Abstract
Background and objectives: Fabry disease is a progressive X-linked disorder of glycosphingolipid metabolism that typically presents in childhood and progresses to heart failure and renal failure in adulthood. This study sought to determine the prevalence of Fabry disease in a multiethnic male chronic kidney disease population, involving dialysis-dependent, non–dialysis-dependent, and transplant patients.
Design, setting, participants, & measurements: A total of 499 patients were screened with assay of α-galactosidase activity using fluorometric enzyme assay on plasma prepared from fresh heparinized blood, followed by leukocyte α-galactosidase activity in the subset of patients with plasma α-galactosidase activity below the second percentile (corresponding to a value <3.0 nmol/h per ml plasma).
Results: This study did not identify any new cases of Fabry disease; however, repeat testing of some of the study patients identified three limitations of the plasma enzyme assay that is commonly used as a high throughput screening method for Fabry disease: (1) False-negative results can occur; (2) these false-negative results are not prevented by use of inhibitors of α-galactosidase B activity; and (3) considerable intraindividual variation in plasma α-galactosidase levels reduces the discriminatory power of the screening test.
Conclusion: Clinicians need to be aware that screening using plasma will fail to detect some patients with Fabry disease.
Fabry disease (FD) is a progressive X-linked disorder of glycosphingolipid metabolism caused by a deficiency of the α-galactosidase lysosomal enzyme. The deficiency of this enzyme leads to progressive accumulation of neutral glycosphingolipids (chiefly globotriaosylceramide) in many tissues throughout the body, particularly the vascular endothelium, heart, and kidney (1–5). Absence of α-galactosidase activity results in cutaneous angiokeratomas, periodic febrile acroparesthesia, decreased or absent sweating, and corneal opacities during childhood. By the second or third decade, patients often manifest progressive proteinuria and renal failure. Eventually patients succumb to nephrologic, cardiac, and cerebrovascular complications in their fourth to fifth decade (3).
Typically the diagnosis of FD is made in male adolescents, but it may be missed or delayed, especially if the classic manifestations are subtle or absent. For example, alternate phenotypes have been described in heterozygous female individuals (3,6) as have cardiac (2,4,7) and renal (5) variants. These “variant” phenotypes usually have a low level of residual α-galactosidase activity that somewhat protects patients from developing microvascular glycosphingolipid accumulation, resulting in a lack of the classic phenotype. Such patients may present solely with progressive end-organ failure in their 40s (1). Because safe and effective enzyme replacement therapy is now available for FD, it is important to identify all patients with the disease earlier, when it is potentially treatable, so that appropriate family screening and genetic counseling can be provided and, if appropriate, enzyme replacement therapy can be started (8,9).
Overall, the prevalence of FD has been estimated to be 1 in 40,000 to 117,000 male individuals (1,3,10); however, several recent studies suggested that the prevalence may be higher in the hemodialysis (HD) population, in which values up to 1.2% have been reported (5,11–17). Most of the available data are from Europe, the United States, and Japan (5,11–13,17); therefore, many ethnic groups are unrepresented in these estimates, and it is possible that the frequency of unrecognized FD will vary by racial, ethnic, and demographic group. This may account in part for the discrepancy in prevalence of FD that has been seen in studies in which screening of dialysis patients was done (13); however, issues of laboratory methods and the choice of appropriate screening cutoff values may also have an impact on the apparent incidence of disease. The widespread assumption that screening for FD by assay of α-galactosidase in plasma is “reliable, relatively simple, and inexpensive” (5) has not been adequately evaluated.
We sought to assess the prevalence of unrecognized FD in our ethnically diverse kidney disease population through a screening program of male patients with chronic kidney disease (CKD). We also sought to validate published screening methods for FD.
CONCISE METHODS
This study was approved by the investigational review boards of our institution. The screening study was supported with an unrestricted educational grant from Genzyme Canada. The sponsor had no role in data collection or interpretation.
Location
Vancouver General Hospital and St. Paul's Hospital are two tertiary care facilities located in Vancouver, BC. Together, these two facilities provide care for approximately two thirds of the 6000 patients with CKD in the province (population approximately 4.25 million).
