Abstract
Background and objectives: Gene-based mutation screening is now available and has the potential to provide diagnostic confirmation or exclusion of autosomal dominant polycystic kidney disease. This study illustrates its utility and limitations in the clinical setting.
Design, setting, participants, & measurements: Using a molecular diagnostic service, genomic DNA of one affected individual from each study family was screened for pathologic PKD1 and PKD2 mutations. Bidirectional sequencing was performed to identify sequence variants in all exons and splice junctions of both genes and to confirm the specific mutations in other family members. In two multiplex families, microsatellite markers were genotyped at both PDK1 and PKD2 loci, and pair-wise and multipoint linkage analysis was performed.
Results: Three of five probands studied were referred for assessment of renal cystic disease without a family history of autosomal dominant polycystic kidney disease, and two others were younger at-risk members of families with autosomal dominant polycystic kidney disease being evaluated as living-related kidney donors. Gene-based mutation screening identified pathogenic mutations that provided confirmation or exclusion of disease in three probands, but in the other two, only unclassified variants were identified. In one proband in which mutation screening was indeterminate, DNA linkage studies provided strong evidence for disease exclusion.
Conclusions: Gene-based mutation screening or DNA linkage analysis should be considered in individuals in whom the diagnosis of autosomal dominant polycystic kidney disease is uncertain because of a lack of family history or equivocal imaging results and in younger at-risk individuals who are being evaluated as living-related kidney donors.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease worldwide affecting one in 500 to 1000 live births (1,2). It is characterized by focal and sporadic development and progressive enlargement of renal cysts, leading to ESRD in late middle age. Typically, only a few renal cysts are detected in most affected individuals before 30 yr of age; however, by the fifth decade of life, hundreds to thousands of renal cysts will be found in the majority of patients. Overall, it accounts for 5 to 8% of ESRD in developed countries (1,2). ADPKD is a systemic disorder associated with multiple extrarenal complications, such as cysts in nonrenal organs, valvular heart disease, colonic diverticula, inguinal hernias, and intracranial arterial aneurysms. It is genetically heterogeneous, with most cases arising from mutations in PKD1 (MIM 601313) and PKD2 (MIM 173910), located on chromosome 16p13.3 and 4q21–23, respectively (1–4). In a linkage-characterized European sample, PKD1 accounts for approximately 85% of the cases, whereas PKD2 accounts for most of the remainder (3,4). Although the clinical manifestations of the two gene types overlap completely, a strong locus effect is evident with more severe renal disease in PKD1 than PKD2 (median age at ESRD 54 versus 74, respectively) (5). In addition, both environmental and genetic modifiers have been implicated to account for the significant intrafamilial renal disease variability observed (6–8), and a mild allelic effect has been suggested for PKD1 but not PKD2 (7,8).
PKD1 is a large gene consisting of 46 exons with an open reading frame of approximately 13 kb and is predicted to encode a protein of 4302 amino acids. Its entire 5′ region up to exon 33 has been duplicated six times more proximally on chromosome 16p, and the presence of these highly homologous pseudogenes has made genetic analysis of PKD1 difficult (1,2). Recent availability of protocols for long-range and locus-specific amplification of PKD1 has enabled the complete mutation screening of this complex gene (9–11). By contrast, PKD2 is a single-copy gene that consists of 15 exons with an open reading frame of approximately 3 kb and is predicted to encode a protein of 968 amino acids (1,2). Marked allelic heterogeneity is evident for ADPKD, with more than 200 different PKD1 and more than 50 different PKD2 mutations reported to date (2,9–11). The majority of these mutations are unique and scattered throughout both genes. Most of them are also predicted to be protein truncating (as a result of frame-shift deletion/insertion, nonsense changes, or splice defects), although a significant number of unclassified variants (UCV; e.g., in-frame deletions, missense changes) have been reported (9–11). Despite sequencing of all of the coding regions and exon-intron splice junctions in both genes only 45 to 63% of pathogenic mutations could be identified in three large clinical series (9–11).
