Abstract
The prorenin/renin receptor is a recently discovered component of the renin-angiotensin system. The effects of aliskiren, a direct inhibitor of human renin, were compared with the handle region decoy peptide (HRP), which blocks the prorenin/renin receptor, in double-transgenic rats overexpressing the human renin and angiotensinogen genes. After 7 wk, all aliskiren-treated rats were alive, whereas mortality was 40% in vehicle-treated and 58% in HRP-treated rats. Aliskiren but not the HRP reduced BP and normalized albuminuria, cystatin C, and neutrophil gelatinase-associated lipocalin, a marker of renal tubular damage, to the levels of nontransgenic controls. In vitro, human renin and prorenin induced extracellular signal–regulated kinase 1/2 phosphorylation, independent of angiotensin II (AngII), in vascular smooth muscle cells. Preincubation with the HRP or aliskiren did not prevent renin- and prorenin-induced extracellular signal–regulated kinase 1/2 phosphorylation, whereas the MAP kinase kinase (MEK1/2) inhibitor PD98059 prevented both. In conclusion, renin inhibition but not treatment with the HRP protects against AngII-induced renal damage in double-transgenic rats. In addition, the in vitro data do not support the use of the HRP to block AngII-independent prorenin- or renin-mediated effects.
A low molecular weight, nonpeptide, direct human renin inhibitor (DRI), aliskiren, is now available to treat hypertension.1 The DRI demonstrated target-organ protection in a double-transgenic rat (dTGR) model of high human renin hypertension.2–4 Nguyen et al.5 cloned a novel receptor (prorenin/renin receptor [(P)RR]) that interacts with both renin and prorenin. The (P)RR, more correctly termed RR/ATP6AP2, is a single-transmembrane-domain protein of 350 amino acids with a large unglycosylated and highly hydrophobic N-terminal domain and a short cytoplasmic tail of approximately 20 amino acids. The (P)RR is highly conserved across species.6 Renin bound to the (P)RR has a three- to five-fold increased catalytic activity.5 Furthermore, when the (P)RR binds prorenin, the latter molecule displays catalytic activity without a proteolytic conversion to active renin (“nonproteolytic activation”).5,7 In contrast to proteolytic activation, whereby the prosegment is cleaved off, nonproteolytic activation is characterized by the unfolding of the prosegment from the enzymatic cleft, leading to a (P)RR-induced conformational change in the prorenin molecule. In addition, renin induces direct (P)RR signaling, leading to extracellular signal–regulated kinase 1/2 (ERK1/2) mitogen-activated protein kinase (MAPK) activation that is independent of angiotensin II (AngII).5,8,9
Ichihara et al.10 and Suzuki et al.11 introduced an epitope of the prorenin prosegment that they termed the handle region peptide (HRP). The HRP presumably blocks binding of prorenin to the (P)RR, thereby functioning as a decoy peptide. Satofuka et al.12 published a schematic of how they envision the HRP to work. The model relies on the handle region and would predict to be confined to prorenin. The group demonstrated that long-term HRP treatment in diabetic mice and rats improved nephropathy without affecting blood glucose levels.10,13 The group also showed that HRP ameliorated renal and cardiac damage in hypertensive spontaneously hypertensive rats (SHR).14,15 We investigated the role of DRI treatment and (P)RR blockade in a transgenic rat model with human high renin and prorenin hypertension. We also conducted studies in human coronary vascular smooth muscle cells (VSMC) to test the hypothesis that renin and prorenin induce signaling independent of AngII and to evaluate DRI and HRP treatment on this signaling.
