Abstract
Background and objectives: Concern about primary fistula failure may contribute to the underuse of arteriovenous fistula. The objective of this study was to investigate the baseline clinical parameters associated with primary fistula success.
Design, setting, participants, & measurements: Consecutive incident patients who commenced dialysis during a 28-mo period in a regional renal program were studied. Data on patient-related variables and on surgical approach (e.g., whether the surgeons routinely assess vessel size during the operation) were collected. Primary fistula success was defined as an arteriovenous fistula that was able to afford successful dialysis for 3 h with blood pump speed of ≥300 ml/min for three consecutive sessions.
Results: A total of 205 (69%) patients had an AVF attempted as their first vascular access. The overall primary success rate was 64% and was similar for radiocephalic and brachiocephalic fistula. Logistic regression was done separately for patients with the two types of fistula because of the presence of statistical interaction. For radiocephalic fistula, male gender was the only parameter associated with primary fistula success (odds ratio 3.57; P = 0.01). The presence of comorbidity was not significantly associated with primary fistula failure.
Conclusions: Despite significant patient comorbidity, there was a high primary fistula success rate among this incident hemodialysis cohort. Given that vessel size may be the ultimate determinant of fistula success, if surgeons assess vessel size perioperatively, then the presence of significant comorbidity might not preclude arteriovenous fistula from being attempted as the initial access.
Given the well-documented advantages of arteriovenous fistula (AVF) over arteriovenous graft and central venous catheter (CVC), the current Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines suggest that AVF should be attempted as the initial vascular access in at least 50% of incident patients and that at least 40% of prevalent hemodialysis (HD) patients should undergo dialysis with an AVF (1–5). Despite these recommendations, it is clear that AVF are underused in many centers in North America, especially when compared with European centers (6,7). Recent data suggest that only 32% of prevalent US HD patients undergo dialysis with an AVF (8). In Canada, AVF are used by 53% of prevalent and only 26% of incident HD patients (7).
One of the fears that may reduce the use of AVF is the high primary fistula failure rate (i.e., failure of AVF to mature) (9). Identifying risk factors that contribute to primary fistula failure may help clinicians and patients make an informed decision as to for whom to attempt an AVF. Only a few studies have prospectively examined the risk factors associated with primary fistula failure, which has been reported in 20 to 50% of patients (9–12).
Most studies that have evaluated factors that are associated with primary fistula failure have typically used a retrospective design. For instance, Miller et al. (10) reported higher primary fistula failure rate in older and female patients with diabetes, although small numbers and a highly selected patient population limit the generalizability of these results. Feldman et al. (13) published data from a larger cohort (348 patients), reporting a primary success rate of 54%. Preexisting cerebrovascular disease, older age, and commencement of dialysis before access creation were noted to be associated with higher primary fistula failure in their study. Ravani et al. (11) noted that cardiovascular disease and late referral to nephrologists were associated with lower primary AVF success in patients from northern Italy.
More recently, Lok et al. (14) studied a cohort of 422 HD patients who were undergoing their first AVF placement to identify preoperative clinical characteristics that are predictive of fistula failure to mature and to use this information to develop a prediction rule to estimate the risk for fistula failure to mature. This prediction rule was validated in an external data set from five North American centers. Age, peripheral vascular disease, coronary artery disease, and white race all were associated with fistula failure to mature in their study; however, in this and the previously mentioned studies, it is not clear whether there was any consistent surgical assessment performed perioperatively to guide fistula placement; as such, the results may not be applicable to programs that use pre- or intraoperative surgical assessment to guide fistula placement.
Given these uncertainties, we sought to determine the association of various baseline clinical parameters with primary fistula success in a cohort that consisted of all incident dialysis patients between July 1, 1999, and November 1, 2001, in Calgary, Alberta, Canada, the majority of whom had an indirect assessment of vessel diameter intraoperatively to guide the location of fistula placement.