The diagnosis of ADPKD is generally straightforward when affected individuals present with a positive family history and enlarged kidneys with multiple cysts (12). Renal ultrasound is a sensitive method for this purpose, and age-dependant criteria based on cyst number have been derived for individuals who are born with 50% risk for PKD1 (13); however, because cyst formation is an age-dependent process, the false-negative rate of ultrasound-based diagnosis is higher in younger at-risk individuals or in those who are affected by PKD2, which is associated with later onset disease (14). Equivocal imaging results can be a source of diagnostic uncertainty in the clinic because the underlying gene type for most patients is unknown. In addition, renal cystic disease without a family history of ADPKD and evaluation of younger at-risk individuals as living-related kidney donors are clinical scenarios that often pose diagnostic challenges (12). Using a case series, we illustrate the utility and limitations of molecular diagnostics for ADPKD in the clinical setting.
Concise Methods
Clinical Assessment
The probands from the study families were assessed at the Hereditary Kidney Disease Clinic of Toronto General Hospital by Y.P. Upon obtaining informed consent, we reviewed the medical records of all of the available family members of the probands. We then screened all of the at-risk individuals for whom a diagnosis of ADPKD had not been made from each family with an abdominal ultrasound scan. We used the following criteria for the diagnosis of ADPKD: (1) The presence of at least two renal cysts (unilateral or bilateral) in an at-risk individual who was younger than 30 yr, (2) the presence of at least two cysts in each kidney in an at-risk individual aged 30 to 59 yr, or (3) the presence of at least four cysts in each kidney in an at-risk individual aged ≥60 yr (13). All study participants provided a blood sample for serum creatinine and DNA genetic analysis. Estimated GFR (eGFR) was calculated from serum creatinine using a formula that adjusted for age, gender, and body weight (15). The institutional review board of the University Health Network in Toronto approved all of the protocols used for this study.
Gene-Based Mutation Screening
Sequence analysis of PKD1 and PKD2 was performed in one clinically affected individual from each family using a commercial diagnostic service (Athena Diagnostics, Worcester, MA) (10,11). Briefly, genomic DNA was used as template for specific long-range PCR amplification of eight segments encompassing the entire PKD1 duplicated region. The long-range PCR products served as templates for 43 nested PCR; the unique region of PKD1 and the entire PKD2 were amplified from genomic DNA in 28 additional PCR. All 71 PCR products were bidirectionally sequenced, including the coding regions and exon-intron splice junctions of both genes (11).
DNA Linkage and Haplotype Analysis
We genotyped all of the available study participants from TOR12 and TOR163 with five simple-sequence repeat (SSR) markers each at the PKD1 and PKD2 loci (16). The locations of these markers relative to the PKD1 locus are shown as follows (the number between markers denotes intermarker distance in cM): HBAP1-2.0-PKD1-0.1-CW4-0.1-SM6-0.6-D16S2618-2.0-D16S423. The locations of these markers relative to the PKD2 locus are shown as follows (the number between markers denotes intermarker distance in cM): D4S231-2.0-D5S1534-2.3-SPP1-0.2-PKD2-0.5-D4S1563-2.0-D4S423. Genotyping was performed by 32P α-dCTP labeling of the PCR products and analyzed by PAGE (16). All genotypes were performed and scored independently by K.R.W. without any knowledge of the clinical status of the study participants. Haplotypes were constructed by hand and using the program GENEHUNTER (v2.1 r5) (17). Two-point and multipoint linkage with “affected-only analysis” was performed using the M-LINK program of the FASTLINK package (version 4.0) (18,19) and GENEHUNTER (v2.1 r5) (17), respectively. An autosomal dominant model with a disease allele frequency of 0.001 and a phenocopy rate of 0.001 was assumed. Marker allele frequencies were obtained from married-in individuals and reconstruction of the genotypes of the founders.
