Abstract
Pan-caspase inhibition reduces tubular apoptosis and proliferation and slows progression of disease in a rat model of polycystic kidney disease (PKD). It is unknown, however, which specific caspases are involved in PKD progression. Because caspase-3 is a major mediator of apoptosis, its role in autosomal recessive PKD was determined. Mice with caspase-3 gene deletion were crossed with mice harboring the congenital polycystic kidney (cpk) mutation to generate double-mutant mice. cpk;casp3−/− mice lived nearly 4 times longer than littermate control cpk mice (mean survival of 117 d versus 32 d, P < 0.01), and cpk;casp3+/− mice lived slightly longer than controls (mean survival of 56 d). In addition, the kidney weight, relative to body weight, was significantly lower in the cpk;casp3−/− mice than in the cpk and cpk;casp3+/− mice. Despite deletion of caspase-3, however, apoptosis occurred and cysts formed; therefore, the alternative pathways of apoptosis in cystic kidneys were investigated. Caspase-7 was up-regulated and the anti-apoptotic protein Bcl-2 was down-regulated in cpk, cpk;casp3+/−, and cpk;casp3−/− mice compared with wild-type controls. In summary, homozygous deletion of caspase-3 markedly prolongs survival of cpk mice, but a caspase-7-mediated pathway may compensate for the deficiency of functional caspase-3. These findings suggest that pan-caspase inhibition may have a greater therapeutic effect than selective caspase inhibition in PKD.
Inherited polycystic kidney disease (PKD) is one of the leading causes of end-stage kidney disease requiring dialysis and kidney transplantation in children and adults.1 Inherited PKD includes both autosomal dominant and autosomal recessive forms. Autosomal dominant polycystic kidney disease (ADPKD) results from mutations in one of two genes, PKD1 or PKD2. The prevalence of ADPKD varies between 1 in 400 and 1 in 1000, thus making it one of the most common hereditary diseases in the United States. ADPKD progresses to end-stage renal disease (ESRD) over a period of decades in 50% to 75% of affected people. Autosomal recessive polycystic kidney disease (ARPKD) results from a mutation in a single gene, PKHD1. ARPKD is less common, affecting about 1 in 20,000 live births and results in ESRD in childhood.2
The congenital polycystic kidney (cpk) mouse is the most extensively characterized mouse model of PKD.2 The inheritance, cyst localization in the kidney, and severity of kidney disease in the cpk mouse resembles ARPKD. Cys1, the cpk gene, encodes a cilia-associated protein called cystin that is disrupted in the cpk mouse.3 Increased apoptosis in polycystic kidneys has been described in cpk mice4–8 as well as in human and other rat and mouse models of PKD.9 In support of a deleterious effect of apoptosis in PKD, we have recently demonstrated that pan-caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in the Han:SPRD rat model of PKD.10 The effect of caspase or apoptosis inhibition in other rat and mouse models of PKD is not known. Caspase-3 is the major mediator of apoptosis (i.e., the so-called “executioner” caspase) and caspase-1 is a pro-inflammatory caspase. However, the effect of inhibition of a specific caspase on PKD is not known. We tested the hypothesis that specific inhibition of caspase-3 would prolong life and reduce cyst formation in PKD.
In the present study, we developed cpk mice that were either heterozygous or homozygous for caspase-3 deletion to determine the effect of specific caspase-3 deletion on the development of PKD.
Results
Genotyping of the cpk and caspase-3 alleles
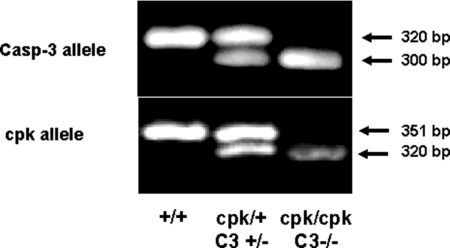
Genomic DNA samples were prepared from the tails of tested mice. The genotyping of the cpk and caspase-3 allele is demonstrated in Figure 1.
Figure 1.
Genotyping of the cpk and caspase-3 allele. The normal and mutated cpk and caspase-3 alleles were amplified by PCR using primers described in “Methods.” For the caspase-3 PCR, the 320 bp band corresponds to normal allele and the 300 bp band corresponds to mutated allele. For the cpk PCR, the 351 bp band corresponds to normal allele and the 320 bp band corresponds to mutated allele.