Results
Aliskiren but not the (P)RR Blocker Ameliorates AngII-Induced Renal Damage
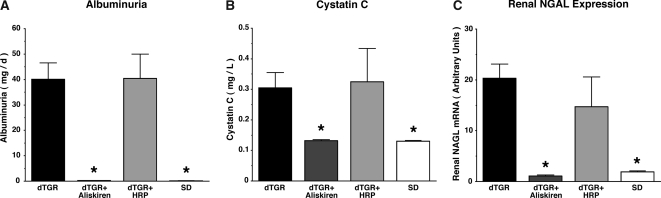
At week 7, no aliskiren-treated dTGR died, whereas mortality was 58% in HRP-treated and 40% in vehicle-treated dTGR (Figure 1A). All nontransgenic Sprague-Dawley (SD) controls survived until the end of the study. Aliskiren normalized systolic BP (106 ± 3 for aliskiren and 111 ± 1 mmHg for SD; Figure 1B). In contrast to aliskiren treatment, HRP-treated rats developed high systolic BP similar to vehicle-treated dTGR (196 ± 9 for HRP versus 200 ± 5 mmHg for dTGR; Figure 1B). Whereas aliskiren normalized albuminuria (Figure 2A) and cystatin C, a marker for GFR (Figure 2B), and renal neutrophil gelatinase-associated lipocalin (NGAL) mRNA expression, a marker for tubular damage (Figure 2C), to SD levels, HRP-treated dTGR were not different from vehicle-treated dTGR. The 30-fold higher dosage of rat and human HRP also showed no protective effect (data not shown). These results demonstrate that the DRI protected fully, whereas the putative competitive (P)RR blocker HRP was ineffective in our dTGR model. We also investigated whether renal (P)RR expression was altered in our study. Vehicle-treated dTGR showed a lower (P)RR expression compared with aliskiren-treated dTGR and nontransgenic SD rats (Figure 3). The (P)RR expression of dTGR and dTGR+HRP treatment were not different. In any event, the (P)RR expression remained robust in all groups throughout the study.
Figure 1.
Effect of aliskiren and HRP on mortality (A) and systolic BP (B). Data are means ± SEM. *P < 0.05 versus vehicle-treated dTGR and HRP-treated dTGR.
Figure 2.
Effect of aliskiren and HRP on albuminuria (A), cystatin C (B), and NGAL (C). All three markers demonstrated that aliskiren but not HRP improved renal damage. Data are means ± SEM. *P < 0.05 versus vehicle-treated dTGR and HRP-treated dTGR.
Figure 3.
(P)RR mRNA expression in the kidney. Vehicle-treated dTGR showed lower renal (P)RR expression compared with aliskiren-treated dTGR and SD rats. HRP and SD rat (P)RR expressions were not different. Data are means ± SEM. *P < 0.05 versus aliskiren-treated dTGR and SD rats. AU, arbitrary units.
Neither Aliskiren nor HRP Affects Renin and Prorenin-Induced ERK1/2 Phosphorylation in Human Coronary VSMC
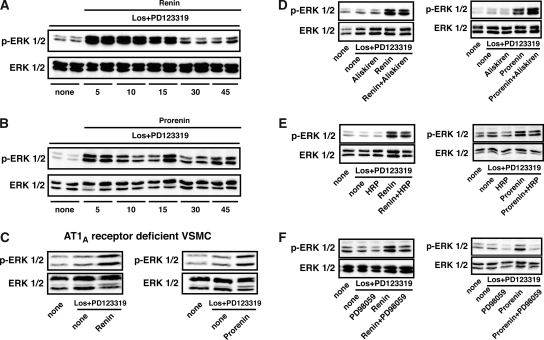
To exclude any AngII-mediated signaling effect, we performed all of our signaling experiments in the presence of the AngII type 1 (AT1) blocker losartan and AT2 blocker PD123319. Time-course analysis revealed that human recombinant renin induced ERK1/2 phosphorylation in a time-dependent manner starting at 5 min with a strong signal up to 15 min and slightly elevated phosphorylation up to 45 min (Figure 4A). Human recombinant prorenin also induced a long-lasting ERK1/2 phosphorylation with a signal up to 45 min (Figure 4B). The final proof that renin- and prorenin-induced ERK1/2 phosphorylation is not AT1A receptor dependent comes from experiments in VSMC deficient for the receptor (Figure 4C), where both stimuli were still active. We subsequently addressed the question of whether aliskiren might also interfere with renin- and prorenin-mediated (P)RR signaling. Our data indicate that aliskiren affected neither renin- or nor prorenin-induced ERK1/2 phosphorylation (Figure 4D). We next investigated the effect of the HRP. To our surprise, we found no evidence that HRP blocked either renin- or prorenin-induced ERK1/2 phosphorylation (Figure 4E). In contrast, the MAP kinase kinase (MEK1/2) inhibitor PD98059, which blocks an upstream kinase of ERK1/2, significantly reduced renin- and prorenin-mediated ERK1/2 phosphorylation (Figure 4F).