Predicting Deleterious Missense Mutations by PolyPhen
We used the software PolyPhen (http://genetics.bwh.harvard.edu/pph/) to evaluate the functional significance of a number of unclassified missense variants that alter a single amino acid residue (20). Upon entry of the protein identification and the wild-type and mutant amino acid variants, PolyPhen performed a comprehensive search to identify all of the homologous protein sequences. On the basis of alignment of these homologous protein sequences, PolyPhen computes profile scores for both allelic variants. Profile scores are logarithmic ratios of the likelihood of a given amino acid occurring at a particular site to the likelihood of this amino acid occurring at any site (background frequency). A variant is predicted to be damaging when the absolute difference between the profile scores of two amino acid variants is >1.7 (20).
Results
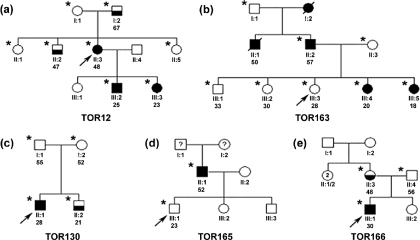
Three of five probands were referred to us for assessment of renal cystic disease without a family history of ADPKD, and the two others were younger members who were at risk for ADPKD and being evaluated as living-related kidney donors. The pedigree structures and clinical findings of these probands and their family members are shown in Figure 1 and Table 1, respectively. The results of their molecular genetic analysis are shown in Table 2.
Figure 1.
Study families. Probands are denoted by the slanted arrow, and individuals who underwent clinical and genetic testing are denoted by *. Filled symbols represent clinically affected individuals, half-filled symbols represent possibly affected individuals, and unfilled symbols represent unaffected individuals. Deceased individuals are denoted by dashed symbols. Individual identification and age are indicated below each symbol.
Table 1.
Clinical findings of study participants
| Family | Individual Identification | Age (yr)a | Ultrasound Findings | Renal Disease Severity, eGFR (ml/min per 1.73 m2) or age at ESRD |
|---|---|---|---|---|
| TOR 12 | I:1 | 70 | Unaffected | |
| I:2 | 67 | Three right renal cysts, one left renal cyst, and four liver cysts; right kidney 13.4 cm and left kidney 11.7 cm in length | 47 | |
| II:1 | 51 | Unaffected | 82 | |
| II:2 | 47 | Single renal cyst | 87 | |
| II:3 | 48 | Affectedb; right kidney 12.1 cm and left kidney 14 cm in length; multiple renal cysts bilaterally | 50 | |
| II:5 | 46 | Unaffected | 86 | |
| III:2 | 25 | Affectedb | N/A | |
| III:3 | 23 | Affectedb | 97 | |
| TOR130 | I:1 | 55 | Unaffected | |
| I:2 | 52 | Unaffected | ||
| II:1 | 28 | Affectedc | 105 | |
| II:2 | 21 | One renal cyst | 102 | |
| TOR163 | I:1 | Unaffected | ||
| I:2 | Affectedb | ESRD at 70 yr | ||
| II:1 | Single kidney, affectedb | ESRD at 48 yr | ||
| II:2 | 57 | Single kidney, affectedb | ESRD at 50 yr | |
| II:3 | 57 | Unaffected | ||
| III:1 | 33 | Unaffected | 105 | |
| III:2 | 30 | Unaffected | ||
| III:3 | 28 | Unaffected | 90 | |
| III:4 | 20 | Affectedb | 88 | |
| III:5 | 18 | Affectedb | 93 | |
| TOR165 | II:1 | 52 | Affectedb; both kidneys >20 cm in length | ESRD at 48 yr |
| III:1 | 23 | Normal | ||
| TOR166 | II:3 | 48 | Normal right kidney; multiple left renal cysts, left enlarged with multiple cysts | 89 |
| II:4 | 56 | Normal | ||
| III:1 | 30 | Affectedb | 108 |
Age at renal ultrasound and laboratory testing.
Renal enlargement with four or more cysts in each kidney.
Two or more cysts in each kidney.
Table 2.
