Abstract
The prevalence, risk factors, and outcome of antibody-mediated rejection (AMR) of the kidney after simultaneous pancreas-kidney transplantation are unknown. In 136 simultaneous pancreas-kidney recipients who were followed for an average of 3.1 yr, 21 episodes of AMR of the kidney allograft were identified. Eight episodes occurred early (≤90 d) after transplantation, and 13 occurred later. Histologic evidence of concomitant acute cellular rejection was noted in 12 cases; the other nine had evidence only of humoral rejection. In 13 cases, clinical rejection of the pancreas was diagnosed simultaneously, and two of these were biopsy proven and were positive for C4d immunostaining. Multivariate analysis identified only one significant risk factor: Female patients were three times more likely to experience AMR. Nearly all early episodes resolved with treatment and did not predict graft loss, but multivariate Cox models revealed that late AMR episodes more than tripled the risk for kidney and pancreas graft loss; therefore, new strategies are needed to prevent and to treat late AMR in simultaneous pancreas-kidney transplant recipients.
Simultaneous pancreas and kidney (SPK) transplantation recipients with type 1 diabetes have a survival advantage equivalent to that of recipients of a living-donor kidney and superior to that of recipients of a deceased-donor kidney alone.1 Excellent short- and long-term patient, kidney, and pancreas survival rates are achieved when the organs are retrieved from young donors.1–5 In recent years, surgical technical improvements6 and the introduction of the new immunosuppressive agents tacrolimus and mycophenolate mofetil (MMF)7 have further improved the short-term results; however, rejection is detrimental to short- and long-term function of any organ transplant. Classical acute T cell rejection (ACR) can be treated effectively with steroids. Despite improvements in immunosuppression and decreasing rejection rates, subsets of patients have rejection episodes that are resistant to traditional therapy.8 Antibody-mediated rejection (AMR) defines all allograft rejection caused by antibodies directed against donor-specific HLA molecules or other cell antigens.9 The most common mechanism underlying AMR is an anamnestic robust antibody response that originates from previous antigenic exposure or de novo development of donor-specific antibody (DSA). Early diagnosis and aggressive treatment of AMR are essential for improving graft and patient outcomes and have been extensively reported in the context of isolated kidney transplantation.10–16 In addition, AMR is a widely known complication after heart transplantation,11,17 and isolated reports suggested that it may also affect the transplanted lung,18 the liver,19 or the pancreas.20,21 No studies have assessed the prevalence and significance of AMR after SPK transplantation.
RESULTS
Demographic Data
The study included 136 SPK transplant recipients; 97 of them received alemtuzumab and 39 basiliximab induction. All of them were treated with tacrolimus, MMF, or mycophenolate sodium (MPS) and steroids. Donor characteristics, recipient profile, and perioperative features are shown in Table 1. During the same period, 979 patients received an isolated kidney transplantation, 353 of whom were treated with a similar maintenance immunosuppression protocol. In this group of patients, alemtuzumab or basiliximab induction was used in 285 and 68 patients, respectively.
Table 1.
Demographic dataa
| Characteristic | Patients(n = 136) |
|---|---|
| Donor characteristics | |
| age (yr; mean ± SD) | 29.2 ± 12.8 |
| gender (% male) | 66.9 |
| nonwhite race (%) | 3.7 |
| deceased cardiac death (%) | 11 |
| serum creatinine before harvesting (mg/dl; mean ± SD) | 0.95 ± 0.39 |
| cold ischemia time (h; mean ± SD) | 15.9 ± 4.9 |
| Recipient characteristics | |
| age at transplantation (yr; mean ± SD) | 40.1 ± 7.0 |
| gender (% male) | 62.5 |
| nonwhite race (%) | 7.3 |
| black (%) | 5.1 |
| other nonwhite (%) | 2.2 |
| transplantation before periodic dialysis (%) | 36 |
| time on dialysis before transplantation (mo; mean ± SD) | 8.1 ± 13.5 |
| duration of diabetes before transplantation (yr; mean ± SD) | 25.8 ± 7.6 |
| donor CMV positive/recipient negative (%) | 29.4 |
| retransplantation (%) | 4.4 |
| pregnant before transplantation | 0 |
| pretransplantation blood transfusion (%) | 0 |
| two mismatches in HLA loci A/B/DR (%) | 38.2/66.9/44.1 |
| peak PRA (%; mean ± SD [range]) | 2.3 ± 5.7 (0 to 45) |
| PRA at transplantation (%; mean ± SD [range]) | 0.6 ± 2.2 (0 to 15) |
| delayed graft function (%; mean ± SD [range]) | 2.9 |
| follow-up after transplantation (yr; mean ± SD [range]) | 3.1 ± 0.8 (0.2 to 4.1) |
CMV, cytomegalovirus; PRA, panel reactive antibody.
Acute Kidney Transplant Rejection in SPK
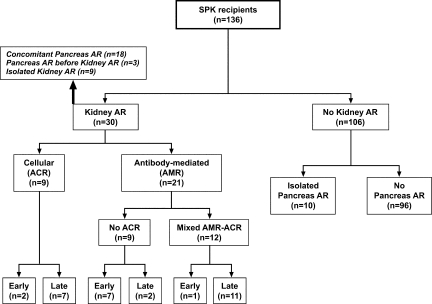
Thirty patients presented with biopsy-proven acute kidney transplant rejection, including 21 (15.4%) patients with AMR (Figure 1). Eight patients had AMR before day 90 (early AMR), and 13 patients had AMR after day 90 (late AMR). No significant differences were detected between patients who received alemtuzumab or basiliximab (data not shown).
Figure 1.
Patient distribution according to kidney and pancreas acute rejection.
Nine of the 21 patients had pure Banff type I AMR (Table 2). The majority of cases (n = 7) were diagnosed within the first 6 wk after transplantation (early AMR), whereas two others were diagnosed on days 211 and 376 (late AMR). All but one biopsy showed acute tubular injury and absence of inflammatory infiltrates (i0 in six and i1 in three). Early cases showed diffuse linear C4d+ staining in peritubular capillaries (PTC); in late cases, C4d staining was only focally positive. Patient 9 developed isolated grade II pancreas rejection 5 mo after transplantation and was treated with steroids. Seven months after this rejection, this patient presented with kidney allograft dysfunction, and the kidney biopsy showed pure AMR, with focally positive C4d staining and later pancreas grade III rejection with kidney and pancreas diffuse C4d staining (Figure 2).
Table 2.