Figure 4.
(A and B) Time-course analysis of renin-induced (10 nM; A) and prorenin-induces (2 nM; B) ERK1/2 phosphorylation (p-ERK1/2) in human VSMC (top) with unphosphorylated ERK as loading control (bottom). (C) Renin and prorenin (10 min) also induced p-ERK1/2 in AT1A receptor–deficient VSMC. (D through F) Effects of 10 μM aliskiren (D), 1 μM HRP (E), and MEK1/2 inhibitor (PD98059; 100 nM; F) on renin- and prorenin-induced (both 10 min) p-ERK1/2 in human VSMC (top) with unphosphorylated ERK as loading control (bottom). All experiments were performed after 24-h serum starvation in the presence of losartan (Los) and the AT2 receptor blocker (PD123319).
Discussion
We provide the first evidence that both prorenin and renin rapidly induce cellular signals in human VSMC and mouse AT1A receptor–deficient mouse VSMC that lead to MAPK ERK1/2 phosphorylation, completely independent of AngII. Prorenin- and renin-induced ERK1/2 phosphorylation was inhibited by MEK1/2 inhibition. Furthermore, we demonstrated that aliskiren affected neither prorenin- nor renin-induced ERK1/2 activation; therefore, aliskiren is a pure DRI that has no (P)RR-blocking potency. Presumably, the enzymatic renin cleft or any conformational changes resulting from occupancy are not involved with any (P)RR interactions. Our results also provided no evidence of a specific (P)RR blockade by the HRP, despite its putative in vivo potency in various experimental diabetic and nondiabetic nephropathy models.10,12–16 HRP also did not improve target-organ damage in dTGR. In contrast, aliskiren completely prevented hypertension, renal damage, and mortality.
In humans, aliskiren exhibits a adverse effect profile no different from placebo,17 while decreasing systolic and diastolic BP alone or in combination with ramipril, valsartan, amlodipine, and hydrochlorothiazide.18–20 In rat models, aliskiren potentiated the BP-lowering effects of angiotensin-converting enzyme inhibitors and AT1 receptor blockers.21 We recently demonstrated that aliskiren improved renal and cardiac damage in a rat model of high human renin hypertension.2–4 Aliskiren offers a novel opportunity to reduce plasma renin activity (PRA) that increases in response to most other antihypertensive drugs. We speculated that the binding of aliskiren in the active cleft of the renin molecule could lead to a conformational change and thus prevent the renin-aliskiren complex from binding to the (P)RR; however, our in vitro signaling experiments suggest that this is not the case.
Our results showed that prorenin, as well as renin, can induce ERK1/2 activation in human primary VSMC. Huang et al.8,9 studied rodent mesangial cells and showed that renin-induced (P)RR stimulation activates ERK1/2 and stimulates TGF-β, collagen, and fibronectin expression. Recently, Saris et al.22 reported that prorenin induced p38 MAPK but not ERK1/2 signaling in rat neonatal cardiomyocytes. Taken together, the results serve to underscore the emphasis that the Ishihara group placed on a possible role of the (P)RR in target-organ damage.10,12–15 The findings give prorenin a substantially greater dimension than provided by early acid activation and cryoactivation studies, which pointed to prorenin's potential but were not relevant in vivo because neither acid activation nor cryoactivation can occur within the body.23–26 Luetscher and colleagues24,27 drew attention to the fact that patients with diabetes and target-organ damage had much higher prorenin levels than healthy individuals. High prorenin levels and not PRA were closely associated with the severity of diabetic complications in these patients.