Summary of molecular genetic analysis
| Family | Individual Identification | Exon Fragment | Nucleotide Changea | Codon Changeb | Detection Method |
|---|---|---|---|---|---|
| TOR12 | II:3 | EX15 | PKD1: 6795C→A | Y2265X | Direct sequencing |
| III:2, III:3 | EX15 | PKD1: 6795C→A | Y2265X | Direct sequencing | |
| I:2, II:2 | EX15 | Normal sequence | NA | Direct sequencing | |
| TOR 130 | II:1 | EX11 | PKD2: 2159ins1bp | Frameshift 720→724X | Direct sequencing |
| II:2 | EX11 | Normal | Direct sequencing | ||
| TOR 163 | II:2 | EX11 | PKD1: 2216A→G c,d | Q739R | Direct sequencing |
| EX23 | PKD1: 8289del3bpc | del2763M; reading frame maintained | |||
| III:3 | EX11 | Normal | Direct sequencing | ||
| EX23 | Normal | ||||
| TOR 165 | II:3 | EX1 | PKD2: 567G→A | W189X | Direct sequencing |
| III:1 | EX1 | Normal | Direct sequencing | ||
| TOR 166 | II:3 | EX15 | PKD1: 6062C→T c,e | L2021P | Direct sequencing |
| III:1 | EX15 | PKD1: 6062C→T | L2021P | Direct sequencing |
Nucleotide numbering starts with the first in-frame ATG codon of the mRNA sequence of PKD1 or PKD2.
Codon numbering starts with the first in-frame methionine of polycystin 1 or 2.
Gene variant of uncertain significance.
Predicted to be benign by PolyPhen.
Predicted to be possibly damaging by PolyPhen.
Probands without a Family History of ADPKD
TOR12.
The proband (II:3) was referred to our center for liver transplantation because of severe polycystic liver disease. At 46 yr of age, she had a massive polycystic liver that extended into her lower abdomen with little normal parenchyma, mildly enlarged kidneys with numerous cysts, and an eGFR of 50 ml/min. She had no family history of ADPKD. Ultrasound screening showed that her mother (I:1) was unaffected, but her 67-yr-old father (I:2) had three right renal cysts, one left renal cyst, four liver cysts, and an eGFR of 47 ml/min. In addition, her 47-yr-old brother (II:2) had a solitary right renal cyst, and two of her children (III:2 and III:3) had multiple renal cysts bilaterally at 25 and 23 yr of age. Haplotype and linkage analysis at both PKD1 and PKD2 loci, assuming that II:3, III:2, and III:3 were affected, were indeterminate (data not shown). Direct sequencing, however, identified a pathogenic PKD1 mutation (C6795A; Y2265X) in II:3, III:2, and III:3 but not in I:1, I:2, and II:2.
The possible diagnoses to be considered in this case include de novo ADPKD in the proband with simple renal cyst(s) in her father and brother versus PKD2 with discordant renal disease severity between family members. DNA linkage analysis was performed initially and was uninformative. Gene-based mutation screening, however, provided unambiguous evidence for de novo PKD1 in the proband.
TOR130.
The proband (II:1) was a 28-yr-old man who was referred for evaluation of renal cystic disease without a family history of ADPKD. He was found to have four cysts in each kidney on ultrasound scan when assessed for nonspecific abdominal pain. Subsequent ultrasound screening showed no renal cysts in either of his parents, who were >50 yr of age, but a single 1.4-cm renal cortical cyst in his 21-yr-old brother (II:2). Gene-based screening revealed a pathogenic mutation in PKD2 (2159ins1bp; FS720→724X) in the proband but not in his brother and parents.
The possible diagnoses to be considered in this case include de novo ADPKD in the proband versus de novo ADPKD with germline mosaicism in one of the proband's parents. In the latter case, the proband's younger brother would be expected to be affected because the presence of even a single renal cyst is highly unusual in his age group (12). Gene-based mutation screening was informative and provided strong evidence suggesting that the proband had de novo PKD2 and that his brother had a simple renal cyst.
TOR166.