Acute rejection diagnosisa
| Patient | Age | Gender | Day after SPKT | Clinical Pancreas Acute Rejection | AMR Histologic Parameters in the Kidney
|
ACR Histologic Parameters in the Kidney
|
Chronic Changes
|
DSA Detected [(Class, Locus)] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C4d | ATI | MAR | PTC Score | HEM | t | i | g | v | ACR | cg | ci | ct | cv | mm | ah | ||||||
| AMR | |||||||||||||||||||||
| 1 | 49 | Female | 7 | Yes | Diff Pos | Yes | Yes | 1 | 0 | 0 | 1 | 0 | 0 | Neg | 0 | 0 | 0 | 1 | 0 | 0 | Yes [II(DR,DQ)] |
| 2 | 46 | Female | 7 | Yes | Diff Pos | Yes | No | 0 | 0 | 0 | 0 | 1 | 0 | Neg | 0 | 1 | 1 | 1 | 1 | 1 | Yes [I(B), II(?)] |
| 3 | 45 | Female | 8 | Yes | Diff Pos | Yes | No | 0 | 0 | 0 | 0 | 0 | 0 | Neg | 0 | 1 | 1 | 0 | 0 | 0 | Yes [I(B)] |
| 4 | 49 | Female | 8 | No | Diff Pos | Yes | No | 0 | 0 | 0 | 0 | 0 | 0 | Neg | 0 | 0 | 0 | 0 | 0 | 0 | Yes [I(B), II(DR,DQ,DP)] |
| 5 | 37 | Female | 10 | Yes | Diff Pos | Yes | No | 0 | 0 | 0 | 0 | 0 | 0 | Neg | 0 | 0 | 0 | 0 | 0 | 0 | Yes [I(A), II(DP)] |
| 6 | 46 | Female | 16 | No | Diff Pos | No | No | 0 | 0 | 0 | 0 | 0 | 0 | Neg | 0 | 0 | 0 | 0 | 1 | 0 | Yes [I(B), II(DQ)] |
| 7 | 41 | Male | 38 | Yes | Diff Pos | Yes | Yes | 1 | 0 | 0 | 1 | 0 | 0 | Neg | 0 | 0 | 0 | 1 | 0 | 0 | Yes [I(B)] |
| 8 | 36 | Male | 211 | Before | Foc Pos | Yes | No | 0 | 0 | 0 | 0 | 0 | 0 | Neg | 0 | 1 | 1 | 0 | 0 | 1 | Yes [I(B), II(DR,DQ)] |
| 9 | 40 | Male | 376 | Beforeb | Foc Posb | Yes | Yes | 1 | 0 | 0 | 1 | 0 | 0 | Neg | 0 | 1 | 1 | 0 | 0 | 0 | Negative |
| Mixed acute AMR-ACR | |||||||||||||||||||||
| 10 | 47 | Female | 13 | No | Diff Pos | Yes | Yes | 2 | 0 | 1 | 1 | 0 | 1 | IIA | 0 | 1 | 0 | 0 | 1 | 0 | Yes [I(B), II(DR)] |
| 11 | 27 | Female | 95 | Yes | Foc Pos | No | No | 0 | 0 | 2 | 2 | 0 | 0 | IA | 0 | 2 | 1 | 0 | 0 | 0 | Not done |
| 12 | 35 | Male | 107 | Yes | Foc Pos | No | Yes | 1 | 0 | 2 | 2 | 0 | 0 | IB | 0 | 0 | 0 | 0 | 0 | 0 | Not done |
| 13 | 26 | Male | 124 | Yes | Diff Pos | No | Yes | 1 | 1 | 1 | 2 | 0 | 0 | Susp | 0 | 2 | 2 | 0 | 0 | 0 | Yes [I(B), II(DQ)] |
| 14 | 48 | Male | 135 | No | Foc Pos | No | Yes | 2 | 0 | 2 | 2 | 1 | 0 | IA | 0 | 1 | 1 | 0 | 1 | 0 | Not done |
| 15 | 28 | Female | 138 | Yes | Foc Pos | Yes | No | 0 | 0 | 3 | 2 | 1 | 0 | IB | 0 | 0 | 0 | 0 | 0 | 0 | Yes [I (A,B), II (DR,DQ)] |
| 16 | 34 | Female | 179 | Yes | Foc Pos | Yes | Yes | 2 | 0 | 3 | 2 | 0 | 0 | IB | 0 | 2 | 1 | 0 | 0 | 1 | Yes [I(B), II(DR)] |
| 17 | 36 | Female | 184 | Yes | Diff Pos | Yes | No | 0 | 1 | 1 | 1 | 0 | 0 | Susp | 0 | 1 | 1 | 1 | 0 | 0 | Yes [I(A,B), II(DR)] |
| 18 | 30 | Female | 237 | No | Diff Pos | Yes | Yes | 2 | 1 | 1 | 1 | 0 | 3 | III | 0 | 1 | 1 | 0 | 0 | 0 | Yes [I (A,B), II (DR)] |
| 19 | 34 | Female | 255 | Yes | Diff Pos | Yes | No | 0 | 0 | 1 | 1 | 0 | 0 | Susp | 0 | 1 | 1 | 0 | 0 | 0 | Yes [I(A), II(DR)] |
| 20 | 28 | Female | 294 | Yes | Foc Pos | Yes | Yes | 2 | 0 | 2 | 3 | 0 | 0 | IA | 0 | 2 | 2 | 0 | 0 | 0 | Not done |
| 21 | 29 | Male | 963 | Before | Foc Pos | Yes | Yes | 2 | 0 | 2 | 2 | 1 | 0 | IA | 0 | 1 | 1 | 1 | 0 | 0 | Yes [I(A), II(DR,DQ)] |
| ACR | |||||||||||||||||||||
| 22 | 28 | Male | 5 | Yes | Neg | Yes | Yes | 1 | 1 | 1 | 2 | 0 | 0 | Susp | 1 | 1 | 1 | 0 | 0 | 1 | Not done |
| 23 | 36 | Male | 20 | No | Neg | Yes | No | 0 | 0 | 1 | 1 | 0 | 0 | Susp | 0 | 0 | 0 | 0 | 0 | 1 | Not done |
| 24 | 49 | Male | 102 | Yes | Neg | Yes | Yes | 1 | 0 | 2 | 2 | 0 | 1 | IIA | 0 | 1 | 1 | 1 | 0 | 1 | Not done |
| 25 | 46 | Male | 111 | No | Neg | No | No | 0 | 1 | 3 | 2 | 0 | 0 | IB | 0 | 2 | 2 | 1 | 1 | 1 | Not done |
| 26 | 45 | Female | 153 | Yes | Neg | No | No | 0 | 0 | 2 | 3 | 0 | 0 | IB | 0 | 0 | 0 | 0 | 0 | 0 | Not done |
| 27 | 35 | Male | 195 | Yes | Neg | Yes | No | 0 | 0 | 3 | 2 | 0 | 0 | IB | 0 | 0 | 0 | 0 | 0 | 0 | Not done |
| 28 | 33 | Male | 299 | No | Neg | Yes | No | 0 | 0 | 3 | 3 | 0 | 0 | IB | 0 | 1 | 1 | 0 | 0 | 0 | Not done |
| 29 | 46 | Male | 435 | Yes | Neg | Yes | No | 0 | 0 | 2 | 2 | 0 | 0 | IA | 0 | 1 | 1 | 0 | 1 | 0 | Not done |
| 30 | 35 | Female | 905 | No | Neg | No | No | 0 | 0 | 2 | 2 | 0 | 0 | IA | 0 | 1 | 1 | 0 | 0 | 0 | Not done |
ah, arteriolar hyalinosis; ATI, acute tubular injury; cg, chronic glomerulopathy; ci, interstitial fibrosis; ct, tubular atrophy; cv, chronic vasculopathy; Diff Pos, diffusely positive; Foc Pos, focally positive; g, glomerulitis; HEM, interstitial hemorrhage; i, inflammation; MAR, capillary margination of inflammatory cells; mm, mesangial matrix expansion; Neg, negative; Pos, positive; SPKT, simultaneous pancreas kidney transplantation; Susp, suspicious; t, tubulitis; v, vasculitis.
For a detailed description of this case, see text and Figure 2.
Figure 2.
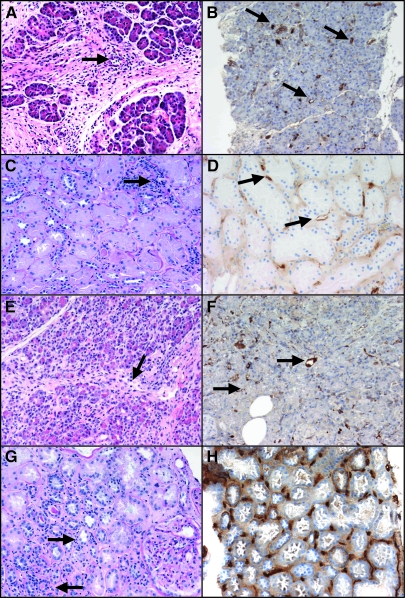
Histopathology of the allografts in a patient with kidney and pancreas AMR. (A) Light micrograph of the transplant pancreas (postoperative day [POD] 155) shows moderate septal mononuclear inflammatory infiltrate with eosinophils and venous endothelialitis (arrow), grade II pancreas acute rejection (hematoxylin and eosin). (B) C4d immunolabeling of the same POD 155 pancreas biopsy reveals interacinar capillaries diffusely positive for C4d (arrows). (C) Periodic acid-Schiff stain section of the transplant kidney (POD 376) shows mild acute tubular injury, mild interstitial mononuclear inflammation (i1), and no tubulitis (t0). There is focal mild capillary margination of leukocytes (PTC score 1, arrows23). (D) C4d focally positive in the same POD 376 kidney biopsy (arrows). (E) Light micrograph of the transplant pancreas (POD 467) shows moderate septal and interacinar (arrows) mononuclear infiltrates with focal endothelialitis (arrow), grade III pancreas acute rejection (hematoxylin and eosin). (F) Interacinar capillaries diffusely positive for C4d in the same POD 467 pancreas biopsy). (G) Light micrograph of the transplant kidney 4 d later (POD 471) shows again mild interstitial inflammation with leukocyte margination (arrows) and no tubulitis. (H) Same POD 471 biopsy shows peritubular capillaries diffusely positive for C4d. Magnifications: ×200 in A and C through G; ×100 in B.
