Abstract
Background and objectives: Cystinosis is an autosomal recessive disease characterized by the intralysosomal accumulation of cystine, as a result of a defect in cystine transport across the lysosomal membrane. Three clinical forms have been described on the basis of severity of symptoms and age of onset: infantile cystinosis, characterized by renal proximal tubulopathy and progression to end-stage renal disease before 12 yr of age; juvenile form, with a markedly slower rate of progression; and adult form, with only ocular abnormalities.
Design, setting, participants, & measurements: Fourteen patients in nine unrelated families with noninfantile cystinosis were studied. Information about clinical outcome, biochemical data, renal histopathologic data, and genotyping was collected.
Results: Eight patients had Fanconi syndrome. Proteinuria was present in all patients. Serum creatinine at last follow-up, without specific treatment, ranged between 69 and 450 μmol/L, at an age of 12 to 56 yr. Four patients reached end-stage renal disease by 12 to 28 yr. Renal biopsies, available in four cases, disclosed focal segmental glomerulosclerosis in three and crystals in three. Genetic screening showed that patients were compound heterozygous for mutations in the CTNS gene in four families and homozygous in two families. Patients had at least one “mild” mutation. A single heterozygous mutation was identified in one family and none in two families (only 72% mutations found).
Conclusion: Renal involvement is heterogeneous in patients with noninfantile cystinosis even within families, and renal disease should be assessed even in families of patients with seemingly isolated ocular forms.
Cystinosis is an autosomal recessive lysosomal storage disease caused by a defect in the carrier-mediated system that normally transports cystine out of lysosomes. This defect results in intralysosomal cystine accumulation, crystal formation, and progressive organ damage (1).
Infantile cystinosis, also known as nephropathic infantile cystinosis, is the most common form. Affected children develop renal proximal tubulopathy (or Fanconi syndrome) at 6 to 12 mo of age. In the absence of treatment, renal failure occurs, with progression to ESRD at approximately 9 yr of age (2). Renal histopathologic changes in infantile nephropathic cystinosis include severe lesions of proximal tubules; typical alterations to the glomerular podocytes, which become multinucleated giant cells; and the presence of cystine crystals, mostly in interstitial cells and podocytes (3). The proximal tubule is the first clinical target of the disease, but cystine crystals are rarely found in the tubular cells of patients with cystinosis. Cystine crystal deposition in the cornea leads to photophobia. Continuous widespread cystine accumulation eventually leads to rickets and retinal, endocrinologic (hypothyroidism and impaired glucose tolerance), hepatic, gastrointestinal, muscular, and neurologic abnormalities. Cysteamine is the only cystine-depleting drug available; it helps to eliminate cystine from lysosomes and slows renal deterioration and other organ dysfunctions (4). Cysteamine hydrochloride eye drops dissolve corneal crystals and improve ocular discomfort (5). CTNS, the gene that is mutated in cystinosis, maps to chromosome 17p13 and encodes a lysosomal membrane protein—cystinosin—that has been shown to act as a cystine transporter (6,7). CTNS mutations have been detected in almost all children with infantile nephropathic cystinosis. More than 50 different CTNS mutations have been described, but approximately 75% of those of European descent carry a homozygous approximately 57-kb deletion encompassing CTNS exons 1 through 10 (6,8–10).
Forms with a later onset have also been identified, accounting for approximately 5% of all cases of cystinosis (1). These forms have been divided in two basic phenotypes: Nephropathic and nonnephropathic (11). Nephropathic forms can be further classified, on the basis of age at presentation, into child-, adolescent-, and adult-onset forms. These noninfantile forms are associated with glomerular impairment but not necessarily Fanconi syndrome. Patients may progress to ESRD. In contrast, renal disease does not develop in the nonnephropathic (or ocular) form of cystinosis, in which deposits are limited to the cornea and conjunctiva. Point mutations in the CTNS gene that do not disrupt the open reading frame of cystinosin are more commonly associated with the late-onset phenotype and generally affect the intertransmembrane loops or the N-terminal region (10,12–15). Affected individuals are usually homozygous for “mild” mutations of this type or compound heterozygous for a mild mutation and a “severe” mutation (i.e., a mutation that completely prevents cystine transport). We studied nine unrelated families with late-onset nephropathic cystinosis, collecting information about clinical outcome, biochemical data, renal histopathologic data, and genotyping.
Concise Methods
All patients included in this study had late-onset nephropathic cystinosis; patients with infantile cystinosis were excluded. None of the patients included presented ESRD before the age of 12 yr. We included 11 patients who had late-onset nephropathic cystinosis and were referred for DNA studies to the Department of Genetics at Necker-Enfants Malades Hospital in Paris between 1999 and 2006. Clinical data were collected with the help of the physicians in charge of these patients in France, Belgium, and Spain. Two patients (8a and 9a) were directly referred to the Adult Nephrology Department of this hospital for diagnostic confirmation and medical care. Family investigation revealed one additional affected individual (8b; Table 1). Thus, this study reflects the experience of the Department of Genetics and of the Adult Nephrology Department at Necker-Enfants Malades Hospital in Paris.