Caspase-3 protein expression in the kidney
There was an increase in caspase-3 protein expression in cpk mice compared with normal control +/+ mice (Figure 2). Caspase-3 expression was decreased in cpk;casp3+/− mouse kidneys. The cpk;casp3+/− mice that had the longest survival (105 d and 113 d) had less expression of caspase-3 than the cpk;casp3+/− mice that had the shortest survival (29 d and 17 d). Caspase-3 expression was absent in cpk;casp3−/− mouse kidneys.
Figure 2.
Caspase-3 immunoblot. Full-length caspase-3 protein expression (32 kDa) was increased in cpk mouse kidneys compared with +/+. Caspase-3 expression was decreased in cpk;casp3+/− mouse kidneys. The cpk;casp3+/− mice that had the longest survival (105 d and 113 d) had less expression of caspase-3 than the cpk;casp3+/− mice that had the shortest survival (29 d and 17 d). Caspase-3 expression was absent in cpk;casp3−/− mouse kidneys. Representative immunoblot of at least 3 separate experiments (100 μg protein loading per well). Equal protein loading was confirmed by Coomassie Blue staining of membranes.
Effect of caspase-3 gene deletion on the development of PKD
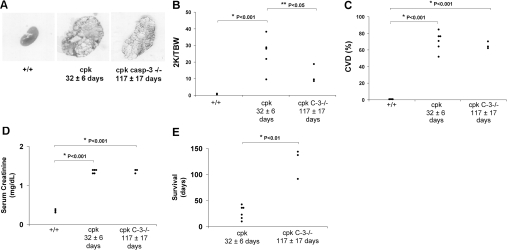
Representative kidney sections, stained with hematoxylin and eosin, at the same magnification are demonstrated in Figure 3A. The two-kidney/total body weight ratio (2K/TBW %) was significantly decreased in cpk;casp3−/− mice compared with cpk. Mean 2K/TBW (%) was 25 ± 5 in cpk and 13 ± 3 in cpk;casp3−/− (P < 0.05 versus cpk). Figure 3B shows the individual data points for 2K/TBW. The cyst volume density (CVD) was the same when comparing 32-d-old cpk mice and 117-d-old cpk;casp3−/− mice. Mean CVD (%) was 0.7 ± 0.1 in +/+, 71 ± 4 in cpk (P < 0.001 versus +/+), and 65 ± 3 in cpk;casp3−/− (P < 0.001 versus +/+). Figure 3C shows the individual data points for CVD. The serum creatinine was the same when comparing 32-d-old cpk mice and 117-d-old cpk;casp3−/− mice. Serum creatinine (mg/dl) was 0.3 ± 0.01 in +/+, 1.3 ± 0.1 in cpk (P < 0.001 versus +/+), and 1.35 ± 0.05 in cpk;casp3−/− (P < 0.001 versus +/+). Figure 3D shows the individual data points for serum creatinine. Homozygous caspase-3 gene deletion markedly prolonged survival. Mean survival (days) was 32 ± 6 in cpk mice and 117 ± 17 in cpk;casp3−/− mice (P < 0.01 versus cpk). Figure 3E shows the individual data points for survival.
Figure 3.
Effects of caspase-3 (C-3) gene deletion on the development of PKD. (A) Representative kidney sections, stained with hematoxylin-eosin, at the same magnification are demonstrated in Figure 3A. (B) The two-kidney/total body weigh ratio (2K/TBW %) was decreased in cpk;casp3−/− mice compared with cpk. *P < 0.001 versus +/+. **P < 0.05 versus cpk. (C) The cyst volume density (CVD) was similar in cpk and cpk;casp3−/− mice. *P < 0.001 versus +/+. (D) Serum creatinine was increased in cpk mice versus +/+ at a mean age of 32 d. Serum creatinine was similar in 117 d old cpk;casp3−/− mice versus 32 d old cpk. *P < 0.001 versus +/+. (E) Survival was markedly increased in cpk;casp3−/− mice compared with cpk mice. *P < 0.01 versus cpk.
