loop perfusion have been described in detail previously.37 “Early proximal” in this study is defined as a proximal segment that is followed by at least five surface segments.
Plasma Determinations
Nonfasting blood glucose concentration was measured with Glucostix (Roche Diagnostics, Indianapolis, IN), and plasma aldosterone was measure by RIA (Coat-A-Count Aldosterone Kit TKAL1; DPC, Los Angeles, CA). Renin concentration was determined as described previously.38
Urine Collection
Twenty-four-hour urine collections were made in metabolic cages (Hatteras Instruments, Cary, NC) at ambient room temperature with unrestricted access to tap water and standard rodent diet. Urine glucose concentration was determined by Glucostix (AccuCheck active; Roche Diagnostics), and urine albumin was determined by ELISA (Albuwell M; Exocell, Philadelphia, PA).
Tissue Sampling and Histochemistry
Kidneys of 26- to 28-wk-old mice were excised, weighed, cut longitudinally, and frozen in liquid nitrogen. Frozen section were cut under a layer of Tissue-Tek OCT. Tissue preparation for histochemistry was provided by Histoserv (Germantown, MD). Three-microgram sections were cut from paraffin-embedded tissue and stained with periodic acid-Schiff, hematoxylin and eosin, and Masson trichrome for light microscopy or retained for immunohistochemistry.
Glomerular Score
Mesangial expansion was assessed semiquantitatively by scoring the extent of glomerular tuft staining in Masson trichrome–stained sections. In a blinded manner, 20 consecutive glomeruli were selected from both superficial and deep cortex of the kidney. The scoring examined the fraction of the glomerular tuft occupied by stained material, including nuclei (black), cytoplasm, and noncollagenous matrix (red) and collagenous matrix (blue).
Immunolabeling
Tissue sections were dewaxed with xylene and rehydrated with graded ethanol. After blocking endogenous peroxidase activity with 0.3% H2O2 for 15 min, sections were boiled in 10 mM sodium citrate solution (pH 6.0) for 20 min. the Sections were stained with primary fibronectin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) using Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA) and counterstained with hematoxylin before being examined.
Electron Microscopy
Kidney tissue for electron microscopy was fixed in 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) overnight at 4°C, washed with cacodylate buffer and postfixed with 1% OsO4 for 2 h, dehydrated in ethanol, and embedded in Eponate 12 resin (Ted Pella, Redding, CA). Sections (80 nm) were obtained with the Leica ultra-cut UCT ultramicrotome (Leica, Deerfield, IL) and stained with saturated uranyl acetate. The grids were viewed in the Philips 410 electron microscope (FEI, Hillsboro, OR) at 80 kV, and images were recorded on Kodak SO-163 film (Rochester, NY).
Three different areas per section were observed by electron microscopy (×5724) and quantified for mesangial and intracapillary areas by tracing function and area calculator using ImageJ 1.35i software. Areas were determined by two masked reviewers using at least three glomeruli per section.
Statistical Analyses
All data are presented as means ± SEM. Multivariable determinations were compared by one-sided ANOVA with Tukey or Bonferroni post hoc testing.
DISCLOSURES
None.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Hostetter TH, Rennke HG, Brenner BM: The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am J Med 72: 375–380, 1982 [DOI] [PubMed] [Google Scholar]
- 2.O'Bryan GT, Hostetter TH: The renal hemodynamic basis of diabetic nephropathy. Semin Nephrol 17: 93–100, 1997 [PubMed] [Google Scholar]
- 3.Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V: Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest 107: 217–224, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods LL, Mizelle HL, Hall JE: Control of renal hemodynamics in hyperglycemia: Possible role of tubuloglomerular feedback. Am J Physiol Renal Physiol 252: F65–F73, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Blantz RC, Konnen KS: Relation of distal tubular delivery and reabsorptive rate to nephron filtration. Am J Physiol Renal Physiol 233: F315–F24, 1977 [DOI] [PubMed] [Google Scholar]
- 6.Blantz RC, Peterson OW, Gushwa L, Tucker BJ: Effect of modest hyperglycemia on tubuloglomerular feedback activity. Kidney Int Suppl 12: S206–S212, 1982 [PubMed] [Google Scholar]
- 7.Yoshioka M, Kayo T, Ikeda T, Koizumi A: A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 46: 887–894, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T: A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 103: 27–37, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M: Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 109: 525–532, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tchekneva EE, Rinchik EM, Polosukhina D, Davis LS, Kadkina V, Mohamed Y, Dunn SR, Sharma K, Qi Z, Fogo AB, Breyer MD: A sensitized screen of N-ethyl-N-nitrosourea-mutagenized mice identifies dominant mutants predisposed to diabetic nephropathy. J Am Soc Nephrol 18: 103–112, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Gartner K: Glomerular hyperfiltration during the onset of diabetes mellitus in two strains of diabetic mice (c57bl/6j db/db and c57bl/ksj db/db). Diabetologia 15: 59–63, 1978 [DOI] [PubMed] [Google Scholar]
