Abstract
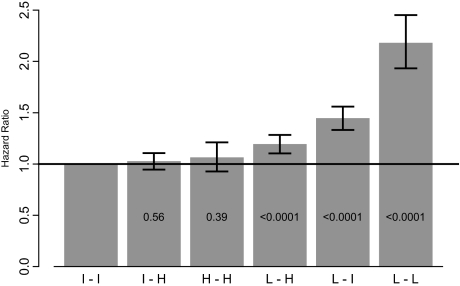
Background/objectives: Awareness of hemoglobin level variability in dialysis patients is increasing, as is interest in its potential implications. In this retrospective, national study of associations between the degree of hemoglobin level variability in the first 6 mo of 2004 and subsequent mortality rates in the following 6 mo, 159,720 hemodialysis patients receiving epoetin therapy were studied. Design, setting, participants, measurements: Monthly hemoglobin values were categorized as low (L; < 11 g/dl), intermediate (I; 11 to 12.5 g/dl), and high (H; >12.5 g/dl). Variability groups were classified on the basis of the lowest and highest hemoglobin categories seen during the 6-mo observation period: low-low (L-L), 1.4%; intermediate-intermediate (I-I), 6.0%; high-high (H-H), 2.3%; low-intermediate (L-I), 18.3%; intermediate-high (I-H), 31.7%, and low-high (L-H), 40.2%.
Results: On multivariate analysis, adjusted hazards ratios for subsequent mortality events were as follows: I-I, 1.0 (reference category); I-H, 1.02 (95% confidence interval [CI] 0.95 to 1.11); H-H, 1.06 (95% CI 0.93 to 1.21); L-H, 1.19 (95% CI 1.10 to 1.28); L-I, 1.44 (95% CI 1.33 to 1.56), and L-L, 2.18 (95% CI 1.93 to 2.45). Persistently and transiently low hemoglobin levels and highly variable hemoglobin levels were associated with increased risk of death; transiently and persistently high hemoglobin levels were not associated with increased risk of death. Bayesian modeling indicated that ≥3 mo with hemoglobin levels <11 g/dl may be associated with of increased risk of death.
Conclusions: Number of months with hemoglobin values below the target range, rather than hemoglobin variability itself, may be the primary driver of increased risk of death. Further research is needed to distinguish cause from effect and to understand the underlying mechanisms.
Anemia treatment in hemodialysis patients has changed significantly since the introduction of epoetin alfa (EPO) into clinical practice in June, 1989. In the early 1990s, anemia correction was viewed simply as treatment with EPO along with correction of iron deficiency to increase hemoglobin levels from below 10 g/dl to within the initial label target range of 10 to 11 g/dl. As experience with anemia correction grew and the potential side effects, including seizures and hypertension, occurred less frequently than anticipated from the initial trials, the United States Food and Drug Administration increased the target hemoglobin range to 10 to 12 g/dl.
Safety and efficacy of anemia correction with erythropoiesis-stimulating agents (synthetic versions of the hormone erythropoietin that stimulate red blood cell production by the bone marrow), have been the subject of numerous observational studies and clinical trials since the mid-1990s (1–7). Observational studies demonstrated increased mortality associated with values <10 g/dl (2,7) and <11 g/dl (4), which suggests the target range should be 11 to 12 g/dl. However, the Normal Hematocrit Study (8) in the mid-1990s showed an adverse effect of targeting hemoglobin levels to 14 versus 10 g/dl, which indicated a safety concern with higher levels.
Clinical performance measures intended to increase the percentage of patients with hemoglobin levels within the target range of 11 to 12 g/dl were adopted from the National Kidney Foundation Kidney Disease Outcomes Quality Initiative clinical practice guidelines (9). Yet, maintaining hemoglobin levels within the 1-g/dl target range proved more difficult than anticipated. Hemoglobin variability became an important issue in practice, and recent studies have reported that only a small percentage of patients remain at any given level over time (10–12). Fishbane and Berns first introduced the concept of hemoglobin cycling by describing the pattern of changes over time in individual patients (10), which provided an important conceptual framework for understanding the frequent fluctuation of hemoglobin levels over time. An analysis by Ebben et al. (11) characterized hemoglobin changes over time for a large sample of US hemodialysis patients and observed considerable variability, with only 10% of patients maintaining stable hemoglobin levels from month to month.