The survival data in the cpk;casp3+/− mice was variable. This variability may be related to the degree of caspase-3 expression (Figure 2). In the cpk;casp3+/−, the mean 2K/TBW (%) was 27 ± 1 and the 2K/TBW (%) for each animal studied was 31, 29, 26, 26, and 23. In the cpk;casp3+/−, the mean CVD (%) was 68 ± 3 and the CVD (%) for each animal studied was 72, 71, 69, 65, and 63. Blood for serum creatinine measurement was not available in cpk;casp3+/− mice. In the cpk;casp3+/−, the mean survival (days) was 56 ± 21 in cpk;casp3+/− and the survival (days) for each animal studied was 113, 105, 29, 17, and 17.
Kidney palpation was performed on all mice at 30 d of age. The two cpk;casp3+/− mice that survived 113 and 105 d and the cpk;casp3−/− mice had smaller kidneys on palpation than cpk mice at 30 d of age.
Apoptosis in PKD kidneys
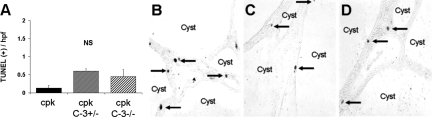
The number of apoptotic (terminal deoxynucleotidyltransferase (TdT) mediated dUTP nick-end labeling (TUNEL)-positive) cells per high power field (HPF) in tubular cells and interstitium was not significantly different in cpk;casp3+/− and cpk;casp3−/− mice compared with cpk mice (Figure 4A). A representative picture of the TUNEL-positive cells in the kidney is demonstrated in Figures 4B, C, and D for cpk, cpk;casp3+/−, and cpk;casp3−/−, respectively.
Figure 4.
Apoptosis in PKD kidneys. (A) The number of apoptotic (TUNEL-positive) tubular and interstitial cells per high power field (HPF) was not statistically different between cpk, cpk;casp3+/−, and cpk;casp3−/− mice. Representative picture of the TUNEL-positive cells (arrows) is demonstrated in cpk (B), cpk;casp3+/− (C), and in cpk;casp3−/− (D). NS, not significant.
Apoptotic pathways in casp3−/− mice
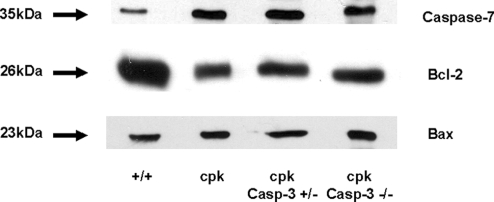
Caspase-3 is the major mediator of apoptosis. Because there was apoptosis and PKD in the absence of caspase-3, alternative pathways of apoptosis were investigated. Caspase-7, like caspase-3, is an “executioner” caspase. There was an increase in caspase-7 protein expression in the kidney in cpk, cpk;casp3+/−, and cpk;casp3−/− mice compared with +/+ mice (Figure 5A). Anti-apoptotic Bcl-2 protein expression was decreased in cpk, cpk;casp3+/−, and cpk;casp3−/− mice compared with +/+ mice (Figure 5B). Pro-apoptotic Bax protein expression was unchanged in these mice (Figure 5C).
Figure 5.
Immunoblots of caspase-7, Bcl-2, and Bax proteins. Full-length caspase-7 protein expression (35 kDa) in the kidney was increased in cpk, cpk;casp3+/−, and cpk;casp3−/− mice compared with +/+ mice. Anti-apoptotic Bcl-2 protein (26 kDa) expression was decreased in cpk, cpk;casp3+/−, and cpk;casp3−/− mice compared with +/+. Pro-apoptotic Bax protein (23 kDa) expression was unchanged. Representative immunoblots of at least 3 separate experiments.
Discussion
ADPKD is one of the most common life-threatening hereditary disorders. ADPKD is more common than sickle cell disease, cystic fibrosis, muscular dystrophy, hemophilia, Down syndrome, and Huntington disease combined.11 Considerable basic and clinical research is underway to find a specific treatment for PKD. There is increased proliferation of cystic tubular epithelial cells in human PKD and in animal models of PKD.12 Therapeutic agents that reduce cystic epithelial cell proliferation have shown promise in reducing cyst formation in animal models of PKD. These agents include lovastatin,13 EGF receptor tyrosine kinase inhibitors,14 rapamycin,15,16 c-myc antisense,17 and most recently the cyclin-dependent kinase inhibitor roscovitine.18 The vasopressin-2 receptor antagonist tolvaptan (OPC-41061) also reduces proliferation and cyst formation in PKD.19,20 Increased intracellular cAMP (which would be the consequence of V2 receptor activation) not only promotes fluid secretion across cystic epithelia, but also promotes cell proliferation in cystic epithelia (in contrast to normal renal epithelia).