Ichihara and colleagues10–12,28 offered an intriguing new concept with their idea of (P)RR blockade. They proposed a 10–amino acid sequence as an effective (P)RR blocker that was efficacious in streptozotocin-induced diabetes.10,11 They showed that the nephroprotective effect of the HRP was independent of AngII in AT1A receptor knockout mice.13 HRP infusion also ameliorated target-organ damage in SHR14,15; however, Susic et al.29 were unable to substantiate these findings. Our dTGR have three- to five-fold increased circulating and tissue AngII levels; have high circulating human renin and prorenin; develop hypertension, cardiac, and renal damage; and die at the age of 7 wk.30 We found no evidence that HRP had any salubrious effects in this model. We took care to apply both human and rat HRP sequences because presumably in the dTGR, the endogenous rat (P)RR is operative. We used the same sequences published by Ishihara and colleagues.14–16 Ishihara et al.15 reported that HRP-treated SHR had normal cardiac AngII like WKY rats, whereas untreated SHR had increased levels. Current notions suggest that cardiac AngII depends on renal renin that is released in its active form.31,32 We took the precaution of studying a dosage of HRP that was 30-fold higher than that used by Ishihara et al. but still could not find any target-organ protection. To address the role of HRP in a simpler experimental system, we performed in vitro signal transduction experiments. These experiments also did not support the notion that HRP blocked prorenin- and renin-induced (P)RR signaling. Our in vitro and in vivo results challenge the notion that HRP acts as a specific (P)RR antagonist. Possibly, HRP efficacy in vivo depends on an undefined mechanism but not on competitive antagonism for the (P)RR.
Ichihara et al.10,13 showed impressive findings in a diabetic model of nephropathy that presumably had high prorenin levels and low PRA. Our rat model is substantially different. We characterized the model earlier and reported AngII concentrations of 100 pg/ml, human PRA values of 60 ng AngI/ml per h, and human angiotensinogen values of approximately 40 μg AngI/ml.30 Kidney AngII concentrations were almost 800 pg/g tissue, approximately four-fold higher than in SD controls. The human prorenin-to-renin ratio in the model is approximately 10:1. Recently, Schefe et al.33 described a novel signal transduction cascade involving direct physical interaction of the (P)RR and the promyelocytic zinc finger protein transcription factor. Their data suggest the existence of a pathway involving ligand, (P)RR, and promyelocytic zinc finger protein, which downregulates (P)RR. We did not investigate this issue; however, wedid find that untreated dTGR had lower (P)RR expression, compared with aliskiren-treated dTGR or SD rats. The expression differences were relatively modest. The (P)RR should have had ample ligand in our study considering the renin and prorenin concentrations in dTGR, compared with SD rats. The issue requires further direct investigation.
The cloning of the (P)RR contributed a new dimension to the renin-angiotensin system. The (P)RR clearly is important in activating prorenin and enhancing renin in terms of angiotensinogen cleavage. This plasma renin activity can be blocked by DRI administration; however, the direct (P)RR signaling resulting in ERK1/2 activation cannot be inhibited by DRI. The possibility remains that the (P)RR contributes to target-organ damage directly. Inhibitors of the (P)RR above and beyond HRP are needed to test this hypothesis.
Concise Methods
Experimental Design
We studied male dTGR (RCC Ltd., Basel, Switzerland) and age-matched nontransgenic SD rats (MDC, Berlin, Germany).2,3,4,30,34 Local authorities approved the studies, which followed American Physiologic Society guidelines for animal care. We studied vehicle-treated dTGR (n = 15), dTGR+aliskiren (3 mg/kg per d subcutaneously by minipump infusion; n = 11), dTGR+HRP (n = 11), and SD rats (n = 8). Because dTGR produce both rat and human renin, we co-infused rat (NH2-RILLKKMPSV-COOH; Biosynthan, Berlin, Germany) and human (NH2-RIFLKRMPSI-COOH; Biosynthan) HRP (3.6 μg/kg per d each, subcutaneously by minipump infusion) to block the (P)RR. The dosage was selected on the basis of previous reports.14–16 We also treated dTGR with a 30-fold higher human and rat HRP dosage. Systolic BP was measured weekly by tail cuff. Twenty-four-hour urine samples were collected in metabolic cages from weeks 5 through 7 for the determination of rat albumin (CellTrend, Lukenwalde, Germany). Rats were killed at age 7 wk by decapitation. Serum was collected for cystatin C analysis. Cystatin C was measured by routine clinical analysis. The kidneys were harvested for RNA isolation and TaqMan reverse transcription–PCR as described previously.2,3 We analyzed NGAL and rat (P)RR in the kidney. For quantification, the target sequences were normalized in relation to the 36B4 product. Biotez (Berlin, Germany) synthesized the primers; all sequences are available upon request. Data are presented as means ± SEM. Statistically significant differences in mean values were tested by ANOVA followed by Scheffé test. Mortality was examined using a Kaplan-Meier analysis. A value of P < 0.05 was considered statistically significant.