Table 1.
Clinical presentation and outcome of patientsa
| Family | Patient | Gender | Age at Onset of Symptoms (yr) | Age at Diagnosis (yr) | Proximal Tubulopathy | SCr at Diagnosis (μmol/L) | Proteinuria | SCr at Last Follow-up (μmol/L) | Granulocyte Cystine Content (nmol half-cystine/mg protein) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | a | F | 7 | 10 | + | 63 | + | 160 (16 yr) | 0.76 |
| 2 | a | F | 5 | 13 | + | 88 | + | 104 (15 yr) | |
| b | F | 4 | 16 | + | 88 | + | 202 (19 yr) | ||
| 3 | a | M | 7 | 7 | + | N | + | 168 (12 yr) | 0.94 |
| 4 | a | F | + | Unknown | + | ESRD (12 yr), KT (17 yr) | 4.80 | ||
| 5 | a | M | 7 | 7 | + | 73 | + | 400 (21 yr) | 1.47 |
| 6 | a | F | 4 | 6 | + | Unknown | + | 450 (35 yr) | 0.91 (1978), 1.96 (1988) |
| 7 | a | M | 14 | 14 | + | Unknown | + | 240 (48 yr) | Increasedb |
| b | M | 13 | 35 | − | Unknown | + | ESRD (27 yr), KT (29 yr) | 1.10 | |
| 8 | a | F | 12 | 12 | − | Unknown | + | 69 (29 yr) | 2.50 |
| b | M | − | Unknown | + | ESRD (21 yr) | Increasedb | |||
| 9 | a | F | 30 | 50 | − | N | − | 91 (56 yr) | 2.00 |
| b | M | 19 | 26 | − | N | + | ESRD (28 yr), KT (29 yr) | 1.02 | |
| c | F | 23 | 24 | − | N | + | N (35 yr) | 1.08 |
KT, kidney transplantation; N, normal; SCr, serum creatinine concentration.
“Increased” according to medical charts; no values were transmitted to us. However family history, clinical presentation, and genetic studies were typical.
Estimated GFR was calculated in adult patients, using the abbreviated Modification of Diet in Renal Disease (MDRD) formula (16,17). Renal failure was defined by GFR <60 ml/min per 1.73 m2. Means ± SD are presented.
Diagnosis
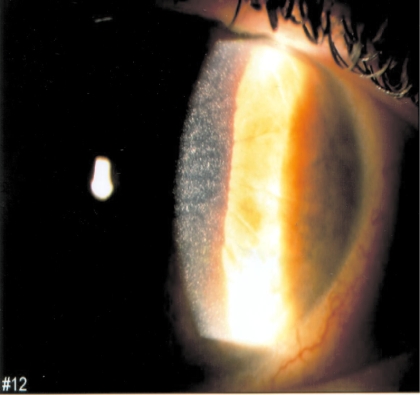
All patients had typical cystine crystal deposits in the cornea, as observed on slit-lamp examination (Figure 1). All had high granulocyte-free cystine content, determined by cystine-binding protein assay, as previously reported (18), expressed as nanomoles of half-cystine per milligram of protein (normal <0.15). The diagnosis of renal proximal tubulopathy (or renal Fanconi syndrome), often incomplete, was based on the usual criteria: Hyperaminoaciduria, glycosuria, tubular proteinuria with the presence of β2-microglobulin, renal loss of potassium, decreased reabsorption of phosphate and hypophosphoremia, reduction in bicarbonate reabsorption producing plasma acidosis, and/or hypouricemia.
Figure 1.
Cystine crystals in the cornea, as seen on slit-lamp examination (patient 9a; courtesy of Dr. Salagnac).
Renal Biopsies
Biopsy specimens for light microscopy were fixed in Bouin's solution, embedded in paraffin, and sectioned at 2 μm. Sections were stained with Masson trichrome, hematoxylin and eosin, periodic acid-Schiff, and silver methenamine. For the detection of cystine crystals, unstained sections or sections briefly stained with methylene blue in absolute alcohol were examined by polarization or phase contrast microscopy. Immunofluorescence studies were carried out as described previously (19). Specimens for electron microscopy were fixed in glutaraldehyde and embedded in Epon resin.
CTNS Mutations
Blood samples for DNA studies were obtained after informed consent had been given by the patients or their parents. Mutations were detected as described previously (6,8,12). In brief, we first looked for the 57-kb deletion. We then screened the 10 coding exons of the CTNS gene and the promoter region encompassing nucleotides −348 to +1 (20) for mutations, by direct sequencing. To determine whether a mutation is also present in a control collective and therefore more likely be a rare polymorphism than a deleterious mutation, we collected a control population that consisted of a panel of 100 locally recruited healthy individuals. These control subjects were analyzed for the presence of all mutations identified in this study with the same technique.