In addition to proliferation, apoptosis of cystic epithelial cells is also dysregulated in PKD.9,12 Inhibition of caspases and apoptosis therefore represents a potential therapy to reduce cyst formation.
We have previously demonstrated that the pan-caspase inhibitor IDN-8050 reduces tubular apoptosis and proliferation and slows disease progression in the Han:SPRD rat model of PKD. IDN-8050 is a pan-caspase inhibitor that inhibits both pro-apoptotic caspases, such as caspase-3, -7, -8, and -9 as well as proinflammatory caspases, such as caspase-1 (formerly known as ICE (IL-1 converting enzyme). The specific caspase that mediates apoptosis in PKD is not known. There are multiple rat and mouse models of both ADPKD and ARPKD.2 Besides, in the Han:SPRD rat model of ADPKD, the effect of caspase and apoptosis inhibition in other models of PKD is not known. Demonstration of an effect of caspase inhibition in more than one model of PKD may highlight the potential therapeutic benefit of either pan-caspase or specific caspase inhibition in human PKD.
Caspase-3−/− mice bred on a C57BL/6 background survive until adulthood21 and are protected against in vivo cerebral ischemia because of decreased apoptotic cell loss in ischemic brain.22 In the present study, we investigated the effect of specific caspase-3 inhibition in PKD by crossing the caspase-3+/− mouse with the cpk/+ mouse to develop double-knockout mice. In the present study, the cpk mouse died from renal failure and PKD at a mean age of 32 d. Complete deficiency of caspase-3 remarkably prolonged the life of the cpk mouse. In only one other study using the microtubule specific agent Taxol has the survival of cpk mice been markedly prolonged.23 In a recent study in cpk mice, administration of the cdk inhibitor roscovitine from 7 to 21 d of age resulted in approximately a 25% reduction in kidney size, cyst volume, and renal function at 21 d of age.18 In this study, survival was not determined.
We have previously described the activation of caspase-3 and dysregulation of the balance between pro- and anti-apoptotic Bcl-2 family proteins in the Han:SPRD rat model of PKD.24,25 In the present study, at the time of demise, the double-knockout mice eventually had as much apoptosis in the polycystic kidneys as cpk mice, but these cpk;casp3−/− mice died after a markedly prolonged life span. Compensatory activation of caspase-7 has been reported when caspase-3 is deficient or absent.22 Thus, we investigated alternative pathways of apoptosis (Figure 6). Caspases-3 and -7 have some similar but also distinct roles in apoptosis.26 Caspase-3, rather than caspase-7, is thought to mediate DNA fragmentation and the morphologic changes of apoptosis. Caspase-7 may be more important in the loss of cellular viability either alone or in combination with caspase-3.26 We demonstrate that, in the absence of caspase-3, the levels of caspase-7 protein in the kidney in caspase-3-deficient mice are as high as in the cpk mice. The activation of caspase-3 is known to be controlled by the balance between pro- and anti-apoptotic Bcl-2 family proteins via cytochrome c release from mitochondria, which results in activation of caspase-9.27 The effect of the balance between pro- and anti-apoptotic Bcl-2 family proteins on caspase-7 is less well described. We studied the anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax. Bcl-2 protein expression was decreased in both cpk mice and caspase-3-deficient cpk mice compared with normal control mice. The pro-apoptotic protein Bax remained unchanged. These findings suggest that the partial or complete absence of caspase-3 in polycystic kidneys has no effect of on Bcl-2 or Bax protein expression in whole kidney.
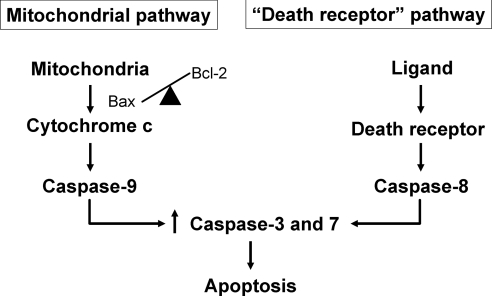
Figure 6.