Patients
In 2004, male patients who had CKD and were registered on HD or attending transplant, peritoneal dialysis, or CKD clinics were approached by a research coordinator during regular clinic visits or dialysis runs for participation in this study. The duration of recruitment was 4 mo to ensure that the majority of the patients were included (based on regular clinic frequency of 3 mo for most patients except those on HD). Once written informed consent was obtained, plasma was drawn for analysis. Reasons for noninclusion in the study sample were logistical: Because the time period for sample gathering was 4 mo, patients who did not attend one of the clinics during that time, either because of geographic or medical reasons, were not approached. The population of patients in British Columbia with CKD is ethnically diverse (47 to 60% white, 18 to 32% Asian, and 11 to 20% Southeast Asian).
Laboratory Analysis
Heparinized blood samples from the patients were collected and placed on ice. Plasma was prepared by cold centrifugation within 1 h of blood collection, promptly frozen, and sent for analysis to the Biochemical Genetics Laboratory (an accredited clinical reference laboratory) at BC's Children's Hospital (Vancouver, BC).
Plasma Assays
In all patients, α-galactosidase activity was measured by fluorometric enzyme assay on plasma. The assay mix included 50 μl of plasma, 4-methylumbelliferyl-α-D-galactopyranoside as substrate (Sigma Chemical Co., St. Louis, MO; final concentration of 6.7 mM), and sodium acetate buffer (pH 4.5; final concentration 0.13 M) in a final volume of 150 μl. This was incubated for 4 h at 30°C. In a subset of patients, the assay was repeated with and without inclusion of N-acetylgalactosamine (GalNAc), at 100 mM final concentration, in the previous assay mixture. GalNAc is an inhibitor of the lysosomal enzyme α-N-acetylgalactosaminidase, also known as α-galactosidase B because in vitro it has some nonspecific activity toward the artificial substrate used for assay of α-galactosidase. Assays including GalNAc inhibitor at a saturating concentration measure exclusively α-galactosidase A, the specific enzyme activity that is deficient in FD (18,19), by eliminating any potential contribution from α-galactosidase B.
Leukocyte Assays
Patients with the lowest plasma α-galactosidase activities (<3.0 nmol/h per ml) were recalled for further testing of leukocyte α-galactosidase activity. The leukocyte enzyme assay was performed fluorometrically, using substrate and buffer concentrations as described previously for the plasma assay. To these was added 50 μl of leukocyte lysate (cell pellet prepared from 3 ml of heparinized blood by Dextran sedimentation followed by sonication in 0.5 to 1.0 ml of water). The reaction mix, in a final volume of 150 μl, was incubated for 1 h at 37°C. In a subset of patients, the assay was repeated with and without addition of the inhibitor GalNAc at 100 mM final concentration to ensure the specific measurement of α-galactosidase A.
Statistical Analyses
Qualitative variables were compared by means of the Fisher exact test. Continuous variables were analyzed using a nonparametric Kruskal-Wallis test. Calculations were performed with aid of the SAS 9.1 (SAS Institute, Cary, NC).
Results
Patients
Of the 603 patients approached by the research coordinator, 523 consented, and 499 patients provided plasma suitable for analysis. Demographic information on the study cohort is shown in Table 1. The research cohort was comparable in race, age, and comorbidities to the entire cohort of patients with CKD in our province (data not shown).
Table 1.
Demographic characteristics of study cohorta
| Variable | CKD | HD | PD | TP |
|---|---|---|---|---|
| Total | 141 | 159 | 59 | 138 |
| Age (yr) | 63 | 67 | 60 | 49 |
| Diabetes (%) | 42 | 38 | 37 | 33 |
| CVD (%) | 48 | 67 | 47 | 23 |
| Race (%) | ||||
| White | 71 | 28 | 43 | 65 |
| Asian | 15 | 59 | 38 | 21 |
| Southeast Asian | 11 | 12 | 16 | 10 |
| Native Canadian | 1 | 1 | 3 | 2 |
| Other | 2 | 1 | 1 | 3 |
There were no significant differences in race, age, and presence of diabetes or cardiovascular disease between the study cohort and the entire male chronic kidney disease (CKD) population in Vancouver, BC. CVD, cardiovascular disease; HD, hemodialysis; PD, peritoneal dialysis; TP renal transplant.