Concise Methods
Patient Population and Data Collection
All patients who commenced dialysis between July 1, 1999, and November 1, 2001, were included in this study. Demographic and clinical data were collected via an electronic database (15). The database was maintained by a team of information technologists. The presence of comorbid illness was assessed as of the date of dialysis initiation by complete review of the patients’ inpatient and outpatient record (containing all information pertaining to medical and surgical consultations and all previous hospital admissions) and by direct patient interview by a trained nursing research assistant. Information was collected for the 19 variables that constitute the Charlson comorbidity index (16), which has been validated for use in patients with ESRD (17,18) (Appendix 1).
Appendix 1.
Weighted scoring system for the Charlson comorbidity indexa
| Assigned Weight for Diseases | Condition |
|---|---|
| 1 | Myocardial infarction |
| CHF | |
| PVD | |
| CVD | |
| Dementia | |
| Chronic pulmonary disease | |
| Connective tissue disease | |
| Ulcer disease | |
| Mild liver disease | |
| Diabetes | |
| 2 | Hemiplegia |
| Moderate or severe renal disease | |
| Diabetes with end-organ damage | |
| Any tumor | |
| Leukemia | |
| Lymphoma | |
| 3 | Moderate or severe liver disease |
| 6 | Metastatic solid tumor |
| AIDS |
The total equals the score. Example: Chronic pulmonary (1), severe renal disease (2), and myocardial infarction (1) = total score (4) (16).
Surgical information was obtained from the ALTRA database, an electronic medical record that captures all vascular access surgical data prospectively. Information was entered by the vascular access surgeons at the time of surgery. Vascular access monitoring was done and information was entered into the database by two full-time vascular access coordinators (both registered nurses). In addition, information on surgical approach to selection of patients for AVF was collected by interview of each surgeon by one of the authors (B. Murphy). Surgeons were classified into two groups on the basis of their surgical approaches. Group 1 surgeons adhered to the following algorithm. If the physical examination of the patients’ nondominant arm revealed an “adequate” radial and ulnar artery pulsation at the wrist, then they went on to explore surgically the distal cephalic vein. If this vein was patent and allowed the insertion of a 3-mm-diameter garret dilator 10 cm proximally, followed by the insertion of an umbilical catheter 30 cm proximally, then they proceeded to explore the distal radial artery. They carried out exactly the same sequence of catheter insertion in the artery. If the artery was of sufficient internal diameter to allow comfortable catheter insertion (≥3 mm), then they proceeded to create an end-to-side radiocephalic anastomosis. If any of these vessels were too small, then they abandoned radiocephalic fistula surgery and commenced a similar process with the cephalic vein at the elbow. Between the time of determination of a suitable vein and before arterial exploration, the patient was given 3000 to 5000 U of heparin intravenously. If a suitable cephalic vein at the elbow was discovered (based on the previous criteria), then a brachiocephalic (upper arm) fistula was created and, if not, then a brachiobasilic loop graft was created. This algorithm depended predominantly on vessel size, rather than demographic or comorbid characteristics; however, in “better” surgical candidates, borderline vessel size might not preclude a radiocephalic fistula attempt, whereas it might in “poorer” surgical candidates. The other surgical approach (group 2) relied on the perioperative appraisal of the arterial and venous anatomy without the aid of dilators or catheters. This more liberal approach allowed some patients with smaller vessels to receive radiocephalic fistulas. End-to-side anastomosis was carried out in AVF that were created by both surgical approaches.
We defined primary fistula success as a fistula that afforded an extracorporeal blood flow of at least 300 ml/min, for at least 3 h, using an arterial and a venous needle, for a minimum of three consecutive dialysis sessions. The research nurse reviewed all of the HD run sheets to assess the status of the fistula using a standard form. In a minority of cases, usually when patients underwent dialysis in satellite units remote from Calgary, run sheets could not be reviewed. In these cases, outcomes were assessed by one of the authors’ directly contacting the dialysis center. Ethical approval was obtained for the study from the Conjoint Research Ethics Board at the University of Calgary.