Results
Clinical Presentation and Outcome
We included 14 patients in nine unrelated families in this study; all had late-onset nephropathic cystinosis (i.e., infantile cystinosis cases were excluded). The first symptoms were ocular discomfort and photophobia as a result of corneal cystine deposits (Figure 1) in 10 patients; however, four patients (7a, 7b, 8a, and 8c) did not complain of ocular symptoms, and corneal deposits were identified later in life, by systematic screening, after cystinosis had been diagnosed based on family investigation. Cystine crystals were also found in the conjunctiva in patient 1a (Table 1). None of the patients had retinal crystals or impaired visual acuity. Cysteamine eye drops were prescribed to the 10 symptomatic patients.
The clinical data for the 14 patients are summarized in Table 1. Age at onset of symptoms ranged from 3 to 50 yr, and age at diagnosis ranged from 6 to 50 yr (mean 18.3 yr ± 13.3). In two families (7 and 9), some patients received the diagnosis late in life, after the age of 30 yr. In one of these patients (7b), the diagnosis was established only after kidney transplantation. Eight patients (from seven families) presented renal Fanconi syndrome, which was often incomplete. In family 7, patient 7a had Fanconi syndrome, whereas patient 7b did not. β2-Microglobinuria was determined and found to be high, at 11,706 μg/L (normal 0 to 350 μg/L), in patient 5a, and normal in patients 8a (130 μg/L) and 9a (30 μg/L). Patients without Fanconi syndrome received the diagnosis significantly later than those with Fanconi syndrome (mean 10.4 ± 3.9 versus 29.4 ± 14.1 yr; P < 0.01). All patients but one (9a) had proteinuria (approximately 1 g/d). At last follow-up, 12 patients displayed renal failure, which led to ESRD in four (patients 4a, 7b, 8b, and 9b) at the ages of 12, 27, 21, and 28 yr, respectively. GFR at last follow-up in patients 2b, 5a, 6a, 7a, 8a, and 9a was calculated and ranged from 10 to 93 ml/min per 1.73 m2. These patients were aged between 19 and 56 yr.
Patients 2a and 2b presented with growth retardation, blond hair, a fair complexion, and poor tanning on exposure to the sun (like many patients with infantile nephropathic cystinosis). Hair color and skin tanning were normal in the 12 other patients. Patients 2a and 2b were sisters and had two brothers and one sister, who died during infancy with rickets. Hypothyroidism was observed in patient 3a. None of the other patients showed hypothyroidism, impaired glucose tolerance, or any other extrarenal involvement. Patient 4 was included in this series because she developed ESRD only at 12 yr of age, unlike patients with infantile form, who reach ESRD earlier. Hair color and skin tanning were normal, and she had no rickets. Seven patients were treated by oral cysteamine. In patient 8a, oral cysteamine treatment was initiated at the age of 29 yr, in association with lisinopril, according to the results of a renal biopsy (see the Renal Biopsy Data section).
Of interest, renal disease progression was highly heterogeneous within families. In family 7, one patient (without Fanconi syndrome) presented ESRD at the age of 27 yr, whereas his brother (with Fanconi syndrome) showed severe renal failure (GFR of 27 ml/min per 1.73 m2) significantly later in life (48 yr of age). In family 8, one patient presented ESRD at the age of 21 yr (8b), whereas his sister (8a) had normal renal function at the age of 29 yr (GFR 93 ml/min per 1.73 m2). The situation was similar in family 9: Patient 9b presented ESRD at the age of 28 yr, whereas his affected older sister had no proteinuria and a GFR of 59 ml/min per 1.73 m2 at the age of 56 yr.
Granulocyte Cystine Content
Granulocyte cystine content ranged from 0.76 to 4.80 nmol half-cystine/mg protein (mean 1.63 ± 1.34) in all 12 patients for whom data were available (normal <0.3). It is interesting that patient 4 had the highest levels of cystine in the granulocytes and also displayed the most rapid progression to ESRD. In patient 8a, granulocyte cystine content decreased from 2.5 to 0.12 nmol half-cystine/mg protein after the introduction of oral cysteamine therapy (daily dosage 1200 mg).
Renal Biopsy Data
Renal biopsies were available in four cases. In patient 1a, light microscopy of a renal biopsy specimen obtained at 10 yr of age disclosed intact glomeruli, dilated tubules, and intratubular crystals. Immunofluorescence study for IgG, IgA, IgM, C3, and C1q was negative.
In patient 7a, renal biopsy carried out at the age of 33 yr revealed glomerular lesions, including FSGS and cystine crystals within endothelial cells and podocytes. Tubules were atrophic with focal loss of the brush border. Cystine crystals appeared as empty rectangular structures by light microscopy. Their presence was confirmed by electron microscopy.