To assess the relationship of hemoglobin variability and mortality and to examine the relationship with mortality of excursions into higher versus lower hemoglobin levels compared with maintaining hemoglobin levels within target, we assessed fluctuations and patterns of change over time, looking for the patterns associated with the lowest mortality rates.
Conise Methods
Study Population
The study population included all hemodialysis patients who survived the first 6 mo of 2004, had Medicare as primary payer, and had outpatient EPO claims in each of the first 6 mo of 2004 (n = 159,720). The first 6 mo of 2004 were considered the exposure assessment period. Patients were followed from the first day after the end of the exposure assessment period until the first of date of death, loss to follow-up for reasons including renal transplantation or switching to peritoneal dialysis, or December 31, 2004.
Defining Hemoglobin Variability
Hemoglobin variability was defined by two systems. By the first classification system, monthly hemoglobin values, ascertained from epoetin claims, were categorized as low (L; <11 g/dl), intermediate (I; 11 to 12.5 g/dl), and high (H; >12.5 g/dl). A 6-group classification system was used, based on the lowest and highest categories seen during the 6-mo observation period: low-low (L-L), intermediate-intermediate (I-I), high-high (H-H), low-intermediate (L-I), intermediate-high (I-H), and low-high (L-H).
Although useful in characterizing hemoglobin change over time, this classification system ignores time sequence. For example, a low hemoglobin level at the start of the observation period carries the same weight as a low level at the end. The system also ignores the number of similar categorizations within the observation period. For example, the sequences L-L-L-L-I-H and L-L-I-I-H-H fall in the same category.
Although the mortality hazard ratios (HR) associated with individual monthly hemoglobin patterns were also of interest, meaningful quantification of mortality associations for each possible pattern was limited by sparseness. With six successive observations of a variable and three possible levels, 729 (36) patterns are possible. Of the 728 observed patterns, 419 (57.6%) applied to <100 patients and 223 (30.6%) applied to <30 patients. To address this sparseness, a Bayesian Poisson model (13) was used to identify patterns significantly associated with relatively high or low mortality rates. The most extreme of those relative rates (shown in Table 2) suggested that that the number and timing of hemoglobin values <11 g/dl were the factors most strongly associated with mortality risk. Thus, the second classification system for defining variability included the total number of months with hemoglobin levels <11 g/dl and an indicator of whether low values were seen in the first 3 mo (first, F), second 3 mo (second, S), or both (F-S). This resulted in the following groups: 1-F, 1-S; 2-F, 2-S, 2-F-S; 3-F, 3-S, 3-F-S; 4-F-S; 5-F-S; 6-F-S. For example, 1-S refers to 1 hemoglobin reading <11 in the second 3-mo period, and 3-F-S refers to 3 hemoglobin readings <11 in both the first and second 3-mo periods.
Table 2.
Significant and most extreme results from Bayesian model assessing adjusted associations of hemoglobin fluctuation patterns and mortality
| Adjusted Relative Rate (RR) Associationsa
| |||||||
|---|---|---|---|---|---|---|---|
| Lowest
|
Highest
|
||||||
| RR | 95% CI | Patternb | Variability Groupc | RR | 95% CI | Patternb | Variability Groupc |
| 0.71 | 0.55 to 0.90 | ILIIHH | L-H | 1.83 | 1.49 to 2.22 | LLIILL | L-I |
| 0.75 | 0.56 to 0.90 | HHLIHH | L-H | 1.71 | 1.57 to 1.86 | LLLLLL | L-L |
| 0.78 | 0.61 to 0.97 | IILIHH | L-H | 1.56 | 1.26 to 1.89 | LLILLL | L-I |
| 0.78 | 0.66 to 0.91 | IIIIHI | I-H | 1.48 | 1.21 to 1.79 | LLLILL | L-I |
| 0.79 | 0.63 to 0.96 | HIIIIH | I-H | 1.46 | 1.24 to 1.69 | IILLLL | L-I |
| 0.79 | 0.65 to 0.95 | LIHHHH | L-H | 1.44 | 1.10 to 1.83 | HLLLLLL | L-H |
| 0.80 | 0.66 to 0.94 | IIHHHH | I-H | 1.38 | 1.07 to 1.76 | HHLLLL | L-H |
| 0.81 | 0.75 to 0.88 | IIIIII | I-I | 1.38 | 1.11 to 1.69 | LILLLL | L-I |
| 0.82 | 0.71 to 0.95 | HHHIII | I-H | 1.34 | 1.08 to 1.63 | LLLLIL | L-I |
| 0.82 | 0.69 to 0.95 | IIIHHH | I-H | 1.33 | 1.15 to 1.53 | IIILLL | L-I |
| 0.82 | 0.70 to 0.97 | HHHHII | I-H | 1.30 | 1.12 to 1.50 | LLLLLI | L-I |
| 0.83 | 0.69 to 0.98 | ILIIII | L-I | 1.30 | 1.01 to 1.64 | IIIHLL | L-H |
| 0.83 | 0.69 to 0.99 | LLIHHH | L-H | 1.28 | 1.09 to 1.49 | ILLLLL | L-I |
| 0.84 | 0.72 to 0.97 | IIIIIH | I-H | 1.26 | 1.02 to 1.54 | LLIIIL | L-I |
| 0.84 | 0.72 to 0.96 | HHIIII | I-H | 1.18 | 1.01 to 1.36 | LLLLII | L-I |
Adjusted for age, gender, race, primary cause of ESRD (diabetes, hypertension, other), an indication of hospital admissions during the entry period, and the 10 comorbid conditions.