In SPK patients with AMR of the kidney allograft, chronic histologic changes at the time of the index biopsy were not prominent. Only four patients showed mild grade I interstitial fibrosis and tubular atrophy, and no patient showed transplant glomerulopathy (Table 2). DSA were detected at the time of the index biopsy in all but one of these nine patients, who tested negative for HLA classes I and II antibodies at the time of index biopsy but became positive 95 d later, during a second AMR episode (Table 2).
Twelve of the 21 SPK patients with AMR of the kidney allograft had mixed AMR-ACR (Table 2). C4d staining was diffuse in five; the remaining patients had focal C4d staining. PTC margination of neutrophils was more frequently observed among biopsies with mixed AMR-ACR than in those with pure AMR. Six patients with mixed AMR-ACR had a PTC score of 2, which was never observed in pure AMR biopsies (P = 0.019). DSA data were available and positive in eight of 12 patients, all of whom had both class I and class II specificities (Table 2). The remaining four patients did not have DSA measured at the time of rejection; therefore, their condition can be diagnosed only as “suspected AMR.”22,23 All of these patients had indirect evidence of AMR: Steroid resistance and/or PTC margination. Nine patients had pure C4d negative ACR (Table 2).
Among the 353 patients who received a kidney-alone allograft during the same period and were treated with a similar immunosuppressive regimen, 90 presented with biopsy-proven acute kidney transplant rejection, including 26 (7.3%) with ACR and 64 (18.1%) with AMR. These rates are not significantly different from those observed in SPK patients (6.6 and 15.4%, respectively).
Acute Pancreas Transplant Rejection
Eighteen of the 30 patients with biopsy-proven acute kidney rejection had elevation of amylase and/or lipase and were assumed to have concomitant acute pancreas allograft rejection (Figure 1, Table 2). Of the remaining 12 patients, three developed clinical acute rejection of the pancreas before kidney allograft rejection (patients 8, 9, and 21) and nine did not have clinical evidence of pancreas rejection. Two patients (9 and 15) showed positive PTC C4d staining in pancreas biopsies performed later than the index kidney biopsy (Figure 2). Histologic findings in patient 9 are summarized in Figure 2; patient 15 showed focally positive (<50% of biopsy surface) C4d staining in both interacinar capillaries and PTC during a recurrent acute rejection episode that led to kidney and pancreas losses (Tables 3 and 4).
Table 3.
Treatment for rejection and clinical evolutiona
| Patient | Day after SPKT | C4d | ACR | Treatment | Kidney Status (Post-SPKT Time) | Last SCr | Pancreas Status (Post-SPKT Time) | Patient Status (Post-SPKT Time) |
|---|---|---|---|---|---|---|---|---|
| AMR | ||||||||
| 1 | 7 | Diff Pos | Neg | ST-IVIg-rituximab | Functioning (3.2 yr) | 1.0 | Functioning (3.2 yr) | Alive (3.2 yr) |
| 2 | 7 | Diff Pos | Neg | ST-IVIg-rituximab-pheresis | Functioning (4 yr) | 1.7 | Lost (22nd day): Acute rejection | Alive (4.0 yr) |
| 3 | 8 | Diff Pos | Neg | No | Lost (2.6 yr): Chronic active AMR | HD | Functioning (3.1 yr) | Alive (3.1 yr) |
| 4 | 8 | Diff Pos | Neg | ST-IVIg-rituximab-pheresis | Functioning (3.7 yr) | 1.4 | Functioning (3.7 yr) | Alive (3.7 yr) |
| 5 | 10 | Diff Pos | Neg | ST-IVIg-rituximab | Functioning (2.0 yr) | 0.8 | Functioning (2.0 yr) | Alive (2.0 yr) |
| 6 | 16 | Diff Pos | Neg | ST-IVIg-rituximab | Functioning (3.1 yr) | 1.2 | Functioning (3.1 yr) | Alive (3.1 yr) |
| 7 | 38 | Diff Pos | Neg | ST-IVIg-rituximab | Functioning (3.2 yr) | 1.6 | Functioning (3.2 yr) | Alive (3.2 yr) |
| 8 | 211 | Foc Pos | Neg | No | Functioning (2.4 yr) | 2.3 | Lost (5 mo): Acute rejection | Alive (2.4 yr) |
| 9 | 376 | Foc Posb | Neg | ST-Thymoglobulinb | Functioning (2.1 yr) | 2.4 | Functioning (2.1 yr) | Alive (2.1 yr) |
| Mixed Acute AMR-ACR | ||||||||
| 10 | 13 | Diff Pos | IIA | ST-Thymoglobulin-pheresis | Functioning (3.6 yr) | 1.0 | Functioning (3.6 yr) | Alive (3.6 yr) |
| 11 | 95 | Foc Pos | IA | ST-IVIg-Thymoglobulin-SRL | Functioning (1.5 yr) | 1.8 | Functioning (1.5 yr) | Alive (1.5 yr) |
| 12 | 107 | Foc Pos | IB | ST-Thymoglobulin | Functioning (2.5 yr) | 1.8 | Functioning (2.5 yr) | Alive (2.5 yr) |
| 13 | 124 | Diff Pos | Susp | ST-IVIg-Thymoglobulin | Functioning (2.4 yr) | 2.4 | Functioning (2.4 yr) | Alive (2.4 yr) |
| 14 | 135 | Foc Pos | IA | ST-IVIg-Thymoglobulin | Functioning (3.7 yr) | 2.9 | Lost (13th day): Primary nonfunction | Alive (3.7 yr) |
| 15 | 138 | Foc Pos | IB | ST-Thymoglobulin | Lost (1.1 yr): Acute rejection | HD | Lost (1.2 yr): Acute rejection | Alive (2.0 yr) |
| 16 | 179 | Foc Pos | IB | ST-Thymoglobulin | Lost (1.3 yr): Chronic active AMR | HD | Functioning (2.7 yr) | Alive (2.7 yr) |
| 17 | 184 | Diff Pos | Susp | ST-IVIg-Thymoglobulin-SRL | Lost (1.6 yr): IF/TA and TV | HD | Lost (1.7 yr): Chronic rejection | Alive (1.8 yr) |
| 18 | 237 | Diff Pos | III | ST-IVIg-Thymoglobulin | Lost (11 mo): Acute rejection | HD | Functioning (1.7 yr) | Alive (1.7 yr) |
| 19 | 255 | Diff Pos | Susp | ST-IVIg-rituximab-Thymoglobulin-SRL | Functioning (2.8 yr) | 1.5 | Lost (10 mo): Acute rejection | Alive (2.8 yr) |
| 20 | 294 | Foc Pos | IA | ST-Thymoglobulin | Lost (3.4 yr): Chronic active AMR | HD | Lost (1.2 yr): Acute rejection | Alive (3.6 yr) |
| 20 | 294 | Foc Pos | IA | ST-Thymoglobulin | Lost (3.4 yr): Chronic active AMR | HD | Lost (1.2 yr): Acute rejection | Alive (3.6 yr) |
| 21 | 963 | Foc Pos | IA | ST-SRL | Lost (3.1 yr): Death with function | 2.0 | Lost (3.1 yr): Death with function | Dead (3.1 yr): Pulmonary embolism |
| ACR | ||||||||
| 22 | 5 | Neg | Susp | ST-Thymoglobulin | Functioning (3.4 yr) | 1.3 | Functioning (3.4 yr) | Alive (3.4 yr) |
| 23 | 20 | Neg | Susp | ST | Functioning (4.1 yr) | 1.1 | Functioning (4.1 yr) | Alive (4.1 yr) |
| 24 | 102 | Neg | IIA | ST-Thymoglobulin | Lost (4 mo): Infection | HD | Lost (1.6 yr): Death with function | Dead (1.6 yr): Fungal sepsis |
| 25 | 111 | Neg | IB | ST | Lost (1.6 yr): Death with function | 1.5 | Lost (1.6 yr): Death with function | Dead (1.6 yr): Bacterial sepsis |
| 26 | 153 | Neg | IB | ST-Thymoglobulin | Functioning (1.3 yr) | 1.3 | Functioning (1.3 yr) | Alive (1.3 yr) |
| 27 | 195 | Neg | IB | ST-Thymoglobulin | Functioning (1.8 yr) | 2.8 | Functioning (1.8 yr) | Alive (1.8 yr) |
| 28 | 299 | Neg | IB | ST-Thymoglobulin | Functioning (3.2 yr) | 1.4 | Functioning (3.2 yr) | Alive (3.2 yr) |
| 29 | 435 | Neg | IA | ST-Thymoglobulin | Functioning (3.9 yr) | 1.9 | Functioning (3.9 yr) | Alive (3.9 yr) |
| 30 | 905 | Neg | IA | ST | Functioning (3.4 yr) | 3.0 | Functioning (3.4 yr) | Alive (3.4 yr) |
HD, hemodialysis; IF/TA, interstitial fibrosis and tubular atrophy; SCr, serum creatinine; SRL, sirolimus; ST, steroids; TV, transplant vasculopathy.