Statistical Analyses
A two-sided t test and a two-sample test of proportions were used to compare differences in continuous and categorical variables, respectively. χ2 tests were used to test for the association between primary fistula success and individual demographic characteristics and comorbid variables. Age and Charlson score were categorized into <65 or ≥65 and less than the mean or more than or equal to the mean for analysis, respectively. The association between individual components of the Charlson score and primary fistula success was assessed using univariate and multivariate logistic regression using fistula success as a dependent variable and clinical parameters, surgical location, and surgical approach as independent variables. We assessed for co-linearity between diabetes, ischemic heart diseases, peripheral vascular disease, and cerebral vascular disease. We also tested for significant interactions between radiocephalic or brachiocephalic AVF and gender, diabetes, and surgical approach.
Results
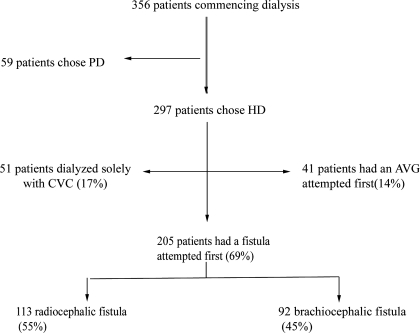
A total of 356 patients commenced renal replacement therapy in the Southern Alberta Renal Program during the study period (Figure 1). The majority of the cohort initiated renal replacement with HD (297 [80%] patients). Of these, 205 (69%) had an attempt at a fistula as their first vascular access. Of the 205 fistula patients, 69 had their access attempted before initiation of HD. A small proportion of the cohort (17%) never had any fistula or graft attempted and remained on HD with a permanent catheter. Fourteen percent of patients had an arteriovenous graft placed as their first vascular access. Three patients died within 6 wk of fistula creation surgery and were classified as having primary fistula failure. One of these cases occurred within 24 h of the access surgery and might have been due to access surgery.
Figure 1.
Trial profile.
Table 1 shows the baseline demographic and comorbid data in patients who commenced HD. Overall, 80% of patients were white. A high proportion of patients had diabetes (47%), with several comorbid illnesses (mean Charlson score 4.7), including ischemic heart disease (40%). Patients who underwent dialysis exclusively with a CVC were older and more likely to be female compared with patients whose first access attempt was an AVF (70.0 versus 62.5 yr [P = 0.0018]; 45 versus 29% female [P = 0.006], respectively). Patients who underwent dialysis exclusively with a CVC also had the highest comorbidity as indicated by higher mean Charlson index scores.
Table 1.
Demographic and comorbid variables in patients who had AVF or AVG attempted as the first vascular access and those who never had an attempt at vascular access and remained on dialysis through a CVCa
| Characteristic | Overall(n = 297) | Any Fistula(n = 205) | AVG(n = 41) | CVC(n = 51) |
|---|---|---|---|---|
| Diabetes (%) | 48.0 | 47.0 | 62.0 | 35.0 |
| Female gender (%) | 45.0 | 29.0 | 61.0b | 45.0c |
| Mean age (yr) | 66.3 | 62.5 | 66.5 | 70.0d |
| Mean Charlson score | 5.1 | 4.7 | 5.2 | 5.3 |
| IHD (%) | 41.3 | 40.0 | 44.0 | 40.0 |
| CHF (%) | 40.3 | 37.0 | 37.0 | 47.0 |
| PVD (%) | 21.3 | 20.0 | 17.0 | 27.0 |
| CVD (%) | 18.3 | 17.0 | 22.0 | 16.0 |
AVF, arteriovenous fistula; AVG, arteriovenous graft; CHF, congestive heart failure; CVC, central venous catheter; CVD, cardiovascular disease; IHD, ischemic heart disease; PVD, peripheral vascular disease.
P = 0.0001, χ2 test of two-sample proportions comparing AVG group with any fistula group.