Plasma α-Galactosidase Activity
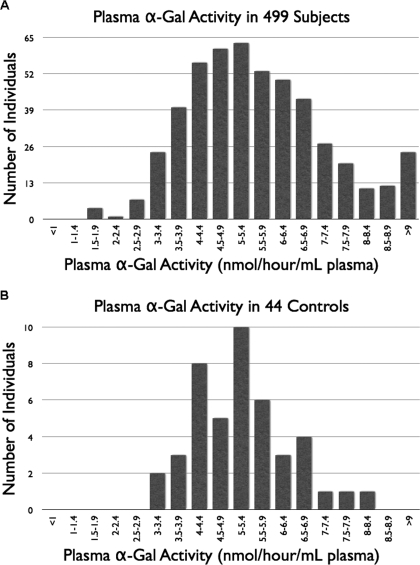
A total of 499 patients who had CKD and had not previously received a diagnosis of FD underwent screening by assay of plasma α-galactosidase activity. Normal control data were obtained for comparison by assay on 44 healthy volunteers (observed range 3.0 to 8.1 nmol/h per ml plasma; median 5.2 nmol/h per ml; mean 5.2 nmol/h per ml; Figure 1B). Two known patients with FD were used as positive control subjects, giving results of 0.0 and 0.2 nmol/h per ml, respectively. The observed range of activities in the renal patient cohort was 1.6 to 23.8 (median 5.5; mean 5.8; Figure 1A). Of the renal patients, 98% (486 patients) had activity ≥3.0 nmol/h per ml. Thus the lowest value seen in the relatively small group of healthy control subjects coincided with the second percentile (55% of the median value) for the 10-fold larger group of renal patients. This activity value was chosen as the screening cutoff below which further diagnostic investigations were indicated. The 11 renal patients who fell below the cutoff had plasma α-galactosidase activities ranging from 1.6 to 2.9 nmol/h per ml plasma. The lowest of these values represented a level of activity corresponding to 29% of the median activity seen in the renal patient group and 31% of the median value in the healthy control subjects.
Figure 1.
(A) Upper histogram. Plasma α-galactosidase results in the study cohort of patients with chronic kidney disease (CKD; n = 499; observed range of activities 1.6 to 23.8 nmol/h per ml plasma; median 5.5; mean 5.8). Eleven patients had α-galactosidase activity below the second percentile of this group (<3.0 nmol/h per ml plasma). (B) Lower histogram. Plasma α-galactosidase in healthy control subjects (n = 44; observed range of activities 3.0 to 8.1 nmol/h per ml plasma; median 5.2; mean 5.2).
These 11 patients who fell below the cutoff for plasma α-galactosidase activity had a mean age of 64.2 yr and did not differ from the whole cohort in ethnicity; presence of comorbidities including peripheral vascular disease, stroke, and diabetes; or classification of CKD (HD, peritoneal dialysis, predialysis, or transplant). The nature of their primary renal disease was diverse, including nephropathy as a result of diabetes and hypertension, interstitial nephritis, postobstructive nephropathy, postnephrectomy, and focal glomerular sclerosis. The prevalence of documented coronary artery disease was lower in these 11 patients than in the entire cohort (9 versus 52%; P = 0.015), but, given the sample size of 11 patients, it is difficult to determine the significance of this finding.
Leukocyte α-Galactosidase Activity
Ten of the 11 renal patients identified as having the lowest plasma α-galactosidase activities in the cohort underwent subsequent α-galactosidase assay on leukocytes. One patient died before leukocyte testing could be undertaken. The control reference range (n = 55) was 42.1 to 112.9 nmol/h per mg protein (median 79.7; mean 78.6). Leukocytes from three known patients with FD, assayed as positive controls, gave activities of 2.0 to 3.8 nmol/h per mg protein. Testing of the recalled renal patients revealed a wide range of leukocyte α-galactosidase activity (38.8 to 125.0 nmol/h per mg protein; mean 67.6; median 64.3), broadly overlapping the normal reference range. Only two renal patients had enzyme activity below the control range (both giving activities of 38.8 nmol/h per mg protein). Although slightly below the lower reference limit, this corresponded to 49% of the control mean and therefore was not suggestive of FD.