Pathways of caspase-mediated apoptosis. In the “mitochondrial” or “intrinsic” pathway of apoptosis, the balance of pro- and anti-apoptotic Bcl-2 family proteins determines cytochrome c release from mitochondria and activation of the “initiator” caspase-9, which in turn activates the “executioner” caspases-3 and -7. In the “death receptor” or “extrinsic” pathway of apoptosis, the binding of a ligand to its death receptor activates the “initiator” caspase-8, which in turn activates the “executioner” caspases-3 and -7. Compensatory activation of caspase-7 has been reported when caspase-3 is deficient or absent.22
There is much evidence that apoptosis is abnormally persistent in PKD and may result in cyst formation:9,12 1) Tubular cell apoptosis occurs in most animal models of PKD and in kidneys from humans with PKD.28 2) Mice overexpressing the proto-oncogene c-myc (SBM mice),29 mice lacking the transcription factor AP-2 beta,30 and Bcl-2 deficient mice31 have increased apoptosis and develop cysts in the kidney. 3) Apoptosis is essential for MDCK cell cyst cavitation in collagen type 1 matrix and cystogenesis in this system is inhibited by overexpression of the anti-apoptotic gene, Bcl-2.32 4) Expression of PKD1 in MDCK cells results in tubule formation, whereas control cells undergo apoptosis and form cysts.33 5) A direct cause-and-effect relationship between apoptosis and cyst formation is demonstrated by our previous study in a rat model of PKD that caspase and apoptosis inhibition with IDN-8050 decreases apoptosis and proliferation in cystic and noncystic tubules, inhibits renal enlargement and cystogenesis, and attenuates the loss of kidney function.10 The present study provides further evidence of a deleterious effect of apoptosis in PKD. Specifically, we demonstrate for the first time in a mouse model of PKD that specific caspase-3 gene deletion markedly prolongs survival.
Because of the complexity of the breeding of double-knockout mice, we were unable to generate enough numbers of mice to sacrifice and compare kidney size at equivalent ages as well as perform the survival study. However, we think that homozygous caspase-3 deletion decreased cyst formation for the following reasons: 1) on kidney palpation at 30 d of age, the two cpk;casp3+/− mice that survived 113 and 105 d and the cpk;casp3−/− mice had smaller kidneys than cpk mice at the same age; and 2) the 2K/TBW ratio of the cpk;casp3−/− mice at a mean age of 117 d was significantly less than cpk and cpk;casp3+/− mice of a much younger age.
In summary, our data demonstrate that the specific genetic deletion of caspase-3 prolongs life in cpk mice. These data also suggest that pan-caspase inhibition rather than inhibition of a single caspase may potentially have a better therapeutic effect in PKD because, in the absence of caspase-3, the function of another “executioner” caspase, caspase-7, may continue.
Concise Methods
Generation of cpk;casp3+/− and cpk;casp3−/− mice
Caspase-3−/− mice in the C57BL/6 background were obtained from Richard A. Flavell, Howard Hughes Medical Institute, Yale University School of Medicine. The caspase-3−/− mice were originally bred on a B6/129 background.22 The C57BL/ 6 caspase-3−/− mice used in the present study were obtained from litters that were originally backcrossed to C57BL/6 for more than 10 generations, ensuring a uniform genetic background. In addition, the caspase-deficient mice were crossed with cpk mice (in the pure C57BL/6 background) at least an additional 5 times before double-knockout mice were obtained.
We confirmed that the kidneys of the C57BL/6 caspase-3−/− mice used in the present study were normal. The kidneys of 130-d-old caspase-3−/− mice were histologically normal. The renal function of caspase-3−/− mice as determined by BUN and serum creatinine was normal and not different from wild-type controls. The total body weights and kidney weights were the same as aged-matched wild-types. In this regard, Kuida et al. also described that the kidneys of young caspase-3−/− mice were histologically normal.34
cpk/+ mice in the C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, ME). The cpk mouse is a model of ARPKD. Thus, heterozygous cpk mice (cpk/+) do not have PKD, whereas homozygous cpk mice develop PKD. In the manuscript, the term “cpk” is used to refer to homozygous mice carrying 2 copies of the cpk gene (cystic cpk/cpk mice). The study protocol was approved by the University of Colorado Health Sciences Center Animal Care and Use Committee. Mice had free access to tap water and standard mouse chow.