In patient 7b, renal biopsy disclosed tubular lesions, with focal disappearance of the brush border, and cytoplasmic vacuolization. In most glomerular structures, a diffuse increase in the mesangial matrix without cell proliferation was associated with focal lesions. Small nonspecific IgA glomerular deposits were observed by immunofluorescence, initially leading to misdiagnosis. By electron microscopy, cystine crystals were observed within interstitial and tubular cells.
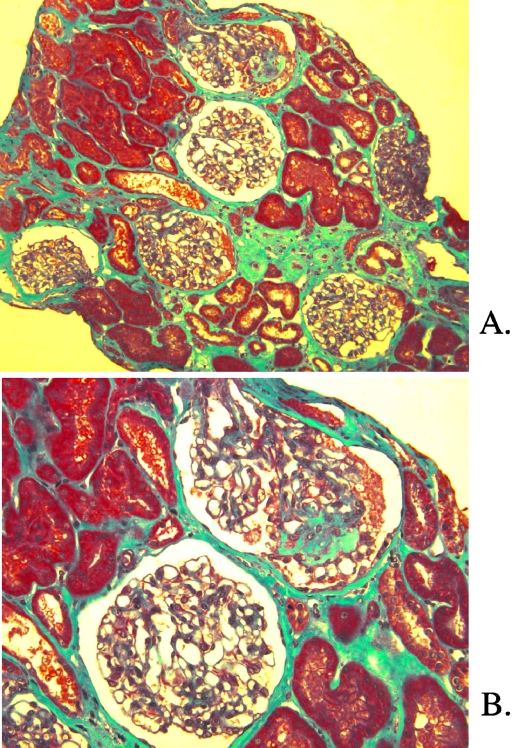
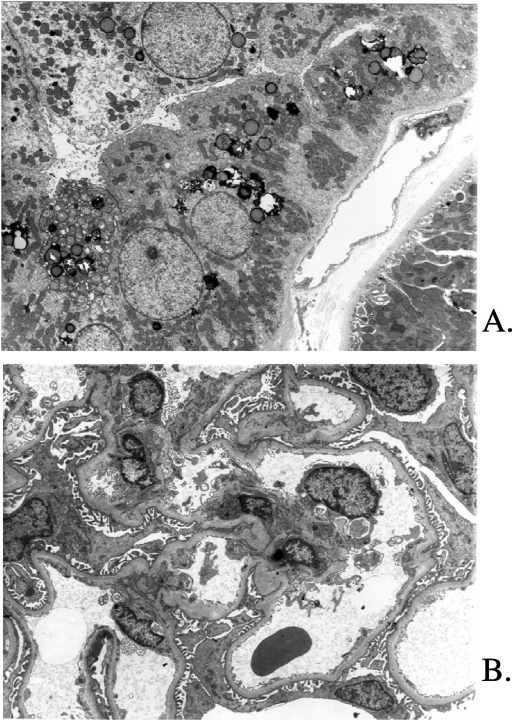
In patient 8a, the first renal biopsy, performed at 15 yr of age, was normal. A second biopsy was performed at 29 yr of age and showed focal tubulointerstitial lesions (Figure 2A), including mild interstitial fibrosis, tubular atrophy with focal thickening of the basement membrane, and focal arteriolar thickening. Six of 19 glomeruli displayed global sclerosis. One glomerulus showed FSGS (Figure 2B). No crystals were identified by polarization. Electron microscopy showed an accumulation of phagolysosomes and lysosomal alterations in distal tubules, without cystine crystals (Figure 3A). Cytoplasmic vacuolization was observed in the proximal tubules. Foot processes were effaced along the glomerular basement membrane (Figure 3B). No multinucleated giant podocytes were found in any of the biopsy samples.
Figure 2.
Patient 8a. (A) Light microscopy. Trichrome light green. Focal tubulointerstitial lesions with mild interstitial fibrosis, tubular atrophy, and focal arteriolar thickening. (B) Light microscopy. Trichrome light green. FSGS on one glomerulus. Magnifications: ×100 in A; ×200 in B.
Figure 3.
Patient 8a. (A) Electron microscopy. Alterations in distal tubules without cystine crystals. Dilation and atrophy of distal tubules. (B) Electron microscopy. Effacement of foot processes along the glomerular basement membrane. Magnification, ×5000.
Genotyping
Genetic screening (Table 2) showed that patients were compound heterozygous for two mutations in the CTNS gene in four families (1, 6, 7, and 9) and homozygous for one mutation in two families (4 and 8). Only one heterozygous mutation was identified in family 2, and no mutations were detected in families 3 and 5. Most patients had at least one “mild” mutation. The newly identified p.T334N missense mutation is located in the sixth intertransmembrane loop. It results in the substitution of asparagine for threonine and has never been reported before. This mutation is thought to be a mild mutation, because it affects an amino acid in a transmembrane loop, like most of the identified mild mutations (21). No mutation was found in the first two noncoding exons or in the promoter. On the whole, 72% mutations were found (13 mutations detected among 18 alleles tested). No patient carried the approximately 57-kb deletion in the homozygous state.