Evaluation of Screening Methods
The initial screening study did not identify any new cases of FD among our renal patient cohort. We sought to evaluate the possibility that methodologic issues might have interfered with our ability to detect patients with FD. We considered two issues related to the enzyme assays and their interpretation: (1) Did interference from α-galactosidase B lead to false-negative results in our screening methods (because GalNAc inhibitor was not used in our initial studies)? (2) Do all male patients with FD show clearly low α-galactosidase A activity on the plasma screening assay?
We performed assays in the presence and absence of 100 mM inhibitor on plasma samples from 20 healthy control subjects. Results are shown in Table 2 and indicate that of the total α-galactosidase assayed in normal plasma, α-galactosidase B typically composes only a small minority (mean 6%). Corresponding studies were performed on stored plasma samples from 10 randomly selected renal patients, with similar results (data not shown). We also carried out corresponding α-galactosidase assays, in the presence and absence of an inhibitor, on leukocytes from 30 healthy individuals (Table 2). α-Galactosidase B on average composed only 3% of the total measured α-galactosidase activity in leukocytes.
Table 2.
Comparison of α-galactosidase activity assayed from healthy individuals in the presence and absence of GalNAc inhibitora
| Parameter | Total α-Galactosidase (Assayed without Inhibitor) | α-Galactosidase A (Assayed with 100 mM GalNAc) | α-Galactosidase B (by subtraction) | α-Galactosidase B as % of Total |
|---|---|---|---|---|
| Plasma (nmol/h per ml plasma; n = 20 individuals) | ||||
| mean | 5.3 | 5.0 | 0.3 | 6.0 |
| median | 5.3 | 4.9 | 0.3 | 6.1 |
| observed range | 3.4 to 7.1 | 3.1 to 7.0 | 0.0 to 1.0 | 0.0 to 18.1 |
| Leucocytes (nmol/h per mg protein; n = 30 individuals) | ||||
| mean | 81.7 | 79.3 | 2.3 | 2.8 |
| median | 77.3 | 77.7 | 2.0 | 3.0 |
| observed range | 49.9 to 126.3 | 47.2 to 124.3 | 0.0 to 8.0 | 0.0 to 7.6 |
GalNAc, N-acetylgalactosamine.
We attempted a second recall of the 10 renal patients who were previously recalled on the basis of low total α-galactosidase in plasma, for the purpose of repeat leukocyte and plasma enzyme assays with and without inhibitor. Five of the 10 were available for further testing. Results are summarized in Table 3. It is interesting that four of these five patients (previously selected on the basis of low plasma enzyme activity) now had plasma enzyme activities well within reference range (Table 3). This discrepancy is unlikely to reflect simply imprecision of the plasma α-galactosidase assay for the following reasons: (1) Day-to-day imprecision (test variability) of the assay, assessed using aliquots from a normal sample in every run, showed a between-assay variation of 10.9% (mean 4.51 nmol/h per ml; SD 0.49; n = 29); this is considered analytically acceptable for a manual enzyme assay; (2) the positive control sample (from a confirmed patient with FD) consistently gave abnormally low results (mean 0.2 nmol/h per ml; range 0.0 to 0.6; n = 16); and (3) samples from some of the renal study patients that had given low results were re-assayed in a subsequent batch, with similar results (1.6 → 1.4; 1.2 → 1.6; 1.9 → 1.9). Therefore the shift in plasma values on recall of the patients shown in Table 3 was much greater than the expected between-assay variation and thus suggests a significant degree of biologic (and/or pathologic) variability in plasma α-galactosidase activity. In addition, various checks for between-assay variation were likewise built into the leukocyte assays throughout the study and gave acceptable analytical performance (data not shown).
Table 3.