For a detailed description of this case, see text and Figure 2 (ST-Thymoglobulin treatment was not received until day 476 after SPKT, when repeated pancreas and kidney biopsies showed AMR).
Table 4.
Acute AMR diagnosis, first and subsequent biopsy-proven episodesa
| Patient | Episode (POD) | C4d | t | i | g | v | ACR | cg | ci | ct | cv | mm | ah | Chronic Banff Grade | Chronic Rejection | Pancreas Biopsies | DSA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 (7) | Diff Pos | 0 | 1 | 0 | 0 | Neg | 0 | 0 | 0 | 1 | 0 | 0 | Neg | Neg | – | Yes [II(DR,DQ)] |
| 2 | 1 (7) | Diff Pos | 0 | 0 | 1 | 0 | Neg | 0 | 1 | 1 | 1 | 1 | 1 | I | Pure Ab | – | Yes [I(B), II(?)] |
| 2 (814) | Foc Pos | 2 | 2 | 1 | 0 | IA | 1 | 1 | 1 | 0 | 1 | 1 | I | Mixed | Pancreas lost POD | Yes [I(A,B)] | |
| 3 | 1 (8) | Diff Pos | 0 | 0 | 0 | 0 | Neg | 0 | 1 | 1 | 0 | 0 | 0 | I | Pure Ab | – | Yes [I(B)] |
| 2 (729) | Diff Pos | 0 | 0 | 0 | 0 | Neg | 2 | 2 | 2 | 1 | 1 | 1 | II | Pure Ab | – | Yes [IB] | |
| 3 (809) | Diff Pos | 0 | 0 | 0 | 0 | Neg | 3 | 2 | 2 | 1 | 1 | 1 | II | Pure Ab | – | Yes [IB] | |
| 4 | 1 (8) | Diff Pos | 0 | 0 | 0 | 0 | Neg | 0 | 0 | 0 | 0 | 0 | 0 | Neg | Neg | – | Yes [I(B), II(DR,DQ,DP)] |
| 5 | 1 (10) | Diff Pos | 0 | 0 | 0 | 0 | Neg | 0 | 0 | 0 | 0 | 0 | 0 | Neg | Neg | – | Yes [I(A), II(DP)] |
| 6 | 1 (16) | Diff Pos | 0 | 0 | 0 | 0 | Neg | 0 | 0 | 0 | 0 | 1 | 0 | Neg | Neg | – | Yes [I(B), II(DQ)] |
| 7 | 1 (38) | Diff Pos | 0 | 1 | 0 | 0 | Neg | 0 | 0 | 0 | 1 | 0 | 0 | Neg | Neg | – | Yes [I(B)] |
| 8 | 1 (211) | Foc Pos | 0 | 0 | 0 | 0 | Neg | 0 | 1 | 1 | 0 | 0 | 1 | I | Pure Ab | – | Yes [I(B), II(DR,DQ)] |
| 9 | 1 (376) | Foc Pos | 0 | 1 | 0 | 0 | Neg | 0 | 1 | 1 | 0 | 0 | 0 | I | Pure Ab | Grade II ACR; C4d Diff Pos | Neg |
| 2 (471) | Diff Pos | 0 | 1 | 0 | 0 | Neg | 0 | 1 | 1 | 0 | 0 | 0 | I | Pure Ab | Grade III ACR; C4d Diff Pos | Yes [I(B), II(DR)] | |
| 10 | 1 (13) | Diff Pos | 1 | 1 | 0 | 1 | IIA | 0 | 1 | 0 | 0 | 1 | 0 | Neg | Neg | – | Yes [I(B), II(DR)] |
| 11 | 1 (95) | Foc Pos | 2 | 2 | 0 | 0 | IA | 0 | 2 | 1 | 0 | 0 | 0 | II | Mixed | – | Not done |
| 2 (199) | Foc Pos | 2 | 2 | 0 | 0 | IA | 0 | 2 | 1 | 1 | 0 | 0 | II | Mixed | – | Not done | |
| 3 (296) | Diff Pos | 0 | 1 | 0 | 0 | Neg | 1 | 2 | 2 | 2 | 0 | 1 | II | Pure Ab | – | Not done | |
| 12 | 1 (107) | Foc Pos | 2 | 2 | 0 | 0 | IB | 0 | 0 | 0 | 0 | 0 | 0 | Neg | Neg | – | Not done |
| 13 | 1 (124) | Diff Pos | 1 | 2 | 0 | 0 | Susp | 0 | 2 | 2 | 0 | 0 | 0 | II | Mixed | – | Yes [I(B), II(DQ)] |
| 14 | 1 (135) | Foc Pos | 2 | 2 | 1 | 0 | IA | 0 | 1 | 1 | 0 | 1 | 0 | I | Mixed | – | Not done |
| 15 | 1 (138) | Foc Pos | 3 | 2 | 1 | 0 | IB | 0 | 0 | 0 | 0 | 0 | 0 | Neg | Neg | – | Yes [I (A,B), II (DR,DQ)] |
| 2 (388) | Foc Pos | 0 | 1 | 0 | 0 | Neg | 0 | 1 | 1 | 0 | 0 | 0 | I | Pure Ab | No ACR; C4d Foc Pos | Yes [I (A,B), II (DR,DQ)] | |
| 16 | 1 (179) | Foc Pos | 3 | 2 | 0 | 0 | IB | 0 | 2 | 1 | 0 | 0 | 1 | II | Mixed | – | Yes [I(B), II(DR)] |
| 2 (257) | Foc Pos | 1 | 1 | 0 | 0 | Susp | 1 | 2 | 2 | 0 | 0 | 0 | II | Mixed | – | Yes [I(B), II(DR)] | |
| 3 (294) | Foc Pos | 1 | 1 | 0 | 0 | Susp | 1 | 2 | 2 | 0 | 0 | 0 | II | Mixed | – | Yes [I(B), II(DR)] | |
| 17 | 1 (184) | Diff Pos | 1 | 1 | 0 | 0 | Susp | 0 | 1 | 1 | 1 | 0 | 0 | I | Mixed | – | Yes [I(A,B), II(DR)] |
| 2 (218) | Foc Pos | 3 | 2 | 0 | 0 | IB | 0 | 2 | 2 | 1 | 0 | 0 | II | Mixed | – | Yes [I(A,B), II(DR)] | |
| 3 (300) | Neg | 1 | 1 | 0 | 0 | Susp | 0 | 3 | 2 | 1 | 0 | 0 | III | Pure cellular | – | Yes [II(DR)] | |
| 18 | 1 (237) | Diff Pos | 1 | 1 | 0 | 3 | III | 0 | 1 | 1 | 0 | 0 | 0 | I | Mixed | – | Yes [I (A,B), II (DR)] |
| 19 | 1 (255) | Diff Pos | 1 | 1 | 0 | 0 | Susp | 0 | 1 | 1 | 0 | 0 | 0 | I | Mixed | – | Yes [I(A), II(DR)] |
| 20 | 1 (294) | Foc Pos | 2 | 3 | 0 | 0 | IA | 0 | 2 | 2 | 0 | 0 | 0 | II | Mixed | – | Not done |
| 2 (466) | Neg | 2 | 2 | 0 | 0 | IA | 0 | 2 | 1 | 1 | 0 | 0 | II | Pure cellular | – | Not done | |
| 21 | 1 (963) | Foc Pos | 2 | 2 | 1 | 0 | IA | 0 | 1 | 1 | 1 | 0 | 0 | I-Mixed | Mixed | – | Yes [I(A), II(DR,DQ)] |
POD, postoperative day.