Table 2.
Mutation analysis in patients with juvenile and adult cystinosisa
| Patient | Mutation 1
|
Mutation 2
|
||||||
|---|---|---|---|---|---|---|---|---|
| Position | Nucleotide Changeb | Amino Acid Change | Transport Activityc | Position | Nucleotide Changeb | Amino Acid Change | Transport Activityc | |
| 1a | Exon 10 | 57-kb deletion | 0 | Exon 5 | 537–557 del21bp | ITILELP67–73del | 19 ± 6.1 | |
| 2a | Exon 10 | c.1178A>G | p.K280R | −0.7 ± 0.9 | NF | NF | NF | |
| 2b | Exon 10 | c.1178A>G | p.K280R | −0.7 ± 0.9 | NF | NF | NF | |
| 3a | NF | NF | NF | NF | NF | NF | ||
| 4a | Exon 6 | c.668G>T | p.G110Vd | 120.0 ± 27.0 | Exon 6 | c.668G>T | p.G110Vd | 120 ± 27 |
| 5a | NF | NF | NF | NF | NF | NF | ||
| 6a | Exon 7 | c.755C>T | p.S139F | −2.9 ± 1.0 | Exon 9 | 985–986insA | p.T216fsX227 | ND |
| 7a | Exon 10 | 57-kb deletion | 0 | Exon 9 | c.938C>T | p.P200L | 15.0 ± 4.5 | |
| 7b | Exon 10 | 57-kb deletion | 0 | Exon 9 | c.938C>T | p.P200L | 15.0 ± 4.5 | |
| 8a | Exon 7 | c.755C>T | p.S139F | −2.9 ± 1.0 | Exon 7 | c.755C>T | p.S139F | −2.9 ± 1.0 |
| 8b | Exon 7 | c.755C>T | p.S139F | −2.9 ± 1.0 | Exon 7 | c.755C>T | p.S139F | −2.9 ± 1.0 |
| 9a | Exon 10 | 57-kb deletion | 0 | Exon 12 | c.1340C>A | p.T334N | ND | |
| 9b | Exon 10 | 57-kb deletion | 0 | Exon 12 | c.1340C>A | p.T334N | ND | |
| 9c | Exon 10 | 57-kb deletion | 0 | Exon 12 | c.1340C>A | p.T334N | ND | |
Mutations in patients 4a and 7a were described by Kalatzis et al. (21) and in patient 6a by Attard et al. (case P41) (12). ND, not determined; NF, not found.
The first nucleotide of the cDNA sequence is considered as +1; the ATG start codon is situated at +340. The nucleotide changes indicated here are those proposed in the original publications, although they do not follow the recommendations that the A of the ATG start codon should be considered as +1.
Effect on transport activity (expressed as a percentage of wt cystinosin activity ± SEM) reported by Kalatzis et al. (21).
Discussion
Cystinosis provides a good example of a “pediatric” disease with a spectrum extending into adult medicine. Early oral cysteamine therapy slows the deterioration of renal function. Patients with infantile cystinosis would therefore be expected to go on to develop renal failure later in life, during adulthood. Kidney transplantation makes it possible for patients with infantile cystinosis to reach adulthood. These patients are likely to be followed up in adult units and to experience serious extrarenal complications of the disease (1,22). Patients with late-onset cystinosis are particularly likely to be followed by adult nephrologists.
Late-onset nephropathic cystinosis is rare; therefore, no large series of patients has yet been reported (13). We studied 14 patients from nine families with noninfantile cystinosis. Early detection was associated with a higher probability of Fanconi syndrome but was not necessarily associated with a poor prognosis. The rate of renal progression was heterogeneous: Age at last follow-up and age at ESRD (in four patients) ranged from 12 to 56 and from 12 to 28 yr, respectively. CTNS mutations were found in only 72% of the alleles tested, and at least one mild mutation was found in most cases in which two CTNS mutations were identified.
Our findings show that nephropathic cystinosis may be difficult to diagnose and that patients may receive the diagnosis late in life, even after kidney transplantation. Some patients may have been unnecessarily treated with immunosuppression for proteinuria before arriving at the diagnosis of nephropathic cystinosis, thus emphasizing the importance to screen for cystinosis. Indeed, clinical presentation may be nonspecific in adolescents or adults. Renal Fanconi syndrome was lacking in six of 14 patients. Proteinuria was often the only abnormality. The detection of typical cystine corneal deposits could be diagnostic, but some adult patients did not complain of ocular discomfort. Renal biopsy showed in three of the four cases nonspecific FSGS, with no crystal deposits in one case. Granulocyte cystine content was higher than normal, but this increase was less marked than that in children with infantile nephropathic cystinosis: Approximately 8 nmol in the study by Gahl et al. (23) and 4 nmol half-cystine/mg protein in the study by Cochat et al. (24). In our patients with noninfantile forms, it generally did not exceed 2.5 nmol half-cystine/mg protein, as previously reported (23).