Activities of α -galactosidase in leukocytes and plasma of five renal patients who had low plasma α-galactosidase activity in the initial screening phase of the studya
| Patient | Leukocytes (nmol/h per mg protein)
|
Plasma (nmol/h per ml)
|
||||
|---|---|---|---|---|---|---|
| Initial Result (No GalNAc Inhibitor)
|
Results on Recall
|
Initial Result (No GalNAc Inhibitor)
|
Results on Recall
|
|||
| Total | Total | A | Total | Total | A | |
| 1 | 66.7 | 51.0 | 51.3 | 1.6 | 2.3 | 1.5 |
| 2 | 125.0 | 89.7 | 87.2 | 2.8 | 5.8 | 5.6 |
| 3 | 73.6 | 60.1 | 55.9 | 2.9 | 5.9 | 5.6 |
| 4 | 94.1 | 60.3 | 60.0 | 2.9 | 4.5 | 4.1 |
| 5 | 58.8 | 34.7 | 33.7 | 2.5 | 4.5 | 4.1 |
In the initial phase, only total α-galactosidase activity was measured. When these patients were recalled for a second time, assays were performed both without and with 100 mM GalNAc inhibitor (thus measuring “Total” and “A” activity, respectively). Reference range for total activity in leukocytes 42.1 to 112.9 nmol/h per mg protein; reference range for total activity in plasma 3.0 to 8.1 nmol/h per ml.
We also obtained plasma samples from three additional patients with known FD and assayed α-galactosidase in the presence and absence of inhibitor. Table 4 shows the results in plasma compared with those in leukocytes. As with the plasma samples from two other patients who had FD and were included as positive control subjects during phase 1 of the study, patient 1's total α-galactosidase activity was clearly low in plasma, as well as in white blood cells; however, the plasma activities for patients 2 and 3 both fell within the reference range, despite clearly low activities in leukocytes. Neither of these patients would have been detected as affected in our screening study protocol, irrespective of the presence or absence of inhibitor. Aliquots of both of these plasma samples were sent “blind” to an international diagnostic reference laboratory for independent verification. The reference laboratory used a similar α-galactosidase A assay protocol to ours (including GalNAc as inhibitor), except that assay was at 37°C rather than 30°C, leading to a higher reference range of activity. With a reference range in plasma of 6.2 to 18.6 nmol/h per ml (mean 12.0), their result for patient 3 was 16.0 and that for patient 2 was 5.8 (48% of control mean). The former was regarded as a normal result and the latter interpreted with caution as slightly below the nonaffected range but well above the affected range of <2.0 nmol/h per ml. The external laboratory confirmed our finding that plasma assays of α-galactosidase A activity can produce false-negative results, even with the use of an inhibitor of α-galactosidase B activity.
Table 4.
α-Galactosidase activities in plasma and leukocytes from three known patients with Fabry diseasea
| Patient | Plasma (nmol/h per ml)
|
Leukocytes (nmol/h per mg protein)
|
||||
|---|---|---|---|---|---|---|
| Total | A | B | Total | A | B | |
| 1 | 0.6 | 0.0 | 0.6 | 12.6 | 6.9 | 5.7 |
| 2 | 3.6 | 3.6 | 0.0 | 9.3 | 5.2 | 4.1 |
| 3 | 8.0 | 7.2 | 0.7 | 3.9 | 2.3 | 1.7 |
Activities were assayed in the absence and presence of 100 mM GalNAc inhibitor (thus measuring “Total” and “A,” respectively). Reference range for total activity in leucocytes 42.1 to 112.9 nmol/h per mg protein; reference range for total activity in plasma 3.0 to 8.1 nmol/h per ml.
Discussion
Recent studies have reported that the prevalence of undiagnosed FD can range from 0.16 to 1.2% in patients with CKD (5,11–17). These studies have focused on homogeneous European, American, and Japanese populations. No study has specifically examined large populations of Chinese and Asian descent. This study population describes a diverse group of individuals including white and Asian; given that many studies have focused on single ethnicities, we had considered it feasible that the multiethnicity of our population might provide broader opportunities to detect FD; however, despite this diversity, no new cases of FD were detected, in a province of 4.25 million.
The majority of previous screening studies that looked for FD in patients with renal disease or cardiac disease used plasma α-galactosidase assay as the first-line test, whereas some used bloodspots. Fluorometric assays with the same artificial substrate have been virtually universally used, and presumably generally similar laboratory methods. Most, although not all, studies included GalNAc as inhibitor in the assay. It is interesting that some studies (5,20) used the inhibitor at concentrations several orders of magnitude lower than the level required for effective inhibition of isolated α-galactosidase B (18). We recognize that the use of GalNAc at suitable concentrations does eliminate the small degree of assay interference from α-galactosidase B. Nonetheless, the data from our method evaluations (Table 2) indicate that this factor is relatively minor and is unlikely to have affected the outcome of our study.