P = 0.006, χ2 test of two-sample proportions comparing CVC group with any fistula group.
P = 0.0018, two-sample t test comparing CVC group with any fistula group.
A detailed comparison of patients who had an attempt at a radiocephalic fistula or brachiocephalic fistula is presented in Table 2. There were 113 radiocephalic fistulas and 92 brachiocephalic fistulas attempted as the first vascular access. The overall primary success rate was 64% and was similar in both types (63.7% in radiocephalic fistula and 64.1% in brachiocephalic fistula). Surgical approaches 1 and 2 were used in 133 and 72 fistula surgeries, respectively. Surgeons who used approach 1 were more likely to attempt a brachiocephalic fistula as the first vascular access compared with surgeons who used approach 2 (64.6 versus 26.4%, respectively). Among patients who had a brachiocephalic fistula attempted, the primary fistula success rate was higher in surgeons who used approach 1, compared with approach 2 (70 versus 42%; P = 0.025). The proportion of patients who had primary fistula success (unadjusted) was high in all patient subgroups (range 44–86%; mean 64%; Table 3).
Table 2.
Baseline demographic and comorbid variables for patients for whom fistula were attempted as the first vascular access (n = 205)
| Characteristic | Any Fistula(%; n = 205) | Radiocephalic Fistula(%; n = 113) | Brachiocephalic Fistula(%; n = 92) |
|---|---|---|---|
| Age (yr) | |||
| <65 | 51 | 50 | 48 |
| ≥65 | 49 | 50 | 52 |
| Gender | |||
| male | 71 | 76 | 65 |
| female | 29 | 24 | 35 |
| Mean Charlson score | 4.7 | 4.6 | 5.0 |
| Diabetes | 53 | 42 | 53 |
| Surgical approach | |||
| 1 | 65 | 53 | 79a |
| 2 | 35 | 47 | 21 |
| IHD | 40 | 35 | 46 |
| Hypertension | 87 | 88 | 86 |
| PVD | 20 | 19 | 22 |
| CHF | 37 | 34 | 40 |
| CVD | 17 | 17 | 16 |
P < 0.0001, χ2 test of two-sample proportions comparing number of patients with radiocephalic and brachiocephalic fistula attempted.
Table 3.
Comparison of unadjusted primary fistula success in radiocephalic and brachiocephalic fistulas
| Characteristic | Radiocephalic Fistula (%) | Brachiocephalic Fistula (%) |
|---|---|---|
| Age (yr) | ||
| <65 | 70 | 68 |
| ≥65 | 57 | 60 |
| Gender | ||
| male | 70 | 63 |
| female | 44a | 66 |
| Diabetes | ||
| absent | 58 | 67 |
| present | 71 | 61 |
| Surgical approach | ||
| 1 | 62 | 70b |
| 2 | 66 | 42 |
| IHD | ||
| absent | 62 | 70 |
| present | 67 | 57 |
| Hypertension | ||
| absent | 86 | 62 |
| present | 61 | 65 |
| PVD | ||
| absent | 65 | 65 |
| present | 57 | 60 |
| CHF | ||
| absent | 64 | 64 |
| present | 63 | 65 |
| CVD | ||
| absent | 63 | 62 |
| present | 68 | 73 |
P = 0.017, χ2 two-sample test comparing men and women with radiocephalic fistula.
P = 0.025, χ2 two-sample test comparing surgical approaches 1 and 2 in brachiocephalic fistula.