- 12.Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, Epstein PN: Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes 53: 3248–3257, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Levine DZ, Iacovitti M, Robertson SJ, Mokhtar GA: Modulation of single-nephron GFR in the db/db mouse model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 290: R975–R981, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC: Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol 17: 2664–2669, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD: Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Kakoki M, Takahashi N, Jennette JC, Smithies O: Diabetic nephropathy is markedly enhanced in mice lacking the bradykinin B2 receptor. Proc Natl Acad Sci U S A 101: 13302–13305, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM: Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290: F214–F222, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Schwieger J, Fine LG: Renal hypertrophy, growth factors, and nephropathy in diabetes mellitus. Semin Nephrol 10: 242–253, 1990 [PubMed] [Google Scholar]
- 19.Hostetter TH, Troy JL, Brenner BM: Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int 19: 410–415, 1981 [DOI] [PubMed] [Google Scholar]
- 20.Vallon V, Blantz RC, Thomson S: Homeostatic efficiency of tubuloglomerular feedback is reduced in established diabetes mellitus in rats. Am J Physiol Renal Physiol 269: F876–F883, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Komers R, Lindsley JN, Oyama TT, Allison KM, Anderson S: Role of neuronal nitric oxide synthase (NOS1) in the pathogenesis of renal hemodynamic changes in diabetes. Am J Physiol Renal Physiol 279: F573–F583, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Thomson SC, Deng A, Komine N, Hammes JS, Blantz RC, Gabbai FB: Early diabetes as a model for testing the regulation of juxtaglomerular NOS I. Am J Physiol Renal Physiol 287: F732–F738, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J: Mediation of tubuloglomerular feedback by adenosine: Evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A 98: 9983–9988, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pflueger AC, Schenk F, Osswald H: Increased sensitivity of the renal vasculature to adenosine in streptozotocin-induced diabetes mellitus rats. Am J Physiol 269: F529–F535, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Pawelczyk T, Grden M, Rzepko R, Sakowicz M, Szutowicz A: Region-specific alterations of adenosine receptors expression level in kidney of diabetic rat. Am J Pathol 167: 315–325, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto S, Huang Y, Briggs J, Schnermann J: Reduced autoregulatory effectiveness in adenosine 1 receptor-deficient mice. Am J Physiol Renal Physiol 290: F888–F891, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Faulhaber-Walter R, Huang YG, Jou W, Pack S, Gavrilova O, Briggs J, Schnermann J: Adenosine A1 receptor deficiency in C57Bl/6 mice is associated with abnormal glucose tolerance, reduced insulin sensitivity, increased body weight and body fat fraction [Abstract]. Mid Atlantic Diabetes Research Symposium, Bethesda, June 2006
- 28.Sallstrom J, Carlsson PO, Fredholm BB, Larsson E, Persson AE, Palm F: Diabetes-induced hyperfiltration in adenosine A(1)-receptor deficient mice lacking the tubuloglomerular feedback mechanism. Acta Physiol (Oxf) 190: 253–259, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Pollock CA, Lawrence JR, Field MJ: Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol Renal Physiol 260: F946–F952, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Vallon V, Huang DY, Deng A, Richter K, Blantz RC, Thomson S: Salt-sensitivity of proximal reabsorption alters macula densa salt and explains the paradoxical effect of dietary salt on glomerular filtration rate in diabetes mellitus. J Am Soc Nephrol 13: 1865–1871, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Thomson SC, Vallon V, Blantz RC: Kidney function in early diabetes: The tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol 286: F8–F15, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Fujita H, Haseyama T, Kayo T, Nozaki J, Wada Y, Ito S, Koizumi A: Increased expression of glutathione S-transferase in renal proximal tubules in the early stages of diabetes: A study of type-2 diabetes in the Akita mouse model. Exp Nephrol 9: 380–386, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M: Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 55: 2502–2509, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Nasrallah R, Xiong H, Hebert RL: Renal prostaglandin E2 receptor (EP) expression profile is altered in streptozotocin and B6-Ins2Akita type I diabetic mice. Am J Physiol Renal Physiol 292: F278–F284, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Kakoki M, Kizer CM, Yi X, Takahashi N, Kim HS, Bagnell CR, Edgell CJ, Maeda N, Jennette JC, Smithies O: Senescence-associated phenotypes in Akita diabetic mice are enhanced by absence of bradykinin B2 receptors. J Clin Invest 116: 1302–1309, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD: Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J: Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest 114: 634–642, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SM, Chen L, Mizel D, Huang YG, Briggs JP, Schnermann J: Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol 292: F415–F422, 2007 [DOI] [PubMed] [Google Scholar]