Cell Culture and Western Blot
Human VSMC (Cambrex, Rockland, USA) were cultured in SmBM medium (Lonza, Basel, Germany) with human fibroblast growth factor, EGF (both Biochrome, Berlin, Germany), insulin (Sigma, Deisenhofen, Germany), 5% FBS, and Pen/Strep. All stimulation experiments were performed under 24-h serum-free conditions. To exclude AngII signaling effects, we also investigated AT1A receptor–deficient mouse VSMC. For stimulation experiments, cells were preincubated with the AT1 receptor blocker losartan (10 μM) and AT2 receptor blocker PD123319 (10 μM) for 30 min. Thereafter, cells were treated with human recombinant renin (10 nM) or human recombinant prorenin (2 nM) as indicated in the legend to Figure 4. In all inhibitor protocols, cells were preincubated with the respective blocker for 30 min. The following blockers were used: Aliskiren (10 μM; Novartis, Basel, Switzerland), the HRP (1 μM; NH2-RIFLKRMPSI-COOH; Biosynthan), and the MEK1/2 inhibitor PD98059 (100 nM; Calbiochem, Darmstadt, Germany). After stimulation, cells were lysed with lysis buffer containing protease inhibitor Complete (Roche, Mannheim, Germany) and phosphatase inhibitor cocktail (Sigma). For Western blotting, we used ERK1/2 and phosphorylated ERK1/2 (both Cell Signaling, Frankfurt, Germany), which were detected by standard technique. At least three different cell stimulation experiments were performed for each protocol.
Disclosures
F.C.L. and D.N.M. have served as advisors for Novartis and have lectured on aliskiren. F.C.L. is a member of the renin academy.
Acknowledgments
Grants-in-aid from the European Union (EuReGene), the Novartis Foundation, and the Deutsche Forschungsgemeinschaft to D.N.M. and F.C.L. supported the studies.
We thank Astrid Schiche, Jutta Meisel, and Gabriele N′diaye for excellent technical assistance.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Muller DN, Luft FC: Direct renin inhibition with aliskiren in hypertension and target organ damage. Clin J Am Soc Nephrol 1: 221–228, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Dechend R, Shagdarsuren E, Gratze P, Fiebeler A, Pilz B, Meiners S, Derer W, Feldman DL, Webb R, Muller DN: Low-dose renin inhibitor and low-dose AT(1)-receptor blocker therapy ameliorate target-organ damage in rats harbouring human renin and angiotensinogen genes. J Renin Angiotensin Aldosterone Syst 8: 81–84, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Pilz B, Shagdarsuren E, Wellner M, Fiebeler A, Dechend R, Gratze P, Meiners S, Feldman DL, Webb RL, Garrelds IM, Jan Danser AH, Luft FC, Muller DN: Aliskiren, a human renin inhibitor, ameliorates cardiac and renal damage in double-transgenic rats. Hypertension 46: 569–576, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Shagdarsuren E, Wellner M, Braesen JH, Park JK, Fiebeler A, Henke N, Dechend R, Gratze P, Luft FC, Muller DN: Complement activation in angiotensin II-induced organ damage. Circ Res 97: 716–724, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD: Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burckle C, Bader M: Prorenin and its ancient receptor. Hypertension 48: 549–551, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Nabi AH, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F: Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med 18: 483–488, 2006 [PubMed] [Google Scholar]
- 8.Huang Y, Noble NA, Zhang J, Xu C, Border WA: Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int 72: 45–52, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W: Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int 69: 105–113, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T: Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest 114: 1128–1135, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki F, Hayakawa M, Nakagawa T, Nasir UM, Ebihara A, Iwasawa A, Ishida Y, Nakamura Y, Murakami K: Human prorenin has “gate and handle” regions for its non-proteolytic activation. J Biol Chem 278: 22217–22222, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Satofuka S, Ichihara A, Nagai N, Yamashiro K, Koto T, Shinoda H, Noda K, Ozawa Y, Inoue M, Tsubota K, Suzuki F, Oike Y, Ishida S: Suppression of ocular inflammation in endotoxin-induced uveitis by inhibiting nonproteolytic activation of prorenin. Invest Ophthalmol Vis Sci 47: 686–2692, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AH, Nishiyama A, Sugaya T, Hayashi M, Inagami T: Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol 17: 1950–1961, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Nakagawa T, Nishiyama A, Kawachi