Hemoglobin level each of months 1 to 6: L, hemoglobin >11g/dl; I, hemoglobin 11 to 12.5 g/dl; H, hemoglobin >12.5 g/dl.
Monthly hemoglobin values were categorized as low (L), intermediate (I), and high (H); variability groups were classified on the basis of the lowest and highest categories seen in the 6-mo observation period.
Covariates
Strong correlations between patient comorbidity and measures of disease severity with hemoglobin variability patterns have been previously reported (11). These same factors were assessed during the 6-mo exposure window for the purpose of adjustment in the multivariate modeling. International Classification of Diseases, Ninth Revision, Clinical Modification codes were used to identify comorbid conditions from Medicare Part A institutional and Part B physician/supplier claims. A comorbid condition was defined as present if a Medicare Part A or Part B claim appeared in the medical record during the exposure assessment period for any of the following: atherosclerotic heart disease, congestive heart failure, dysrhythmia, other cardiac disease (including valvular disease), cerebrovascular accident/transient ischemic attack, peripheral vascular disease, chronic obstructive pulmonary disease, cancer, gastrointestinal bleeding, and hepatic disease. Hospital admission information was obtained from the Medicare Inpatient Standard Analytical File. Demographic data were obtained from the Centers for Medicare & Medicaid Services (CMS) Medical Evidence Report (CMS-2728), which records date of birth, gender, race, renal diagnosis, and first ESRD service date. Mortality information was obtained from the CMS ESRD Death Notification (CMS-2746).
Statistical Analyses
Descriptive statistics were used to examine patient characteristics according to the six hemoglobin variability categories of the first classification system, and mortality rates were calculated for the categories. Cox proportional hazards regression was used to estimate HR and 95% confidence intervals (CI) for the association between the categories and mortality, adjusting for demographic and comorbidity characteristics. The intermediate (11 to 12.5 g/dl) category was the reference group for all analyses. Adjustment was made for age, race, gender, hospital admissions during the entry period, and the 10 comorbid conditions listed above. Because diabetes as cause of renal failure was found to be nonproportional, this variable was used as a strata variable in the proportional hazards model. Mortality was assessed for the second classification system in the same fashion.
The Bayesian Poisson model included adjustments for age, gender, race, primary cause of ESRD (diabetes, hypertension, other), an indication of hospital admissions during the entry period, and the 10 comorbid conditions, along with an effect for hemoglobin fluctuation pattern. Pattern effects were assumed to follow a normal distribution, with mean 0 and variance τ. The prior distributions of each adjustment effect and τ were proper but diffuse, whereas the prior distribution of the model intercept was improper. Three parallel chains, each with 1000 burn-in iterations and 1000 draws, formed the posterior distribution of each pattern effect. The reported relative rates of mortality represent posterior means, and corresponding CIs were formed from the 2.5 and 97.5 percentiles of the posterior distributions.
Interval Poisson regression was used to assess potential changes in the effect of hemoglobin patterns on mortality over the follow-up period, as the proportional hazards model calculates estimates that are an average effect over time. For this modeling approach, follow-up time was discretely categorized as 0 to 30 d, 31 to 60 d, 61 to 90 d, 91 to 180 d, 181 to 365 d, and >365 d. For this analysis, patients were followed until the first of date of death, loss to follow-up for reasons including renal transplantation or switching to peritoneal dialysis, or September 1, 2005. In addition to the basic hemoglobin fluctuation patterns, each detailed pattern was assessed for association with mortality.