The development of the double-knockout of both the caspase-3 and cpk genes was a prolonged and complex 2-step process. Caspase-3−/− mice are not good breeders. The cpk/cpk mice do not breed because they die of PKD at about 32 d of age. In step 1, we crossed cpk heterozygous (cpk/+) mice with caspase-3 deletion heterozygous mice (casp-3+/−). Statistically, one in 4 of the offspring would be cpk heterozygous and caspase-3 deletion heterozygous mice (cpk/+/casp3+/−). In step 2, the (cpk/+/casp3+/−) mice were used as breeding pairs depending on the gender that was not always in equal numbers. The chance of the cpk/+/casp3+/− pairs producing a caspase-3 homozygous deletion cpk homozygous mouse (double-knockout mouse) is 1 in 16. The number of double-knockout mice and littermate controls developed in the more than 4 yr duration of the study was: cpk littermate controls (n = 6), cpk;casp3+/− (n = 5), and cpk;casp3−/− mice (n = 3).
The double-knockout mice were sacrificed when they were looking sick and were expected to die within the next 24 h. The mice were killed at that time to obtain blood and viable tissue for examination.
Genotyping of cpk;casp3+/− and cpk;casp3−/− mice
Genotyping of the offspring was performed by polymerase chain reaction (PCR) of tail DNA extracts. The cpk gene encodes a 145 amino acid protein termed Cystin.3 In cpk mouse, there is a tandem deletion of 12-bp and 19-bp in exon 1 of the cpk gene. Therefore, the cpk mutations can be identified using a PCR primer set flanking the deletions. The following exon1 primer set amplified a 351 bp product from the wild-type cpk gene and a 320 bp product from a mutant cpk gene: 5′-CPK: 5′TCC TCC CTC CCT ATC TCT CCA-3′; 3′ CPK: 5′-ATC CAG CAG GCG TAG GGT CTC-3′. The PCR condition was as follows: 95°C for 2 min, then 30 cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 1 min.
Caspase-3 gene-deficient mice were generated by homologous recombination, which replaced part of caspase-3 gene with a neomycin resistance gene.22,34 Caspase-3−/− mice were then bred on a C57BL/6 background. PCR genotyping of wild-type and deficient caspase-3 was performed using three primers, the sense primer for the wild-type allele 5′-CPP32, 5′GGG AAA CCA ACA GTA GTC AGT CCT-3′; the sense primer for the mutant allele derived from the neomycin cassette METPHIL, 5′-TGC TAA AGC GCA TGC TCC AGA CTG-3′; and the antisense primer 3′-CPP32, 5′GCG AGT GAG AAT GTG CAT AAA TTC CPP-3′. The primer sets amplified a 320 bp DNA fragment for wild-type caspase-3 and a 300 bp DNA fragment for deficient caspase-3. The PCR condition was as follows: 33 cycles of 96°C for 45 s, 94°C for 30 s, 55°C for 30 s, and 72°C for 60 s, and then one cycle of 72°C for 5 min.
Western Blotting
Kidney tissue was homogenized in RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM PMSF, 1 mM EDTA, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) plus protease inhibitor cocktail (p-8340, Sigma-Aldrich, St Louis, MO). Equal amounts of protein (100 μg per lane) were separated on 12% SDS-PAGE and then transferred to Immobilon-p membrane (Millipore, Billerica, MA). The membranes were blocked with 5% nonfat dry milk in TBST buffer (50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 0.1% Tween-20) for 2 h. The membranes were incubated with the following primary antibodies for 2 h at room temperature: 1) a caspase-3 antibody (sc-7148, 1:1000, Santa Cruz Biotechnology, Santa Cruz, CA) that detects the full-length caspase-3 (32 kDa); 2) a caspase-7 antibody (1:1000, Cell Signaling Technology, Danvers, MA, catalog no. 9492) that detects the full-length caspase-7 (35 kDa); 3) a Bcl-2 antibody (sc 492, 1:1000, Santa Cruz Biotechnology) that detects a peptide mapping at the N-terminus of Bcl-2 of human origin (26 kDa); and 4) a Bax antibody (sc 493, 1:1000, Santa Cruz Biotechnology) that detects the a peptide mapping at the N-terminus of Bax of human origin (23 kDa). Membranes were washed in TBST buffer and further incubated with goat anti-rabbit IgG-HRP secondary antibody (sc 2030, 1:1000, Santa Cruz Biotechnology) for 1 h at room temperature. Subsequent detection was carried out by enhanced chemiluminescence (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), according to the manufacturer's instructions. Equal protein loading (100 μg per lane was loaded) was confirmed by Coomassie Blue staining of membranes.