Of the 136 SPK transplants included in this study, 106 patients did not have kidney rejection. Of these, 10 patients received a diagnosis of having isolated acute pancreas rejection on the basis of biochemical data (Figure 1). These 10 patients received steroids and Thymoglobulin treatment and have excellent kidney and pancreas function after 1.6 to 3.9 yr of follow-up.
Treatment, Clinical Outcome, and Acute Rejection Recurrence
Four out of the nine pure AMR cases were successfully treated with steroids, intravenous Ig (IVIg), and rituximab (Table 3). In two other very early cases, plasmapheresis was added. One patient was treated with steroids and Thymoglobulin with the goal of controlling recurrent rejection, and two patients did not receive any specific antirejection treatment (Table 3).
All patients with mixed AMR-ACR received steroids, and all but one received Thymoglobulin for the control of kidney and pancreas rejection (Table 3). In addition, one patient with early mixed rejection received plasmapheresis; four patients received combined plasmapheresis and IVIg; and one patient received plasmapheresis, IVIg, and rituximab. Treatment with intravenous steroids was effective in reversal of rejection in three patients with pure ACR; the remaining six required Thymoglobulin (Table 3).
Eight patients had recurrence of acute kidney transplant rejection. Details on timing and type of rejections, acute and chronic Banff scores, C4d staining, and DSA are summarized in Table 4.
Risk Factors for AMR
In the univariate analysis, female gender (hazard ratio [HR] 3.144; 95% confidence interval [CI] 1.302 to 7.575; P = 0.011) and peak PRA (HR 1.043 each 1%; 95% CI 1.000 to 1.089; P = 0.052) were associated with AMR. In the multivariate analysis, only female gender was significantly associated with the development of AMR (adjusted HR 2.967; 95% CI 1.218 to 7.246; P = 0.016). Donor age, gender, race, type of deceased donor (cardiac-dead or brain-dead), donor serum creatinine, cytomegalovirus donor and recipient serology, recipient age and race, HLA A, B, or DR mismatches, time on dialysis, previous transplants, preservation time, delayed graft function, and induction treatment were not significantly associated with AMR, and their inclusion in the multivariate model did not exclude female gender as a risk factor. No differences were detected when the four patients with suspected AMR were excluded.
Graft Loss and Death
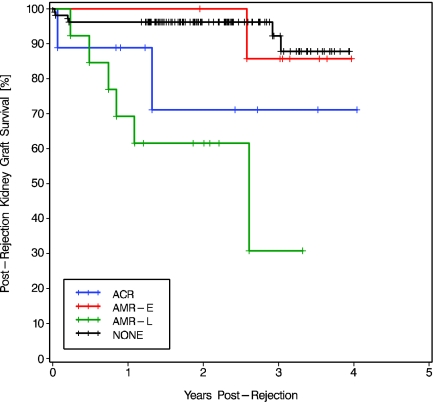
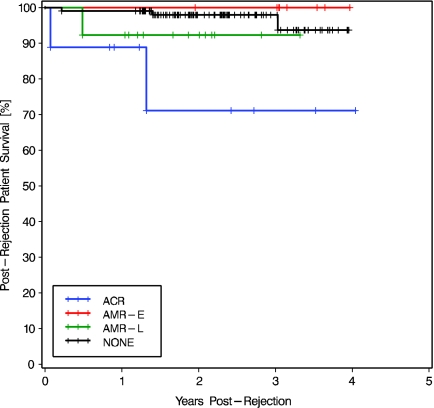
Kidney graft loss occurred in nine (30%) of the 30 patients with kidney allograft rejection (Table 3). During the same period, only six (5.7%) of the 106 nonrejectors lost their kidney grafts. Compared with nonrejectors, postrejection kidney graft survival was lower in patients with late AMR (at 2 yr 61.5 versus 96.2%; log-rank P < 0.0001; Figure 3). Postrejection kidney graft survival was lower in late AMR than in early AMR (P = 0.035).
Figure 3.
Kaplan Meier postrejection kidney graft survival in patients who developed ACR, early AMR (AMR-E), or late AMR (AMR-L), compared with posttransplantation kidney graft survival in nonrejectors (NONE). The overall comparison was significantly different (log rank P < 0.0001, Wilcoxon P < 0.0001). Two-stratum comparisons ACR versus AMR-E and ACR versus AMR-L were NS. Patients with ACR had lower kidney graft survival than no rejectors (log-rank P = 0.059, Wilcoxon P = 0.028). Patients with AMR-L showed lower kidney graft survival than those with AMR-E (log-rank P = 0.035, Wilcoxon P = 0.041) or without rejection (log-rank P < 0.0001, Wilcoxon P < 0.0001).
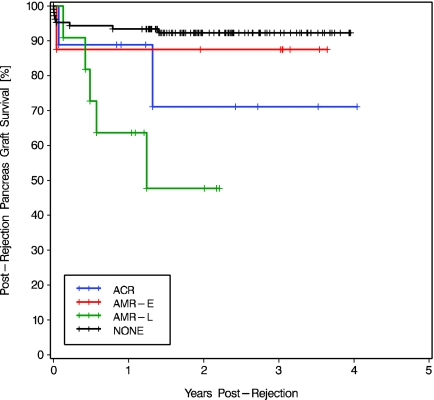
Pancreas graft loss occurred in 10 (33.3%) of the 30 patients with kidney allograft rejection (Table 3). During the same period, only eight (7.5%) of the 106 nonrejectors lost their pancreas. Postrejection pancreas graft survival was lower in patients with late AMR than that of nonrejectors (at 2 yr 47.7 versus 92.3%; log-rank P < 0.0001; Figure 4). The difference in pancreas survival between early and late AMR did not reach statistical significance (P = 0.143).
Figure 4.
Kaplan Meier postrejection pancreas graft survival in patients who developed ACR, AMR-E, or AMR-L, compared with posttransplantation pancreas graft survival in nonrejectors (NONE). The overall comparison was significantly different (log rank P = 0.001, Wilcoxon P = 0.002). Two-stratum comparisons ACR versus AMR-E, ACR versus AMR-L, AMR-E versus AMR-L, and ACR versus NONE were NS. Patients with AMR-L had significantly lower pancreas survival than nonrejectors (log-rank P < 0.0001, Wilcoxon P = 0.0001).
Crude patient survival was 90% during the study period, and three (10%) patients died during the follow-up period (Table 3). Only three of the 106 nonrejectors died. Postrejection patient survival in patients with kidney ACR was significantly lower than posttransplantation patient survival in nonrejectors (at 2 yr 71.1 versus 97.9%; log-rank P = 0.002; Figure 5). Differences in postrejection patient survival between those who developed ACR and AMR were NS. No change was detected when the four patients with suspected AMR were excluded.
Figure 5.
Kaplan Meier postrejection patient survival in patients who developed ACR, AMR-E, or AMR-L, compared with posttransplantation patient survival in nonrejectors (NONE). The overall comparison was significantly different (log rank P = 0.016, Wilcoxon P = 0.006). Two-stratum comparisons ACR versus AMR-E and ACR versus AMR-L were NS, whereas patients with ACR had lower survival than nonrejectors (log-rank P = 0.002, Wilcoxon P = 0.0003).