On review of published studies, it is clear that some authors assume that patients with FD will necessarily have markedly low activities of α-galactosidase A in plasma and hence select only the most marked outliers for further follow-up (17). A review of published screening studies (5,11,13,17,20,21) showed that no authors have set a screening cutoff any higher than 35% of control mean or cohort mean, and most have set it considerably lower. It is therefore very likely that two of our five known control patients with FD (patients 2 and 3; Table 4) would have escaped detection at those centers. Patient 3 (Table 4) is especially noteworthy in showing high-normal activity in plasma despite markedly deficient activity in leukocytes. Assay on leukocytes is more reliable than on plasma, but current methods are too labor-intensive for this to be a feasible high-throughput screening method. The knowledge that some patients with FD, especially those with the “variant” clinical phenotypes, can display significant residual enzyme activities is not new; neither is the identification of certain patients with deficient activity in leukocytes but normal activity in plasma, as a result of variant enzymes reported to be unstable in the acidic lysosome but more stable in the neutral plasma (19); however, this issue has not been taken into account in screening studies done to date, and we have shown that the screening methods currently in use can cause false-negative results. With the introduction of enzyme replacement therapy that has been shown to slow progression of disease, it is important to identify patients early in the course of the disease (21–24). A recent study (21) on newborn screening for FD, using assays on dried blood spots followed up by plasma, suggests an incidence much greater than previously believed and that patients with “classic” FD constitute only the minority. In the newborn study, results on dried blood spots and plasma were concordant. There is insufficient information to determine whether bloodspots would be more reliable than plasma in screening adult patient populations with renal or cardiac disease. It is plausible that circulating enzyme levels in such populations might be more influenced by secondary confounders (dietary, biologic, pathologic, or iatrogenic) than in neonates. Careful attention to sample type, laboratory method design, and data analysis is required, along with recognition that some patients will inevitably elude detection by population screening protocols.
Disclosures
None.
Acknowledgments
We acknowledge the patients who participated in this project, the excellent research assistance from Candy Ho and Lina Sioson, and the laboratory technologists at both St. Paul's Hospital and BC's Children's Hospital (Biochemical Genetics Laboratory). We thank Dr. Marie Grace (Mount Sinai Medical Center, New York, NY) for kindly providing independent enzyme activity assays on selected patients’ plasma samples. We thank Genzyme Canada for provision of an unrestricted educational grant to support this project.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, Grabowski G, Packman S, Wilcox WR: Fabry disease, an under-recognized multisystemic disorder: Expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med 138: 338–346, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, Yoshida A, Kuriyama M, Hayashibe H, Sakuraba S, Tanaka H: An atypical variant of Fabry's disease in men with left ventricular hypertrophy. N Engl J Med 333: 288–293, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Mehta A, Ricci R, Widmer U, Dehout F, Garcia de Lorenzo A, Kampmann C, Linhart A, Sunder-Plassmann G, Ries M, Beck M: Fabry disease defined: Baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest 34: 236–242, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Nagao Y, Nakashima H, Fukuhara Y, Shimmoto M, Oshima A, Ikari Y, Mori Y, Sakuraba H, Suzuki Y: Hypertrophic cardiomyopathy in late-onset variant of Fabry disease with high residual activity of α-galactosidase A. Clin Genet 39: 233–237, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Nakao S, Kodama C, Takenaka T, Tanaka A, Yasumoto Y, Yoshida A, Kanzaki T, Enriquez AL, Eng CM, Tanaka H, Tei C, Desnick RJ: Fabry disease: Detection of undiagnosed hemodialysis patients and identification of a “renal variant” phenotype. Kidney Int 64: 801–807, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Deegan PB, Baehner AF, Barba Romero MA, Hughes