Next, we performed multivariate logistic regression to determine which clinical, demographic, and comorbid factors were associated with primary fistula success. We noted a significant interaction (P < 0.05) between fistula location and gender and between fistula location and surgical approach when predicting the likelihood of fistula success in the overall model, and, as such, we reported the regression model for radiocephalic and brachiocephalic fistula separately. Table 4 reports the results of the logistic regression for radiocephalic fistula. Male gender was the only factor that was significantly associated with success (odds ratio 3.57; 95% confidence interval 1.36 to 9.38; P = 0.01). Notably, the presence of comorbid conditions, when included as the individual components (Tables 4 and 5) or as a continuous variable (i.e., as the Charlson score; data not shown), was not associated with primary fistula success. In brachiocephalic fistula, the only factor associated with primary fistula success was surgical approach. This may have been because surgeons in group 1 selected better candidates for brachiocephalic fistula on the basis of larger vessel sizes (i.e., surgeons in group 2 were less selective and more likely to place radiocephalic fistula in patients). There was no association between age, gender, or comorbidity and fistula success in brachiocephalic fistulas. We constructed a separate model to examine the determinants of choosing brachiocephalic fistula over radiocephalic fistula. This analysis revealed that female patients and patients who were operated on using surgical approach 1 were more likely to have a brachiocephalic AVF placed (data not shown).
Table 4.
Logistic regression of primary fistula success in radiocephalic fistula (n = 113)a
| Independent Variable | OR | P | 95% CI |
|---|---|---|---|
| Gender (male versus female) | 3.57 | 0.010 | 1.36 to 9.38 |
| Surgical approach 2 versus 1 | 1.18 | 0.689 | 0.52 to 2.71 |
| Age <65 versus ≥65 | 1.69 | 0.240 | 0.70 to 4.05 |
| Diabetes | 1.92 | 0.140 | 0.80 to 4.61 |
| IHD | 1.31 | 0.580 | 0.50 to 3.40 |
| CHF | 1.21 | 0.680 | 0.49 to 3.00 |
| PVD | 0.41 | 0.140 | 0.12 to 1.36 |
| CVD | 1.86 | 0.340 | 0.52 to 6.74 |
CI, confidence interval; OR, odds ratio.
Table 5.
Logistic regression of primary fistula success in brachiocephalic fistula (n = 92)
| Independent Variable | OR | P | 95% CI |
|---|---|---|---|
| Gender (male versus female) | 0.79 | 0.659 | 0.29 to 2.19 |
| Surgical approach 2 versus 1 | 0.25 | 0.016 | 0.08 to 0.78 |
| Age <65 versus ≥65 | 1.91 | 0.200 | 0.71 to 5.14 |
| Diabetes | 0.66 | 0.410 | 0.24 to 1.78 |
| IHD | 0.59 | 0.310 | 0.21 to 1.65 |
| CHF | 1.46 | 0.490 | 0.51 to 4.10 |
| PVD | 0.66 | 0.470 | 0.21 to 2.03 |
| CVD | 2.34 | 0.210 | 0.61 to 8.98 |
Discussion
In an incident HD cohort, we report a high primary fistula success rate (64%), which is notable given the high proportion of fistulas attempted in this incident population. Despite higher patient comorbidity compared with other reports in the literature, none of the comorbid diseases was noted to influence primary fistula success in our study. It is likely that comorbid factors do influence primary fistula success through their association with vascular disease; however, appropriate surgical selection of patients on the basis of careful assessment of vasculature may make existence of comorbid diseases on their own less important.
Despite significant patient comorbidities, the proportion of AVF attempted locally during this study (69%) is similar to the rate reported in a study by Allon et al. (12) when venous mapping was routinely performed (64%). Historically, AVF are more commonly used in European centers, as recently demonstrated in the Dialysis Outcomes and Practice Patterns Study (DOPPS) (19). Higher rates of primary fistula success have also been reported in European centers (11), which may be due to lower rates of patient comorbidity, including diabetes, congestive heart failure, and peripheral vascular diseases (7). The results of the DOPPS I survey suggest that the preference of the medical directors of dialysis units in North America might also contribute to underuse of AVF (20); however, AVF are still underused in North American centers despite most medical directors’ and vascular access surgeons’ preference for AVF, as noted in the recent DOPPS II study (7).