Impact of AMR and Other Factors on Kidney and Pancreas Graft Loss
In univariate analysis, a higher donor serum creatinine at procurement, recipient female gender, recipient nonwhite race, and the development of any AMR or late AMR were significant risk factors for kidney allograft loss in SPK recipients (Table 5). Early AMR was not significantly related to kidney graft loss. In multivariate analysis, donor serum creatinine, female gender, and late AMR remained significantly associated with kidney graft loss. Older donor age, higher donor serum creatinine at procurement, and the development of any AMR or late AMR were significant risk factors for pancreas allograft loss in univariate analyses (Table 6). In the multivariate model, donor age and serum creatinine and the development of late AMR remained associated with pancreas graft loss. Early AMR was not significantly associated with pancreas graft loss.
Table 5.
Risk factors for kidney allograft loss after SPKTa
| Variable | Univariate Analysis
|
Multivariate Analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Donor SCr (each mg/dl) | 2.679 | 1.435 to 5.001 | 0.0020 | 2.343 | 0.993 to 5.531 | 0.052 |
| Recipient female gender | 3.333 | 1.140 to 9.803 | 0.0280 | 4.166 | 1.194 to 14.490 | 0.025 |
| Recipient nonwhite | 5.000 | 1.374 to 18.180 | 0.0150 | 3.831 | 0.921 to 15.870 | 0.065 |
| AMR | 3.882 | 1.396 to 10.790 | 0.0090 | 1.883 | 0.612 to 5.800 | 0.270 |
| Late AMR | 6.605 | 2.349 to 18.570 | 0.0003 | 3.339 | 0.999 to 11.190 | 0.050 |
Donor age, gender, cardiac status, and recipient age, CMV status, HLA A, B, and DR mismatches, time on dialysis, retransplantation, total preservation time, the type of induction agent used, and development of acute ACR or early AMR were not significantly associated with kidney graft loss in the univariate analysis. The variables significantly associated (P ≤ 0.05) were included in the multivariate analysis
Table 6.
Risk factors for pancreas allograft loss after SPKTa
| Variable | Univariate Analysis
|
Multivariate Analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Donor age (each year) | 1.036 | 1.001 to 1.073 | 0.042 | 1.047 | 1.008 to 1.088 | 0.018 |
| Donor SCr (each mg/dl) | 2.682 | 1.470 to 4.892 | 0.001 | 2.757 | 1.338 to 5.679 | 0.006 |
| AMR | 2.978 | 1.117 to 7.938 | 0.029 | 2.320 | 0.802 to 6.710 | 0.120 |
| Late AMR | 4.519 | 1.606 to 12.720 | 0.004 | 3.224 | 1.003 to 10.360 | 0.049 |
Donor gender, cardiac status, recipient age, race, gender, CMV status, HLA A, B, and DR mismatches, peak PRA, time on dialysis, retransplantation, total preservation time, the type of induction agent used, and development of acute ACR or early AMR were not significantly associated with pancreas graft loss in the univariate analysis. The variables significantly associated (P ≤ 0.05) were included in the multivariate analysis.
DISCUSSION
We report for the first time a high incidence of AMR (15%) in a homogeneous population of SPK transplant recipients with a low immunologic risk. Prompt and aggressive treatment of early AMR episodes yielded good results, with the resolution of rejection and good outcomes; however, late (>3 mo) episodes were associated with significant immediate and late graft loss. Only late AMR was associated with kidney and pancreas graft loss in the multivariate analysis. The deleterious effects of late AMR episodes were previously reported in isolated kidney allograft recipients.24 The results of this study extend this observation to SPK transplant recipients. Our treatment protocol based on steroids, rituximab, and IVIg was successful to reverse early rejection but had limited benefit for late AMR.
We speculate that the different impact of early versus late AMR stems from the nature of the immune response underlying these disorders. Whereas the robust antibody response in early AMR results in complement-mediated lytic injury of the endothelium and rapidly progressive allograft dysfunction allowing an early diagnosis, the smoldering generation of de novo DSA observed in late AMR results in nonlytic complement-mediated injury, partial accommodation, and slow allograft dysfunction.25–28 Whether the differences in the nature of the immune response influence the response to therapy in these entities has yet to be studied.
Female gender was the only risk factor for AMR and was also an independent risk factor for graft loss. It is noteworthy that no pregnancies and/or pretransplantation transfusions were present in our study population; therefore, this gender-related risk was totally independent of these widely known sensitizing events. In our series, peak panel reactive antibody was marginally associated with AMR, but the significance was lost when we adjusted for female gender. Although the association of female gender with graft loss was previously reported in a registry-based study,5 all cases of pancreas AMR published to date have occurred in men.20,21
In addition to female gender, a high donor serum creatinine was an independent risk factor for both kidney and pancreas graft loss. This finding is not surprising because it is widely known that donor age and donor kidney function are closely related to graft survival after SPK transplantation.5,29
Our study suggests that most AMR episodes in SPK transplant recipients can be diagnosed in a similar way as in isolated kidney transplantation, with the triad of allograft dysfunction, C4d positivity in PTC, and detectable DSA in recipient serum.9,30,31 Some of the cases reported here with C4d staining and allograft dysfunction are almost undoubtedly AMR episodes, despite the lack of DSA at the time of diagnosis. Furthermore, some cases of pancreas transplant dysfunction with stable kidney function can be antibody mediated, as shown in patient 9. Hence, adequate DSA surveillance and C4d immunolabeling of pancreas biopsies may yield a prompt diagnosis of AMR and allow adequate and early treatment of isolated pancreas rejection.
Most of our patients did not undergo pancreas biopsies; consequently, our study is mainly a study of AMR in kidney allografts. This raises the natural question as to whether our SPK experience is any different from that of our kidney transplant recipients using comparable immunosuppression. To address this issue, we compared our SPK experience with a homogeneous group of 353 patients who received a kidney alone transplant during the same period and received similar immunosuppression. In this last group of patients, both ACR and AMR rates were very similar to those observed in our SPK patients. We therefore conclude that no relevant differences may be expected between SPK and isolated kidney recipients with regard to rate of AMR in the kidney allograft.
In light of these results, our awareness of the possibility of concomitant, consecutive, or asynchronous AMR in SPK recipients has led to an increase in the number of pancreas biopsies performed at our institution; however, given the paucity of pancreas biopsies in this study, we are unable to provide any important conclusions regarding the natural history of histologically proven pancreas AMR. Clearly, a consensus recommendation concerning the diagnosis of pancreas AMR and management is needed.
To our knowledge, only two cases of AMR have been reported independently in two pancreas allograft recipients.20,21 The first one was a 44-yr-old male patient who developed kidney AMR early after SPK transplantation, 7 yr after first isolated deceased-donor kidney transplantation. After intensive treatment and during kidney allograft function recovery, pancreas function began to deteriorate and a biopsy showed diffuse C4d staining in the interacinar capillaries.20 Finally, pancreas and kidney rejections were resolved after repeated rituximab and plasmapheresis sessions. No pancreas biopsy was performed at the time of kidney AMR, so it is difficult to interpret the overlapping of both rejections.
The second case was a 46-yr-old male patient who developed pancreas AMR 6 mo after pancreas transplantation, following isolated deceased-donor kidney transplantation 7 yr before.21 Notably, both AMR cases developed despite intense induction therapy with Thymoglobulin and maintenance immunosuppression with tacrolimus, MMF, and steroids. In both cases, diffuse C4d deposition in interacinar capillaries was shown by immunofluorescence.
Our study is a retrospective analysis of our SPK AMR population and is subjected to the biases inherent to all retrospective surveys. The lack of protocol biopsies and surveillance of DSA limits our ability to assess subclinical rejection, both cellular and antibody mediated, because the diagnosis of rejection in our population was made only after clinical evidence of acute graft dysfunction. This bias is somewhat reduced by including only patients with biopsy-proven ACR or AMR and excluding all cases without a histologic diagnosis. In addition, the lack of posttreatment DSA testing and kidney and pancreas biopsies may have led to inadequate treatment of some patients and continuous kidney and pancreas injury. These limitations might explain some of the late kidney and pancreas graft losses. Some focally positive C4d kidney biopsies were not treated and may have been responding to therapeutic intervention. Despite these deficiencies, our patient sample is large and treated in a single institution, thereby avoiding problems inherent to registry data analysis that cannot account for center-related variations in patient care. In particular, induction and maintenance immunosuppression were consistent throughout the study in all patients.