DA, Kampmann C, Beck M: Natural history of Fabry disease in females in the Fabry Outcome Survey. J Med Genet 43: 347–352, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Scheidt W, Eng CM, Fitzmaurice TF, Erdmann E, Hubner G, Olsen EG, Christomanou H, Kandolf R, Bishop DF, Desnick RJ: An atypical variant of Fabry's disease with manifestations confined to the myocardium. N Engl J Med 324: 395–399, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ: International Collaborative Fabry Disease Study Group. Safety and efficacy of recombinant human alpha-galactosidase A: Replacement therapy in Fabry's disease. N Engl J Med 345: 9–16, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Schiffmann R, Kopp JB, Austin HA 3rd, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO: Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 285: 2743–2749, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Meikle PJ, Hopwood JJ, Clague AE, Carey WF: Prevalence of lysosomal storage disorders. JAMA 281: 249–254, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Kotanko P, Kramar R, Devrnja D, Paschke E, Voigtlander T, Auinger M, Pagliardini S, Spada M, Demmelbauer K, Lorenz M, Hauser AC, Kofler HJ, Lhotta K, Neyer U, Pronai W, Wallner M, Wieser C, Wiesholzer M, Zodl H, Fodinger M, Sunder-Plassmann G: Results of a nationwide screening for Anderson-Fabry disease among dialysis patients. J Am Soc Nephrol 15: 1323–1329, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Utsumi K, Kase R, Takata T, Sakuraba H, Matsui N, Saito H, Nakamura T, Kawabe M, Iino Y, Katayama Y: Fabry disease in patients receiving maintenance dialysis. Clin Exp Nephrol 4: 49–51, 2000 [Google Scholar]
- 13.Linthorst GE, Hollak CE, Korevaar JC, Van Manen JG, Aerts JM, Boeschoten EW: Alpha-galactosidase A deficiency in Dutch patients on dialysis: A critical appraisal of screening for Fabry disease. Nephrol Dial Transplant 18: 1581–1584, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Spada M, Pagliardini S: Screening for Fabry disease in end-stage nephropathies. J Inherit Metab Dis 25[Suppl I]: 113, 2002 [Google Scholar]
- 15.Walters BA, Prichard M, McCardle H, Richards SM, Bosch JP: Prevalence of reduced plasma alpha-galactosidase activity in a cohort of male patients on hemodialysis (HO) in the United States [Abstract]. Presented at the annual meeting of the American College of Medical Genetics; March 14–17, 2002; New Orleans, LA
- 16.Bekri S, Enica A, Ghafari T, Plaza G, Champenois I, Choukroun G, Unwin R, Jaeger P: Fabry disease in patients with end-stage renal failure: The potential benefits of screening. Nephron 101: c33–c38, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Ichinose M, Nakayama M, Ohashi T, Utsunomiya Y, Kobayashi M, Eto Y: Significance of screening for Fabry disease among male dialysis patients. Clin Exp Nephrol 9: 228–232, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Mayes J, Scheerer, Sifers RN, Donaldson ML: Differential assay for lysosomal alpha-galactosidases in human tissues and its application to Fabry's disease. Clin Chim Acta 112: 247–251, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Desnick RJ, Ioannou YA, Eng CM: Alpha-galactosidase A deficiency: Fabry disease. In: The Molecular and Metabolic Bases of Inherited Disease, 8th Ed., edited by Scriver CR, Sly WS, Childs B, Beaudet AL, New York, McGraw-Hill, 2001, pp 3733–3774
- 20.Sachdev B, Takenaka T, Teraguchi H, Tei C, Lee P, McKenna WJ, Elliott PM: Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation 105: 1407–1411, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H, Ponzone A, Desnick RJ: High incidence of later-onset Fabry disease revealed by newborn screening. Am J Hum Genet 79: 31–40, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosch M, Koch HG, Oliveira JP, Soares C, Bianco F, Breuning F, Rasmussen AK, Schaefer RM: Enzyme replacement therapy administered during hemodialysis in patients with Fabry disease. Kidney Int 66: 1279–1282, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Wilcox WR, Banikazemi M, Guffon N, Waldek S, Lee P, Linthorst GE, Desnick RJ, Germain DP, International Fabry Disease Study Group: Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet 75: 65–74, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sessa A, Meroni M, Battini G, Righetti M, Mignani R: Chronic renal failure, dialysis, and renal transplantation in Anderson-Fabry disease. Semin Nephrol 24: 532–536, 2004 [DOI] [PubMed] [Google Scholar]