The proband (III:1) was a 30-yr-old man who was referred for evaluation of enlarged kidneys with multiple cortical cysts bilaterally on ultrasound scan when he was investigated for abdominal pain. He had no family history of ADPKD. Ultrasound screening showed no renal abnormality in his father, but his 48-yr-old mother (II:3) was found to have numerous left renal cysts and a normal right kidney. Although the renal ultrasound findings of the proband are highly suggestive of ADPKD, this case is confounded by the atypical renal ultrasound findings in his mother. Gene-based screening was therefore performed in the proband but failed to identify a definitively pathogenic mutation. However, one UCV (L2021P) in PKD1 was identified in both the proband and his mother and is predicted as possibly damaging by PolyPhen (i.e., the absolute difference between the profile scores of two amino acid variants for L2021P was 1.837). The clinical significance of this UCV is presently unclear.
Evaluation of Living-Related Kidney Donors
TOR163.
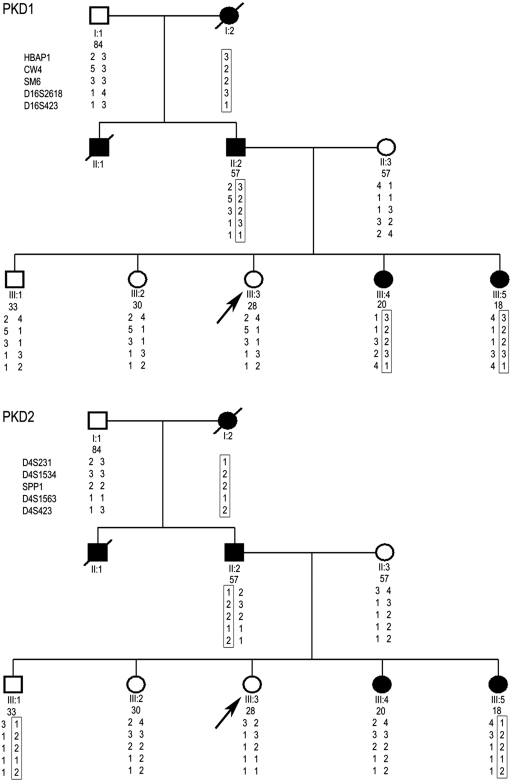
The proband (III:3) was a 28-yr-old woman who was referred for evaluation as a living-related kidney donor to her father. She had a strong family history of ADPKD that traced back to her paternal grandmother (I:2), who died of ESRD at 70 yr of age. Both her father (II:2) and paternal uncle (II:1), who were born with a solitary kidney, were affected and developed ESRD by 50 yr of age. Her two youngest sisters (III:4 and III:5) were also affected with normal renal function. Ultrasound screening of the proband and her two older siblings was normal. A negative scan at her age, however, cannot completely rule out ADPKD, especially for the milder PKD2. Direct sequencing of PKD1 and PKD2 was therefore performed on her father, which identified two PKD1 UCV (Q739R and del2763M) but no definitively pathogenic mutation. The missense variant was predicted to be benign by PolyPhen (i.e., the absolute difference between the profile scores of two amino acid variants for Q739R was 0.155), whereas the clinical significance of the in-frame deletion is unknown. Given the indeterminate results of the gene-based screening, we genotyped the family with SSR markers at both PKD1 and PKD2 loci. Pair-wise and multipoint “affected-only” linkage analysis of TOR163 yielded exclusionary logarithm of odds scores (less than −2) at the PKD2 locus and maximal multipoint logarithm of odds score of 1.1 at the PKD1 locus (data not shown). Haplotype inspection showed that only the PKD1 haplotype (3-2-2-3-1) but no single PKD2 haplotype co-segregated with all of the affected individuals (Figure 2). These data indicate that TOR163 is PKD1 linked, and the absence of the putative PKD1 disease haplotype (3-2-2-3-1) in our proband provides strong evidence that she is not affected by ADPKD. She was therefore cleared as a living-related donor.
Figure 2.
Haplotype analysis of TOR163. Only the PKD1 haplotype 3-2-2-3-1 (top) but no single PKD2 haplotype (bottom) co-segregated with the affected members of TOR163. These data are consistent with this family's being PKD1 linked. The finding that the proband (III:3) did not carry the putative PKD1 disease haplotype provided further reassurance that she was unaffected.