The renal changes observed in late-onset nephropathic cystinosis have been less extensively described than in the infantile form. Renal biopsy data are available for approximately 15 patients from published studies (13,25–33). Crystals were seen in three of the four biopsies carried out in our series. Our results are concordant with other reports in which cystine crystals were found in only one third of the patients who underwent renal biopsy (26,28,30); however, cystine crystals are water soluble and may be missed if certain precautions are not taken. The most striking lesion was FSGS, found in most biopsy specimens in published studies and in three of our four biopsies. The patients concerned had proteinuria, probably unrelated to proximal tubular dysfunction. FSGS may thus play a role in renal progression in late-onset cystinosis. Multinucleated podocytes have been reported in some patients (3) but were not observed in our patients. Multinucleation may lead to podocyte death, glomerular basement membrane denudation, and subsequent events that trigger the development of FSGS.
The spectrum of mutations found in these late-onset cystinosis cases differs from that found in infantile-type cystinosis. Mutations are found in all families with infantile cystinosis. Individuals have “severe” mutations in both alleles, leading to the complete loss of cystinosin function. The most common mutation is an approximately 57-kb deletion, found in the homozygous state in approximately 75% of patients of European descent. In contrast, we found point mutations that do not disrupt the open reading frame of cystinosin in our late-onset cystinosis cases. No patient was found to carry the homozygous approximately 57-kb deletion. Patients were usually homozygous for a mild mutation of this type or compound heterozygous for a mild mutation and a severe mutation (12). Mild mutations impair but do not completely abolish cystine transport (21). Moreover, the level of transport inhibition often correlates with the severity of symptoms. The new missense mutation p.T334N is located in the sixth intertransmembrane loop. We believe this mutation to be mild because it affects a loop (21). As in patient 4a, some of the tissue-sparing seen in benign cystinosis may result from tissue-specific splicing factors, mitigating the effect of splice-site mutations in renal tissue by favoring the expression of residual normal message (14,15,21). Mutations in the promoter region have also been described in patients with ocular cystinosis (20), but we did not detect such mutation in our population. In addition, contrary to what is found in infantile cystinosis, in which virtually all mutations are detected, CTNS mutations were found in only 72% of the alleles tested here. Mutations in the noncoding regions of CTNS, such as the regulatory regions, not tested here, may be involved in these late-onset forms. Alternatively, other genes, such as genes encoding proteins that interact with cystinosin, may be involved.
The rate of renal disease progression differed considerably between the affected siblings of three kindreds reported here (families 7, 8, and 9; Table 2). Similar interfamily variability in renal disease progression was reported by Thoene et al. (13) in a family with intermediate cystinosis, whereas renal progression was remarkably similar in two other patients from another kindred. Thus, renal progression cannot be accurately predicted from the rate of progression in affected relatives. The “benign” ocular form seen in adults is usually differentiated from late-onset nephropathic cystinosis (14). Our findings challenge this distinction. Indeed, patient 9a, at the age of 59 yr, had typical corneal deposits and high free cystine granulocyte content, with little or no renal involvement (no proteinuria, borderline GFR of 59 ml/min per 1.73 m2; no renal biopsy carried out), whereas her brother had reached ESRD at the age of 28 yr. These observations suggest that environmental and/or modifier genes may influence renal progression in patients with late-onset cystinosis.
There are several possible reasons for the slow progression of renal disease in patients with late-onset nephropathic cystinosis. Mild mutations are usually found, preserving often some transport activity of cystine out of lysosomes. The renal toxicity of cystine depends on events downstream from the lysosomes. These pathophysiologic steps may modulate the severity of the phenotype. Some polymorphisms in the genes that control these steps may modify clinical expression of the disease.
The possibilities for treating late-onset cystinosis depend on the putative mechanisms responsible for renal progression. Oral cysteamine can be considered to deplete cystine from lysosomes. Its efficacy against renal damage in late-onset forms is unknown. In patient 8b, oral cysteamine normalized granulocyte cystine content. Cysteamine may also be useful for preventing or reversing cystine deposition in other organs. Inhibitors of the renin-angiotensin system should be administered to reduce proteinuria and to prevent FSGS. Progress in the treatment of cystinosis depends on the design of new compounds to prevent the accumulation of cystine in lysosomes and on improvements in our understanding of the successive steps that lead from the primary lysosomal event to cell damage.
Conclusions
The clinical presentation of late-onset nephropathic cystinosis may be confusing with mild renal insufficiency and proteinuria without extrarenal signs. Progression is heterogeneous, even within families, but may be severe, leading to ESRD; however, severity is not correlated with the presence of Fanconi syndrome. Family investigations are requested in this field. Further investigations might provide deeper insight into the molecular or cellular mechanisms that modulate disease severity and reveal unsuspected aspects of the pathogenic cascade.