In conclusion, clinically significant AMR may develop in SPK transplant recipients, as it has been repeatedly reported in isolated kidney transplants. The incidence of kidney AMR in this SPK population with low immunologic risk was 15%. Female gender was a significant risk factor for AMR, and late (as opposed to early) AMR was associated with a significant decrease in kidney and pancreas survival rates regardless of treatment. Preventive and/or different treatment strategies are required to address late AMR in SPK transplant recipients.
CONCISE METHODS
We performed a retrospective review of all adult (≥18 yr of age) patients who received an SPK transplant at the University of Wisconsin Transplant Program from October 2002 to September 2005. Data were collected using our Health Insurance Portability and Accountability Act–regulated Transplant Database System after internal review board approval. Clinical charts were reviewed as needed for additional data. All transplants were performed with enteric drainage of pancreatic exocrine secretions and systemic venous drainage. All patients were followed until death or November 2006. The SPK patients were compared with all of the patients who received an isolated kidney transplant and a similar immunosuppressive protocol during the same period.
Initial and Maintenance Immunosuppression Treatment
Patients received induction therapy with alemtuzumab (Campath 1-H; ILEX, San Antonio, TX), administered on day 0 and day 1 (30 mg each dose), or the IL-2 receptor antagonist basiliximab (Simulect; Novartis, East Hanover, NJ), administered at a dosage of 20 mg on day 0 and day 4.30 Maintenance immunosuppression consisted of prednisone, either MMF (CellCept; Roche, Nutley, NJ; 1000 mg twice daily since day 1) or enteric-coated MPS (Myfortic; Novartis; 720 mg twice daily since day 1), and tacrolimus (Prograf; Astellas, Deerfield, IL) usually started when the serum creatinine level declined to <3.0 mg/dl, with a target level of 6 to 10 ng/ml.
Diagnosis of Acute Rejection, C4d Labeling, and DSA
All patients who developed a sudden increase in serum creatinine without an obvious reason or experienced delayed graft function for >10 d underwent a kidney transplant biopsy. Pancreas biopsies were performed more selectively, usually after an unexplained increase in serum amylase that did not correlate with kidney graft dysfunction. No protocol biopsies were performed, and at least two needle cores were obtained. One of them was fixed in 10% neutral buffered formalin for at least 1 h for paraffin embedding; the other one was fixed in cold acetone to obtain frozen sections. Eight serial paraffin sections (2 to 3 μ) per biopsy were stained with hematoxylin and eosin, Masson's trichrome, periodic acid-Schiff, and argyrophilic impregnations. Staining for the split product of complement C4d was performed on 4-μ acetone-fixed frozen sections labeled with a 1:600 dilution of an anti-C4d mAb (Biogenesis, Kingston, NH) at a constant incubation temperature of 42°C for 32 min. Target detection was performed by an indirect biotin-avidin system incorporating diaminobenzidine. Endogenous peroxidase quenching and biotin blocking were done on-instrument with kit reagents (Ventana Medical Systems, Tucson, AZ). The immunolabeled slides were counterstained with hematoxylin and then dehydrated and coverslipped. C4d staining in the kidney was interpreted as positive when a linear staining along the PTC basement membranes was evident in >10 of them. When this pattern was observed in >50% of PTC, this staining was considered diffuse positive; <50% was considered focally positive. A similar approach was used for defining C4d positivity in pancreatic interacinar capillaries.
Biopsies were evaluated for adequacy and rejection scores according to the Banff 97 (Banff 05 update).22,23,31 Patients were classified as having AMR after a kidney allograft biopsy (index biopsy) was identified as diagnostic of acute rejection in the setting of graft dysfunction and positive C4d staining of the PTC.10,22,31 Capillary margination of inflammatory cells was scored as proposed by the updated 2005 Banff Report23: 0, absence; 1, cortical peritubular capillary with three to four luminal inflammatory cells; 2, five to 10 cells; and 3, >10 cells. ACR (classical cellular rejection) was diagnosed when the kidney biopsy showed signs of interstitial inflammation, tubulitis, and/or intimal arteritis and no C4d staining.23 Some cases had features of both AMR and ACR and were defined as mixed.32 Chronic kidney histologic changes were scored (I, II, and III) according to Banff 05 criteria23 and then subclassified into pure Ab (any chronic changes with C4d diffuse or focally positive), mixed (any chronic changes with cellular findings and C4d diffuse or focally positive), pure cellular (any chronic changes with cellular rejection and negative C4d staining), and inactive/scarred (any chronic change without cellular rejection and C4d negative). Pancreas allograft rejection was defined as either grades II through V rejection per the University of Maryland criteria33 on pancreas allograft biopsy or a >50% increase in serum pancreas enzymes in the setting of a kidney allograft biopsy with findings of rejection and the absence of abnormality in the pancreas by imaging techniques. We assigned early AMR when the diagnosis was made before day 91 after transplantation and late AMR when it was after day 90.
The presence of DSA in the recipient's serum was regarded as a supporting criterion for the diagnosis of AMR.34 Since 2004, magnetic microbeads coated with purified class I or class II HLA antigens have been used for the detection of serum anti-HLA antibodies using Luminex xMAP multiplex technology. As this technology has advanced, newer beads became available (LABScreen Single Antigen Class I and Class II; One Lambda, Canoga Park, CA), allowing for the detection of single antigen class I and class II specificities. LABScreen microbeads for single antigen class I (groups 1 and 2) include 79 beads for 29 HLA-A and 50 HLA-B specificities. LABScreen microbeads for single antigen class II (group 1) were introduced at our institution in 2006. This kit includes 57 beads (17 HLA-DRB1, two HLA-DRB3, two HLA-DRB4, two HLA-DRB5, 18 HLA-DQB1, and 13 HLA-DPB1). Sera from patients with C4d+ rejection without detectable anti-HLA specificities at the time of rejection were retested with this most recent assay. Retrospectively, patients who received a diagnosis of C4d+ AMR before 2004 were tested for DSA in previously stored frozen serum samples.
Antirejection Treatment Protocol
Patients with ACR received steroid boluses and/or Thymoglobulin (Genzyme, Cambridge, MA) when suboptimal response to steroid treatment or grade more than IB ACR was detected. In AMR cases, the treatment was based on (1) inhibition of residual antibodies with IVIg (Cytogam [Medimmune, Gaithersburg, MD] or Gammagard Liquid 10% [Baxter Healthcare Corp., Deerfield, IL]) 100 mg/kg weekly for 4 wk, prolonged up to eight doses depending on clinical outcome; (2) suppression or depletion of B cells with the anti-CD20 antibody rituximab (Biogen-IDEC and Genentech Pharmaceuticals, San Francisco, CA) at a dosage of 375 mg/m2 intravenously for one to two doses; and (3) steroid boluses and Thymoglobulin when a vascular C4d− component was detected in the biopsy. The elimination of DSA with plasmapheresis was undertaken only in some early AMR cases, not in late AMR. All analyzed patients were receiving tacrolimus, MMF/MPS, and steroids during the rejection episode, so conversion to this regimen was not a possibility.10
Statistical Analyses
Results are expressed as percentages or means ± SD. Wilcoxon two-sample, χ2, and Fisher exact test were used as needed for particular comparisons. Univariate and multivariate Cox proportional hazard risk models were calculated for detecting risk factors for AMR and graft loss. Kaplan-Meier analysis with log-rank test and Wilcoxon comparison were used for the measurement of kidney and pancreas free of rejection survival and postrejection survival. All P values are two sided, and P ≤ 0.05 was considered statistically significant. All analyses were performed with SAS software (SAS Institute, Cary, NC).
DISCLOSURES
None.
Acknowledgments
J.P. had a temporary appointment at the University of Wisconsin supported by Institute Carlos III-Spanish Health Department grant BA06/90020. M.D.S. was supported by American Heart Association Scientist Development Grant 0235290N and National Institutes of Health UO-1 Grant (AI-050022).