H, Shimizu F, Inagami T: Contribution of nonproteolytically activated prorenin in glomeruli to hypertensive renal damage. J Am Soc Nephrol 17: 2495–2503, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Nishiyama A, Inagami T, Hayashi M: Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension 47: 894–900, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H: Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol 18: 1789–1795, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Weir MR, Bush C, Anderson DR, Zhang J, Keefe D, Satlin A: Antihypertensive efficacy, safety, and tolerability of the oral direct renin inhibitor aliskiren in patients with hypertension: a pooled analysis. J Am Soc Hypertens 1: 264–277, 2007 [DOI] [PubMed] [Google Scholar]
- 18.O'Brien E, Barton J, Nussberger J, Mulcahy D, Jensen C, Dicker P, Stanton A: Aliskiren reduces blood pressure and suppresses plasma renin activity in combination with a thiazide diuretic, an angiotensin-converting enzyme inhibitor, or an angiotensin receptor blocker. Hypertension 49: 276–284, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A: Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: A randomised, double-blind trial. Lancet 370: 221–229, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Villamil A, Chrysant SG, Calhoun D, Schober B, Hsu H, Matrisciano-Dimichino L, Zhang J: Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens 25: 217–226, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Wood JM, Schnell CR, Cumin F, Menard J, Webb RL: Aliskiren, a novel, orally effective renin inhibitor, lowers blood pressure in marmosets and spontaneously hypertensive rats. J Hypertens 23: 417–426, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Saris JJ, t Hoen PA, Garrelds IM, Dekkers DH, den Dunnen JT, Lamers JM, Jan Danser AH: Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Hypertension 48: 564–571, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Hsueh WA, Luetscher JA, Carlson E, Grislis G, Elbaum D, Chavarri M: A comparison of cold and acid activation of big renin and of inactive renin in normal plasma. J Clin Endocrinol Metab 47: 792–799, 1978 [DOI] [PubMed] [Google Scholar]
- 24.Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M: Increased plasma inactive renin in diabetes mellitus: A marker of microvascular complications. N Engl J Med 312: 1412–1417, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Sealey JE, Moon C, Laragh JH, Alderman M: Plasma prorenin: Cryoactivation and relationship to renin substrate in normal subjects. Am J Med 61: 731–738, 1976 [DOI] [PubMed] [Google Scholar]
- 26.Weinberger MH, Wade MB, Aoi W, Usa T, Dentino M, Luft F, Grim CE: An extrarenal source of “renin-like” activity in anephric man. Circ Res 40: I1–I4, 1977 [PubMed] [Google Scholar]
- 27.Wilson DM, Luetscher JA: Plasma prorenin activity and complications in children with insulin-dependent diabetes mellitus. N Engl J Med 323: 1101–1106, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Luft FC: Renin and its putative receptor remain enigmas. J Am Soc Nephrol 18: 1989–1992, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Susic D, Lippton H, Knight M, Frohlich ED: Cardiovascular effects of nonproteolytic activation of prorenin. Hypertension 48: e113, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Mervaala E, Muller DN, Schmidt F, Park JK, Gross V, Bader M, Breu V, Ganten D, Haller H, Luft FC: Blood pressure-independent effects in rats with human renin and angiotensinogen genes. Hypertension 35: 587–594, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Muller DN, Fischli W, Clozel JP, Hilgers KF, Bohlender J, Menard J, Busjahn A, Ganten D, Luft FC: Local angiotensin II generation in the rat heart: role of renin uptake. Circ Res 82: 13–20, 1998 [DOI] [PubMed] [Google Scholar]
- 32.van Kesteren CA, Danser AH, Derkx FH, Dekkers DH, Lamers JM, Saxena PR, Schalekamp MA: Mannose 6-phosphate receptor-mediated internalization and activation of prorenin by cardiac cells. Hypertension 30: 1389–1396, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H: A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res 99: 1355–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Ganten D, Wagner J, Zeh K, Bader M, Michel JB, Paul M, Zimmermann F, Ruf P, Hilgenfeldt U, Ganten U, Kaling M, Bachmann S, Fukamizu A, Mullins J, Murakami K: Species specificity of renin kinetics in transgenic rats harboring the human renin and angiotensinogen genes. Proc Natl Acad Sci U S A 89: 7806–7810, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]