Disclosures
None.
Acknowledgments
This work was supported by the Cystinosis Research Foundation, Vaincre les Maladies Lysosomales and the Cystinosis Research Network.
This work was presented as an abstract at the 39th annual meeting of the American Society of Nephrology; November 14 through 19, 2006; San Diego, CA.
We thank the following physicians who took care of the patients and provided medical information: Dr. Broyer, CHU Necker, France; Dr. Fischbach, CHU Strasbourg, France; Dr. Legrand, CHR Annonay, France; Dr. McGreggor, CHU Lyon, France; Dr. Anglicheau, CHU Necker, France; Dr. Bedet, France; Dr. Dehenaut, CHU Lille, France; Dr. Lombaerts, University Hospital Leuven, Belgium; Dr. Vilaseca, Hospital Sant Joan de deu, Barcelona, Spain; Dr. Godefroid, Cliniques universitaires St Luc et Hôpital des enfants Reine Fabiola, Brussels, Belgium; Dr. Soulami, CHU Ibn Rochd, Casablanca, Morocco. We thank Dr. Touchard, N. Quellard, and B. Fernandez in the ultrastructural and experimental pathology unit in Poitiers. We are very grateful to Dr. Marie-Claire Gubler for critical reading of this article and advice.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gahl WA, Thoene JG, Schneider JA: Cystinosis. N Engl J Med 347: 111–121, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Gretz N, Manz F, Augustin R, Barrat TM, Bender-Gotze C, Brandis M, Bremer HJ, Brodehl J, Broyer M, Bulla M, Callis L, Chantler C, Diekmann L, Dillon MJ, Egli F, Ehrich JH, Endres W, Fanconi A, Feldhoff C, Geisert J, Gekle D, Gescholl-Bauer B, Grote K, Gruttner R, Hagge W, Haycock CB, Hennemann H, Klare B, Leupold D, Lohr H, Michalk D, Oliveira A, Ott F, Pistor K, Rau J, Scharer K, Schindera F, Schmidt H, Schulte-Wissermann H, Verrier-Jones K, Weber HP, Willenbockel U, Wolf H: Survival time in cystinosis: A collaborative study. Proc Eur Dial Transplant Assoc 19: 582–589, 1983 [PubMed] [Google Scholar]
- 3.Gubler MC, Lacoste M, Sich M, Broyer M: The Pathology of the Kidney in Cystinosis, Paris, France, Elsevier, 1999
- 4.Gahl WA: Early oral cysteamine therapy for nephropathic cystinosis. Eur J Pediatr 162[Suppl 1]: S38–S41, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Gahl WA, Kuehl EM, Iwata F, Lindblad A, Kaiser-Kupfer MI: Corneal crystals in nephropathic cystinosis: Natural history and treatment with cysteamine eyedrops. Mol Genet Metab 71: 100–120, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP, van’t Hoff W, Antignac C: A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet 18: 319–324, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Kalatzis V, Cherqui S, Antignac C, Gasnier B: Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter. EMBO J 20: 5940–5949, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forestier L, Jean G, Attard M, Cherqui S, Lewis C, van’t Hoff W, Broyer M, Town M, Antignac C: Molecular characterization of CTNS deletions in nephropathic cystinosis: Development of a PCR-based detection assay. Am J Hum Genet 65: 353–359, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Touchman JW, Anikster Y, Dietrich NL, Maduro VV, McDowell G, Shotelersuk V, Bouffard GG, Beckstrom-Sternberg SM, Gahl WA, Green ED: The genomic region encompassing the nephropathic cystinosis gene (CTNS): Complete sequencing of a 200-kb segment and discovery of a novel gene within the common cystinosis-causing deletion. Genome Res 10: 165–173, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anikster Y, Shotelersuk V, Gahl WA: CTNS mutations in patients with cystinosis. Hum Mutat 14: 454–458, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Gahl WS, Pertti A: Lysosomal transport disorders: Cystinosis and sialic acid storage disorders. In: The Metabolic and Molecular Bases of Inherited Disease, Vol. 3, edited by Scriver CB, Sly W, Valla D, New York, McGraw-Hill, 1995, pp 3780