We thank the transplant coordinators, nursing staff, and transplant fellows who provided excellent care to these patients.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Rayhill SC, D'Alessandro AM, Odorico JS, Knechtle SJ, Pirsch JD, Heisey DM, Kirk AD, Van der Werf W, Sollinger HW: Simultaneous pancreas-kidney transplantation and living related donor renal transplantation in patients with diabetes: Is there a difference in survival? Ann Surg 231: 417–423, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sollinger HW, Odorico JS, Knechtle SJ, D'Alessandro AM, Kalayoglu M, Pirsch JD: Experience with 500 simultaneous pancreas-kidney transplants. Ann Surg 228: 284–296, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Carlo A, Odorico JS, Leverson GE, Fernandez LA, Chin LT, Becker YT, Knechtle SJ, Pirsch JD, D'Alessandro AM, Sollinger HW. Long-term outcomes in simultaneous pancreas-kidney transplantation: Lessons relearned. Clin Transpl 215–220, 2003 [PubMed]
- 4.Fernandez LA, Di Carlo A, Odorico JS, Leverson GE, Shames BD, Becker YT, Chin LT, Pirsch JD, Knechtle SJ, Foley DP, Sollinger HW, D'Alessandro AM: Simultaneous pancreas-kidney transplantation from donation after cardiac death: Successful long-term outcomes. Ann Surg 242: 716–723, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvalaggio PR, Schnitzler MA, Abbott KC, Brennan DC, Irish W, Takemoto SK, Axelrod D, Santos LS, Kocak B, Willoughby L, Lentine KL: Patient and graft survival implications of simultaneous pancreas kidney transplantation from old donors. Am J Transplant 7: 1561–1571, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Pirsch JD, Odorico JS, D'Alessandro AM, Knechtle SJ, Becker BN, Sollinger HW: Posttransplant infection in enteric versus bladder-drained simultaneous pancreas-kidney transplant recipients. Transplantation 66: 1746–1750, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Odorico JS, Pirsch JD, Knechtle SJ, D'Alessandro AM, Sollinger HW: A study comparing mycophenolate mofetil to azathioprine in simultaneous pancreas-kidney transplantation. Transplantation 66: 1751–1759, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Colvin RB: Antibody-mediated renal allograft rejection. J Am Soc Nephrol 18: 1046–1056, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, Kupiec-Weglinski J, Matas A, Montgomery RA, Nickerson P, Platt JL, Rabb H, Thistlethwaite R, Tyan D, Delmonico FL: National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant 4: 1033–1041, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Pascual M, Saidman S, Tolkoff-Rubin N, Williams WW, Mauiyyedi S, Duan JM, Farrell ML, Colvin RB, Cosimi AB, Delmonico FL: Plasma exchange and tacrolimus-mycophenolate rescue for acute humoral rejection in kidney transplantation. Transplantation 66: 1460–1464, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Jordan SC, Quartel AW, Czer LS, Admon D, Chen G, Fishbein MC, Schwieger J, Steiner RW, Davis C, Tyan DB: Posttransplant therapy using high-dose human immunoglobulin (intravenous gammaglobulin) to control acute humoral rejection in renal and cardiac allograft recipients and potential mechanism of action. Transplantation 66: 800–805, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Crespo M, Pascual M, Tolkoff-Rubin N, Mauiyyedi S, Collins AB, Fitzpatrick D, Farrell ML, Williams WW, Delmonico FL, Cosimi AB, Colvin RB, Saidman SL: Acute humoral rejection in renal allograft recipients: I. Incidence, serology and clinical characteristics. Transplantation 71: 652–658, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Bohmig GA, Regele H, Exner M, Derhartunian V, Kletzmayr J, Saemann MD, Horl WH, Druml W, Watschinger B: C4d-positive acute humoral renal allograft rejection: Effective treatment by immunoadsorption. J Am Soc Nephrol 12: 2482–2489, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Rocha PN, Butterly DW, Greenberg A, Reddan DN, Tuttle-Newhall J, Collins BH, Kuo PC, Reinsmoen N, Fields T, Howell DN, Smith SR: Beneficial effect of plasmapheresis and intravenous immunoglobulin on renal allograft survival of patients with acute humoral rejection. Transplantation 75: 1490–1495, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Shah A, Nadasdy T, Arend L, Brennan J, Leong N, Coppage M, Orloff M, Demme R, Zand MS: Treatment of C4d-positive acute humoral rejection with plasmapheresis and rabbit polyclonal antithymocyte globulin. Transplantation 77: 1399–1405, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Becker YT, Becker BN, Pirsch JD, Sollinger HW: Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant 4: 996–1001, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Reed EF, Demetris AJ, Hammond E, Itescu S, Kobashigawa JA, Reinsmoen NL, Rodriguez ER, Rose M, Stewart S, Suciu-Foca N, Zeevi A, Fishbein MC, International Society for Heart and Lung Transplantation: Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant 25: 153–159, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Miller GG, Destarac L, Zeevi A, Girnita A, McCurry K, Iacono A, Murray JJ, Crowe D, Johnson JE, Ninan M, Milstone AP: Acute humoral rejection of human lung allografts and elevation of C4d in bronchoalveolar lavage fluid. Am J Transplant 4: 1323–1330, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Watson R, Kozlowski T, Nickeleit V, Woosley JT, Schmitz JL, Zacks SL, Fair JH, Gerber DA, Andreoni KA: Isolated donor specific alloantibody-mediated rejection after ABO compatible liver transplantation. Am J Transplant 6: 3022–3029, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Melcher ML, Olson JL, Baxter-Lowe LA, Stock PG, Posselt M: Antibody-mediated rejection of a pancreas allograft. Am J Transplant 6: 423–428, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Carbajal R, Karam G, Renaudin K, Maillet F, Cesbron A, Rostaing L, Cantarovich D, Soulillou JP, Blancho G: Specific humoral rejection of a pancreas allograft in a recipient of pancreas after kidney transplantation. Nephrol Dial Transplant 22: 942–944, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K: Antibody-mediated rejection criteria: An addition to the Banff'97 classification of renal allograft rejection. Am J Transplant 3: 708–714, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ: Banff '05 Meeting Report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7: 518–526, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Sun Q, Liu ZH, Ji S, Chen J, Tang Z, Zeng C, Zheng C, Li LS: Late and early C4d-positive acute rejection: Different clinico-histopathological subentities in renal transplantation. Kidney Int 70: 377–383, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Terasaki P, Ozawa M: Predicting kidney graft failure by HLA antibodies: A prospective trial. Am J Transplant 4: 438–443, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Hourmant M, Cesbron-Gautier A, Terasaki P, Mizutani K, Moreau A, Meurette A, Dantal J, Giral M, Blancho G, Cantarovich D, Karam G, Follea G, Soulillou JP, Bignon JD: Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol 16: 2804–2812, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lee P-C, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, Chen YL, Tsai A, Lei HY: All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation 74: 1192–1194, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Mauiyyedi S, Della Pelle P, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, Williams WW, Cosimi AA, Schneeberger EE, Colvin RB: Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol 12: 574–582, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Andreoni KA, Brayman KL, Guidinger MK, Sommers CM, Sung RS: Kidney and pancreas transplantation in the United States, 1996–2005. Am J Transplant 7: 1359–1375, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Knechtle SJ, Fernandez LA, Pirsch JD, Becker BN, Chin LT, Becker YT, Odorico JS, D'alessandro AM, Sollinger HW: Campath-1H in renal transplantation: The University of Wisconsin experience. Surgery 136: 754–760, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y, et al.: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Nickeleit V, Andreoni K: The classification and treatment of antibody-mediated renal allograft injury: Where do we stand? Kidney Int 71: 7–11, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Drachenberg CB, Papadimitriou JC, Klassen DK, Racusen LC, Hoehn-Saric EW, Weir MR, Kuo PC, Schweitzer EJ, Johnson LB, Bartlett ST: Evaluation of pancreas transplant needle biopsy: Reproducibility and revision of histologic grading system. Transplantation 63: 1579–1586, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Mauiyyedi S, Crespo M, Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Tolkoff-Rubin NE, Williams WW, Delmonico FL, Cosimi AB, Colvin RB: Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol 13: 779–787, 2002 [DOI] [PubMed] [Google Scholar]