TOR165.
The proband (III:1) was a 23-yr-old man who was referred for evaluation as a living-related kidney donor to his father. His father (II:1) had grossly enlarged polycystic kidneys and developed ESRD at 48 yr of age. Because his father was adopted, it was not possible to ascertain whether there was a family history of ADPKD. Although the proband had a normal renal ultrasound scan, it was considered as insufficient for disease exclusion because of his young age. Direct sequencing of both PKD1 and PKD2 was performed in the proband's father and, surprisingly, revealed a pathogenic PKD2 mutation (W189X). Because the proband did not carry this mutation, he was cleared as a living-related kidney donor.
Discussion
Renal ultrasonography is commonly used for presymptomatic screening of at-risk individuals with a positive family history of ADPKD, whose pretest probability of disease is 50% at birth. Highly sensitive and specific diagnostic criteria based on renal cyst number have been derived for this purpose (12–14). A family history, however, may be absent in 10 to 20% of new patients in whom the diagnosis of ADPKD is first suspected from imaging studies performed to evaluate otherwise unexplained hematuria, abdominal mass, flank pain, or renal insufficiency (1,2,12). In these cases, the finding of ADPKD can be due to a de novo mutation or ascertainment of a small family with PKD2 and mild renal disease, which is often underdiagnosed (12,21). In the latter instance, ultrasound screening may reveal multiple renal cysts in one of the parents. In the case of when one or both parents are deceased, review of autopsy reports (if available) may also be helpful. Without a definite family history, the pretest probability of ADPKD can be assumed to be only one in 500 to 1000 (population risk), and the ultrasound-based diagnostic criteria established for individuals with a positive family history are not likely valid. Gene-based molecular diagnostics are useful in this clinical scenario; however, a definitely pathogenic mutation only be identified may in up to two thirds of cases. As seen in TOR166, the interpretation of potentially pathogenic UCV remains uncertain as a result of a lack of a valid assay to determine their functional consequences (10,11).
Individuals who are born with 50% risk for ADPKD often are evaluated as potential living-related kidney donors to their affected relatives. The issue in these cases becomes one of disease exclusion, which can be difficult in younger individuals using an imaging-based approach (12–14). Gene-based mutation screening plays an increasingly important role in the evaluation of these individuals, such as the proband from TOR165. In this particular family, we were surprised to find a pathogenic PKD2 mutation in the proband's father (II:1), who had severe renal disease typical of PKD1. This finding illustrates that renal disease severity may not be a reliable indicator of the underlying gene type. As seen in TOR163 and TOR166, gene-based mutation screening may not be informative in 47 to 58% of cases (9–11). In such instances, DNA linkage-based diagnostics using microsatellite markers and single-nucleotide polymorphisms should be considered, as in TOR163, when multiple affected individuals are available for evaluation.
Molecular diagnostics should be considered in individuals in whom the diagnosis of ADPKD is uncertain because of a lack of family history or equivocal imaging results and in younger at-risk individuals who are being evaluated as living-related kidney donors. Although gene-based mutation screening is simple and requires only a blood sample from the test individual, definitive mutations are identified in only 47 to 58% of cases (9–11). In pedigrees with multiple affected members, DNA linkage analysis may provide useful information and should be considered.
Disclosures
None.
Acknowledgments
This work was supported by a grant from the Kidney Foundation of Canada (to Y.P.).
We are indebted to all of the participating members of the families with ADPKD.