Quantitation of TUNEL positive cells
The TUNEL method was used to detect in situ DNA strand breaks. TACS 2 TdT-blue label in situ apoptosis detection kit (Trevigen, Gaithersburg, MD) was used. Briefly, the tissue sections were incubated with proteinase K for 1 h at 37°C for permeabilization. Endogenous peroxidase activity was quenched by incubating the tissue sections with 3% hydrogen peroxide in methanol for 5 min. The sections were then incubated with labeling reaction mix for 1 h at 37°C. After incubating with streptavidin-horseradish peroxidase, the apoptotic cells were detected with Blue Label. The number of apoptotic cells per tubule was counted by using a Nikon Eclipse E400 microscope equipped with a digital camera connected to SPOT ADVANCED 3.5 imaging software by an observer blinded to the treatment modality. At least 15 areas per sample in the cortex were randomly selected.
Quantitation of Cystic Development
The 2K/TBW % and cyst volume density were used as indicators of cyst progression. Hematoxylin and eosin-stained kidney sections were used to determine the cyst volume density. This was performed by a reviewer, blinded to the identity of the rat kidney, using point counting stereology.24 Areas of the cortex at 90°, 180°, and 270° from the hilum of each section were selected to guard against field selection variation.
Statistical Analysis
Values are expressed as mean ± SEM. Non-normally distributed data were analyzed by the nonparametric unpaired Mann-Whitney test. Multiple group comparisons were performed using a one-way analysis of variance with post test according to Newman-Keuls. A P value of <0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
This work was supported by NIH grants RO1-DK074835, PO1-DK-34039, and K08-DK067191.
Portions of this study were presented at the World Congress of Nephrology Meeting in 2006 and the American Society of Nephrology Meetings in 2005 and 2006.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Ecder T, Fick-Brosnahan G, Schrier RW: Polycystic kidney disease. In: Diseases of the Kidney and Urinary Tract, Vol. 2, 8th Ed., edited by Schrier RW, Philadelphia, Lippincott Williams and Wilkins, 2007, pp 502–539
- 2.Guay-Woodford LM: Murine models of polycystic kidney disease: molecular and therapeutic insights. Am J Physiol Renal Physiol 285: F1034-F1049, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D'Eustachio P, Beier DR, Guay-Woodford LM: Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest 109: 533–540, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma M, Brantley JG, Alcalay NI, Zhou J, Heystek E, Maser RL, Vanden Heuvel GB: Differential expression of Cux-1 and p21 in polycystic kidneys from Pkd1 null and cpk mice. Kidney Int 67: 432–442, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Gattone VH, Ricker JL, Trambaugh CM, Klein RM: Multiorgan mRNA misexpression in murine autosomal recessive polycystic kidney disease. Kidney Int 62: 1560–1569, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Ali SM, Wong VY, Kikly K, Fredrickson TA, Keller PM, DeWolf WE Jr, Lee D, Brooks DP: Apoptosis in polycystic kidney disease: involvement of caspases. Am J Physiol Regul Integr Comp Physiol 278: R763-R769, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Ostrom L, Tang MJ, Gruss P, Dressler GR: Reduced Pax2 gene dosage increases apoptosis and slows the progression of renal cystic disease. Dev Biol 219: 250–258, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Winyard PJ, Nauta J, Lirenman DS, Hardman P, Sams VR, Risdon RA, Woolf AS: Deregulation of cell survival in cystic and dysplastic renal development. Kidney Int 49: 135–146, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Edelstein CL: What is the role of tubular epithelial cell apoptosis in polycystic kidney disease (PKD)? Cell Cycle 4: e141-e145, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Tao Y, Kim J, Faubel S, Wu JC, Falk SA, Schrier RW, Edelstein CL: Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease (PKD). Proc Natl Acad Sci U S A 102: 6954–6959, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabow PA: Autosomal dominant polycystic kidney disease. N Engl J Med 329: 332–342, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Wilson