Both our data and the observations of Feldman et al. (13) are in keeping with the notion that the ultimate predictor of primary fistula success is likely the vessel size. In addition to previous reports, Feldman and colleagues (13,21) demonstrated that vessels >3 mm in diameter are associated with higher rates of primary fistula success. Although Feldman et al. reported that a history of cerebrovascular disease and cardiovascular disease, increasing age, and dependence on dialysis at the time of fistula creation predicted primary fistula success, it should be noted that whether to attempt an AVF in the study by Feldman et al. was not based on vessel size measurement (as it was in our study for surgeons who used approach 1). As illustrated, these comorbid factors may be surrogates for vessel size. If the surgeons are able to measure vessel size either pre- or intraoperatively, then fistula success may not be associated with the presence of comorbidities. Although we did not have information on specific vessel size pre- or intraoperatively, we did note that surgeons in our study who followed an algorithm of vessel size measurement using catheters had better results in brachiocephalic fistulas. This is consistent with evidence from retrospective, nonrandomized studies that suggested that preoperative vein mapping by duplex ultrasonography may improve primary fistula success rate (12,22). Preoperative venous mapping is able to identify patients with adequate arterial and venous diameter and therefore may increase the likelihood of fistula success. Our results are consistent with those of Mendes et al. (23), who used preoperative ultrasound vein mapping in eight different locations of the arm. In a single center of 44 patients, they found that vein diameter ≤2.0 mm was associated with high (76%) failure rate to maturation regardless of age, race, and gender. Our findings may be more generalizable to the majority of North American centers, where venous mapping by preoperative ultrasound is not routinely performed.
Lok et al. (14) presented the largest multicenter study to date that examined the predictors of fistula failure to mature. A prediction model for fistula failure to mature was derived from a cohort of 422 patients and then validated in five different centers. Age, white race, peripheral vascular disease and coronary artery disease, but not age or diabetes were found to be significant predictors of fistula failure to mature. No detailed preoperative radiologic mapping or intraoperative assessment of vessel size was performed, and this may explain the differences in results noted between their study and ours.
Although many studies suggest that male gender is associated with higher rates of primary fistula success (24,25), other studies, including the one by Lok et al. (14), have not noted such an association. Moreover, Caplin et al. (26) reported equal AVF success rates and prevalence in both women and men. In their center, preoperative vascular mapping was routinely performed using duplex ultrasound. There was no difference in arterial sizes between women and men, but venous sizes were not reported. This might further support the notion that vessel size might be the ultimate determinant of AVF success. As noted, the controversy may be due to variable patient selection methods for choosing men and women for AVF surgery.
Our study has several limitations. Although this study is the third largest cohort study to examine the predictors of AVF failure, our study enrolled a relatively small number of patients. The risk of type II error (i.e., declaring that no risk factors for fistula success exist when in fact they do) would be reduced if we had more study patients. Perhaps the biggest limitation of our study was that we did not have a direct measure of vessel size in patients. We did have an indirect measure of vessel size but only in patients who were in surgeon group 1. As such, we can only postulate that comorbid conditions affect AVF success through vessel size. Future studies should measure patient comorbidity and vessel size accurately, thereby enabling this hypothesis to be tested. Finally, although there is some evidence that late referral to nephrologists is a risk factor for primary fistula failure, information on this factor was not collected in this study.
Conclusions
Our study demonstrates that a higher proportion of AVF success is achievable in a North American HD population and strongly supports the recommendations made by K/DOQI. In addition, our data suggest that vessel size is likely the ultimate determinant of fistula success. If surgeons assess vessel size perioperatively, then the presence of significant patient comorbidity itself should not preclude AVF from being attempted as the initial access. Better assessment of vessel size either pre- or intraoperatively may improve rates of primary access success, although this requires further study. It is reasonable to consider brachiocephalic fistula in older and female patients.
Disclosures
None.