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Molecular Diagnostics of ADPKD Coming of Age,” on pages 1–2.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Igarashi P, Somlo S: Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol 13: 2384–2398, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Ong A, Harris P: Molecular pathogenesis of ADPKD: The polycystin complex gets complex. Kidney Int 67: 1234–1247, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ravine D, Walker RG, Gibson RN, Forrest SM, Richards RI, Friend K, Sheffield LJ, Kincaid-Smith P, Danks DM: Phenotype and genotype heterogeneity in autosomal dominant polycystic kidney disease. Lancet 340: 1330–1333, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Peters DJ, Sandkuijl LA: Genetic heterogeneity of polycystic kidney disease in Europe. Contrib Nephrol 97: 128–139, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Hateboer N, v Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D: Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353: 103–107, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Paterson AD, Magistroni R, He N, Wang KW, Johnson A, Fain P, Dicks E, Parfrey P, St. George-Hyslop P, Pei Y: Progressive loss of renal function is a heritable trait in type 1 autosomal dominant polycystic kidney disease. J Am Soc Nephrol 16: 755–762, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Rossetti S, Burton S, Stremecki, Pond GR, San Millán JL, Zerres K, Barratt TM, Ozen S, Torres VE, Bergstralh EJ, Winearls CG, Harris PC: The position of the polycystic kidney disease 1 gene mutation correlates with the severity of renal disease. J Am Soc Nephrol 13: 1230–1237, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Magistroni R, He N, Wang KR, Andrew R, Johnson A, Gabow P, Dicks E, Parfrey P, Torra R, San-Millan J, Coto E, v Dijk M, Breuning M, Peters D, Bogdanova N, Ligabue G, Albertazzi A, Hateboer N, Demetriou K, Pierides A, Deltas C, St. George-Hyslop P, Ravine D, Pei Y: Genotype-renal function correlation in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol 14: 1164–1174, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Rossetti S, Chauveau D, Walker D, Saggar-Malik A, Winearls C, Torres V, Harris P: A complete mutation screen of the ADPKD genes by DHPLC. Kidney Int 61: 1588–1599, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Rossetti S, Consugar M, Chapman A, Torres V, Guay-Woodford L, Grantham J, Bennett W, Meyers C, Walker D, Bae KT, Qin Zhang, Thompson P, Miller P, Harris P, the CRISP Consortium: Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 18: 2143–2160, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Gonzalez M, Jones J, Allen S, Palatucci C, Batish S, Seltzer W, Lan Z, Allen E, Qian F, Lens X, Pei Y, Germino G, Watnick T: Evaluating the clinical utility of a molecular genetic test for polycystic kidney disease. Mol Genet Metab 92: 160–167, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pei Y: Diagnostic approach in autosomal dominant poly-cystic kidney disease. Clin J Am Soc Nephrol 1: 1108–1114, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Ravine D, Gibson R, Walker R, Scheffield L, Kincaid-Smith P, Danks D: Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343: 824–827, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Pei Y, Obaji J, Pinto R, Paterson A, Magistroni R, Dicks E, Parfrey P, Coto E, Torra R, San Millan J, Breuning M, St. George-Hyslop P, Peters D, Ravine D: Unified ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease [Abstract]. J Am Soc Nephrol 15: 657A, 2004 [Google Scholar]
- 15.Cockcroft D, Gault H: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 16.Pei Y, Paterson AD, Wang KR, He N, Hefferton D, Watnick T, Germino G, Parfrey P, Somlo S, St. George-Hyslop P: Bilineal disease and trans-heterozygotes in autosomal dominant polycystic kidney disease. Am J Hum Genet 68: 355–363, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruglyak L, Daly M, Reeve-Daly M, Lander E: Parametric and nonparametric linkage analysis: A unified multipoint approach. Am J Hum Genet 58: 1347–1363, 1996 [PMC free article] [PubMed] [Google Scholar]
- 18.Lathrop M, Lalouel J-M: Easy calculations of LOD scores and genetic risks on small computers. Am J Hum Genet 36: 460–465, 1984 [PMC free article] [PubMed] [Google Scholar]
- 19.Schäffer A, Gupta S, Shriram K, Cottingham RW Jr: Avoiding recomputation in linkage analysis. Hum Hered 44: 225–237, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Sunyaev S, Ramensky V, Koch I, Lathe W, Kondrashov A, Bork P: Prediction of deleterious human alleles. Hum Mol Genet 10: 591–597, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Davies F, Coles G, Harper P, Williams A, Evans C, Cochlin D: Polycystic kidney disease re-evaluated: A population-based study. Q J Med 79: 477–485, 1991 [PubMed] [Google Scholar]