PD: Polycystic kidney disease. N Engl J Med 350: 151–164, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Gile RD, Cowley BD Jr, Gattone VH, O'Donnell MP, Swan SK, Grantham JJ: Effect of lovastatin on the development of polycystic kidney disease in the Han:SPRD rat. Am J Kidney Dis 26: 501–507, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Torres VE, Sweeney WE Jr, Wang X, Qian Q, Harris PC, Frost P, Avner ED: EGF receptor tyrosine kinase inhibition attenuates the development of PKD in Han:SPRD rats. Kidney Int 64: 1573–1579, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Tao Y, Kim J, Schrier RW, Edelstein CL: Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease (PKD). J Am Soc Nephrol 16: 46–51, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T: The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103: 5466–5471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricker JL, Mata JE, Iversen PL, Gattone II VH: C-myc antisense oligonucleotide treatment ameliorates murine ARPKD. Kidney Int 61: 125–131, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O: Long lasting arrest of murine polycystic kidney disease with CDK inhibitor rosocovitine. Nature 444: 949–952, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Gattone VH, Wang X, Harris PC, Torres VE: Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH: Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Leonard JR, Klocke BJ, D'Sa C, Flavell RA, Roth KA: Strain-dependent neurodevelopmental abnormalities in caspase-3-deficient mice. J Neuropathol Exp Neurol 61: 673–677, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Le DA, Wu Y, Huang Z, Matsushita K, Plesnila N, Augustinack JC, Hyman BT, Yuan J, Kuida K, Flavell RA, Moskowitz MA: Caspase activation and neuroprotection in caspase-3-deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc Natl Acad Sci U S A 99: 15188–15193, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo DD, Miao SY, Pelayo JC, Woolf AS: Taxol inhibits progression of congenital polycystic kidney disease. Nature 368: 750–753, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Ecder T, Melnikov VY, Stanley M, Korular D, Lucia MS, Schrier RW, Edelstein CL: Caspases, Bcl-2 proteins and apoptosis in autosomal-dominant polycystic kidney disease. Kidney Int 61: 1220–1230, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Tao Y, Kim J, Stanley M, He Z, Faubel SG, Schrier RW, Edelstein CL: Pathways of caspase-mediated apoptosis in autosomal dominant polycystic kidney disease (ADPKD). Kidney Int 67: 909–919, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA: Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311: 847–851, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green DR: Apoptotic pathways: paper wraps stone blunts scissors. Cell 102: 1–4, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Woo D: Apoptosis and loss of renal tissue in polycystic kidney diseases. N Engl J Med 333: 18–25, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Trudel M, Barisoni L, Lanoix J, D'Agati V: Polycystic kidney disease in SBM transgenic mice: role of c-myc in disease induction and progression. Am J Pathol 152: 219–229, 1998 [PMC free article] [PubMed] [Google Scholar]
- 30.Moser M, Pscherer A, Roth C, Becker J, Mucher G, Zerres K, Dixkens C, Weis J, Guay-Woodford L, Buettner R, Fassler R: Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2beta. Genes Dev 11: 1938–1948, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ: Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75: 229–240, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Lin HH, Yang TP, Jiang ST, Yang HY, Tang MJ: Bcl-2 overexpression prevents apoptosis-induced Madin-Darby canine kidney simple epithelial cyst formation. Kidney Int 55: 168–178, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Boletta A, Qian F, Onuchic LF, Bhunia AK, Phakdeekitcharoen B, Hanaoka K, Guggino W, Monaco L, Germino GG: Polycystin-1, the gene product of PKD1, induces resistance to apoptosis and spontaneous tubulogenesis in MDCK cells. Mol Cell 6: 1267–1273, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell R: Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384: 368–372, 1996 [DOI] [PubMed] [Google Scholar]