- 12.Attard M, Jean G, Forestier L, Cherqui S, van’t Hoff W, Broyer M, Antignac C, Town M: Severity of phenotype in cystinosis varies with mutations in the CTNS gene: Predicted effect on the model of cystinosin. Hum Mol Genet 8: 2507–2514, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Thoene J, Lemons R, Anikster Y, Mullet J, Paelicke K, Lucero C, Gahl W, Schneider J, Shu SG, Campbell HT: Mutations of CTNS causing intermediate cystinosis. Mol Genet Metab 67: 283–293, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Anikster Y, Lucero C, Guo J, Huizing M, Shotelersuk V, Bernardini I, McDowell G, Iwata F, Kaiser-Kupfer MI, Jaffe R, Thoene J, Schneider JA, Gahl WA: Ocular nonnephropathic cystinosis: Clinical, biochemical, and molecular correlations. Pediatr Res 47: 17–23, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Kalatzis V, Cohen-Solal L, Cordier B, Frishberg Y, Kemper M, Nuutinen EM, Legrand E, Cochat P, Antignac C: Identification of 14 novel CTNS mutations and characterization of seven splice site mutations associated with cystinosis. Hum Mutat 20: 439–446, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Lewis J, Agodoa L, Cheek D, Greene T, Middleton J, O’Conner D, Ojo A, Phillips R, Sika M, Wright J Jr: Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis 38: 744–753, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Ged C, Jean G, Tete MJ, Broyer M, Kamoun P: Intra-leukocyte cystine in cystinosis treated with cysteamine [in French]. Ann Biol Clin (Paris) 49: 482–486, 1991 [PubMed] [Google Scholar]
- 19.Fakhouri F, Darre S, Droz D, Lemaire M, Nabarra B, Machet MC, Chauveau D, Lesavre P, Grunfeld JP, Noel LH, Knebelmann B: Mesangial IgG glomerulonephritis: A distinct type of primary glomerulonephritis. J Am Soc Nephrol 13: 379–387, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Phornphutkul C, Anikster Y, Huizing M, Braun P, Brodie C, Chou JY, Gahl WA: The promoter of a lysosomal membrane transporter gene, CTNS, binds Sp-1, shares sequences with the promoter of an adjacent gene, CARKL, and causes cystinosis if mutated in a critical region. Am J Hum Genet 69: 712–721, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalatzis V, Nevo N, Cherqui S, Gasnier B, Antignac C: Molecular pathogenesis of cystinosis: Effect of CTNS mutations on the transport activity and subcellular localization of cystinosin. Hum Mol Genet 13: 1361–1371, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Theodoropoulos DS, Krasnewich D, Kaiser-Kupfer MI, Gahl WA: Classic nephropathic cystinosis as an adult disease. JAMA 270: 2200–2204, 1993 [PubMed] [Google Scholar]
- 23.Markello TC, Bernardini IM, Gahl WA: Improved renal function in children with cystinosis treated with cysteamine. N Engl J Med 328: 1157–1162, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Cochat PC, Lacôte C, Saïd MH: Cystinosis: Epidemiology in France. In: Cystinosis, edited by Broyer M, Paris, France, Elsevier, 1999, pp 28–35
- 25.Manz F, Harms E, Lutz P, Waldherr R, Schärer K: Adolescent cystinosis: Renal function and morphology. Eur J Pediatr 138: 354–357, 1982 [DOI] [PubMed] [Google Scholar]
- 26.Pabico RC, Panner BJ, McKenna BA, Bryson MF: Glomerular lesions in patients with late-onset cystinosis with massive proteinuria. Ren Physiol 3: 347–354, 1980 [DOI] [PubMed] [Google Scholar]
- 27.Verougstraete C, Libert J, Toussaint D: Cystinosis in adolescence: Clinical and histopathological study [in French]. Bull Soc Belge Ophtalmol 9–20, 1978 [PubMed]
- 28.Hory B, Billerey C, Royer J, Saint Hillier Y: Glomerular lesions in juvenile cystinosis: Report of 2 cases. Clin Nephrol 42: 327–330, 1994 [PubMed] [Google Scholar]
- 29.Hauglustaine D, Corbeel L, van Damme B, Serrus M, Michielsen P: Glomerulonephritis in late-onset cystinosis: Report of two cases and review of the literature. Clin Nephrol 6: 529–536, 1976 [PubMed] [Google Scholar]
- 30.Hamdoun S, Lebon P, Nivet H, Beaufils H, Babinet F, Kernaonet E: Late onset cystinosis: Apropos of a case [in French]. Nephrologie 8: 193–195, 1987 [PubMed] [Google Scholar]
- 31.Waldherr R, Manz F, Hagge H: Kidney changes in infantile and adolescent (nephropathic) cystinosis [in German]. Verh Dtsch Ges Pathol 66: 297–303, 1982 [PubMed] [Google Scholar]
- 32.Blanc-Brunat N, Berthoux F, Colon S, Janin G: Late diagnosis of cystinosis in 2 brothers: histological and ultrastructural renal study [in French]. Arch Fr Pediatr 35: 486–503, 1978 [PubMed] [Google Scholar]
- 33.Marx M, Plank C, Zimmermann B, Graupner M, Kiehntopf M, Dotsch J: End-stage renal failure as manifestation of adolescent cystinosis. Eur J Pediatr 163: 260–261, 2004 [DOI] [PubMed] [Google Scholar]