Acknowledgments
This research was supported by the Center for Advancement of Health, Calgary Health Region, and the Alberta Heritage Foundation for Medical Research.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ethier JH, Lindsay RM, Barre PE, Kappel JE, Carlisle EJ, Common A: Clinical practice guidelines for vascular access. Canadian Society of Nephrology. J Am Soc Nephrol 10: S297–S305, 1999 [PubMed] [Google Scholar]
- 2.Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK: Type of vascular access and mortality in US hemodialysis patients. Kidney Int 60: 1443–1451, 2001 [DOI] [PubMed] [Google Scholar]
- 3.D’Cunha PT, Besarab A: Vascular access for hemodialysis: 2004 and beyond. Curr Opin Nephrol Hypertens 13: 623–629, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Pastan S, Soucie JM, McClellan WM: Vascular access and increased risk of death among hemodialysis patients. Kidney Int 62: 620–626, 2002 [DOI] [PubMed] [Google Scholar]
- 5.K/DOQI. III. NKF-K/DOQI clinical practice guidelines for vascular access: Update 2000. Am J Kidney Dis 37: S137–S181, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, Wolfe RA, Goodkin DA, Held PJ: Vascular access use in Europe and the United States: Results from the DOPPS. Kidney Int 61: 305–316, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Mendelssohn DC, Ethier J, Elder SJ, Saran R, Port FK, Pisoni RL: Haemodialysis vascular access problems in Canada: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS II). Nephrol Dial Transplant 26: 721–728, 2006 [DOI] [PubMed] [Google Scholar]
- 8.USRDS. 2005 annual report. Available at: http://www.usrds.org/atlas.htm, accessed September 2006
- 9.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Miller PE, Tolwani A, Luscy CP, Deierhoi MH, Bailey R, Redden DT, Allon M: Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int 56: 275–348, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Ravani P, Brunori G, Mandolfo S, Cancarini G, Imbasciati E, Marcelli D, Malberti F: Cardiovascular comorbidity and late referral impact arteriovenous fistula survival: A prospective multicenter study. J Am Soc Nephrol 15: 204–209, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML: Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60: 2013–2020, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Feldman HI, Joffe M, Rosas SE, Burns JE, Knauss J, Brayman K: Predictors of successful arteriovenous fistula maturation. Am J Kidney Dis 42: 1000–1012, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Manns BJ, Mortis GP, Taub KJ, McLaughlin K, Donaldson C, Ghali WA: The Southern Alberta Renal Program database: A prototype for patient management and research initiatives. Clin Invest Med 24: 164–170, 2001 [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML: A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med 108: 609–613, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Young EW, Dykstra DM, Goodkin DA, Mapes DL, Wolfe RA, Held PJ: Hemodialysis vascular access preferences and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 61: 2266–2271, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Rayner HC, Besarab A, Brown WW, Disney A, Saito A, Pisoni RL: Vascular access results from the Dialysis Outcomes and Practice Patterns Study (DOPPS): Performance against Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines. Am J Kidney Dis 44[Suppl 3]: 22–26, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Reilly DT, Wood RF, Bell PR: Prospective study of dialysis fistulas: problem patients and their treatment. Br J Surg 69: 549–553, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Schuman E, Standage BA, Ragsdale JW, Heinl P: Achieving vascular access success in the quality outcomes era. Am J Surg 187: 585–589, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Mendes RR, Farber MA, Marston WA, Dinwiddie LC, Keagy BA, Burnham SJ: Prediction of wrist arteriovenous fistula maturation with preoperative vein mapping with ultrasonography. J Vasc Surg 36: 460–463, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Hirth RA, Turenne MN, Woods JD, Young EW, Port FK, Pauly MV, Held PJ: Predictors of type of vascular access in hemodialysis patients. JAMA 276: 1303–1308, 1996 [PubMed] [Google Scholar]
- 25.Miller CD, Robbin ML, Allon M: Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int 63: 346–352, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Caplin N, Sedlacek M, Teodorescu V, Falk A, Uribarri J: Venous access: Women are equal. Am J Kidney Dis 41: 429–432, 2003 [DOI] [PubMed] [Google Scholar]