Results
Demographic characteristics of the 159,720 hemodialysis patients who met the inclusion criteria are provided in Table 1 and are similar to the overall Medicare hemodialysis population. Using the first classification system, the percent of patients in each of the six groups was as follows: low-low (L-L), 1.4%; intermediate-intermediate (I-I), 6.0%; high-high (H-H), 2.3%; low-intermediate (L-I), 18.3%; intermediate-high (I-H), 31.7%; and low-high (L-H), 40.2%. Of the 728 observed hemoglobin variability patterns, those significantly and most positively or most negatively associated with mortality as assessed by Bayesian modeling are presented in Table 2. Most patterns associated with a mortality risk below the null included few if any months with hemoglobin levels <11 g/dl; only 5 patterns included ≥1 mo with a value <11 g/dl, and in each case it appeared during the first 3 mo of the 6-mo exposure assessment period. Most patterns associated with the highest mortality risk included at least 3 mo with hemoglobin levels <11 g/dl, and these usually appeared late in the 6-mo period.
Table 1.
Patient characteristics (n = 159,720)
| Characteristic | All | Hemoglobin Variability Groupa
|
P Valueb | |||||
|---|---|---|---|---|---|---|---|---|
| L-L | I-I | H-H | L-I | I-H | L-H | |||
| Hemoglobin category (<11/11 to 12.5/>12.5) | ||||||||
| Month 1 | 22.6/47.7/29.7 | 100/0/0 | 0/100/0 | 0/0/100 | 38.3/61.7/0 | 0/54.6/45.4 | 35.3/32.5/32.2 | — |
| Month 2 | 21.5/48.8/29.8 | 100/0/0 | 0/100/0 | 0/0/100 | 41.0/59.0/0 | 0/51.8/48.2 | 31.3/38.5/30.2 | — |
| Month 3 | 20.6/49.1/30.2 | 100/0/0 | 0/100/0 | 0/0/100 | 42.3/57.7/0 | 0/52.8/47.2 | 28.7/39.2/32.1 | — |
| Month 4 | 19.9/48.4/31.8 | 100/0/0 | 0/100/0 | 0/0/100 | 42.4/57.6/0 | 0/52.5/47.5 | 26.7/37.7/35.6 | — |
| Month 5 | 20.0/49.1/30.9 | 100/0/0 | 0/100/0 | 0/0/100 | 43.8/56.2/0 | 0/55.8/44.2 | 26.5/37.4/36.1 | — |
| Month 6 | 19.2/47.4/33.4 | 100/0/0 | 0/100/0 | 0/0/100 | 39.2/60.8/0 | 0/55.3/44.7 | 26.5/31.6/41.9 | — |
| Gender | ||||||||
| male | 51.3 | 54.0 | 54.1 | 56.3 | 51.0 | 52.7 | 49.5 | <0.0001 |
| female | 48.7 | 46.0 | 45.9 | 43.7 | 49.0 | 47.3 | 50.6 | |
| Race | ||||||||
| white | 53.4 | 48.5 | 58.5 | 51.3 | 54.7 | 53.4 | 52.4 | <0.0001 |
| black | 40.3 | 46.6 | 35.0 | 41.6 | 39.2 | 40.3 | 41.3 | |
| other | 6.3 | 4.9 | 6.5 | 7.1 | 6.2 | 6.3 | 6.3 | |
| Age, years | ||||||||
| <40 | 8.8 | 16.8 | 7.3 | 9.3 | 9.6 | 7.8 | 9.2 | <0.0001 |
| 40 to 64 | 41.2 | 50.1 | 39.0 | 41.0 | 42.8 | 40.6 | 40.9 | |
| 65 to 74 | 26.3 | 19.6 | 27.2 | 26.3 | 25.7 | 26.7 | 26.3 | |
| ≥75 | 23.8 | 13.5 | 26.6 | 23.4 | 22.0 | 24.9 | 23.6 | |
| Cause of renal failure | ||||||||
| diabetes | 42.8 | 35.1 | 43.4 | 42.4 | 42.7 | 42.9 | 43.0 | <0.0001 |
| hypertension | 29.8 | 27.3 | 28.4 | 30.4 | 28.5 | 30.5 | 30.0 | |
| other | 27.4 | 37.6 | 28.2 | 27.3 | 28.8 | 26.7 | 27.0 | |
| Dialysis vintage, yr | ||||||||
| <1 | 20.5 | 18.7 | 14.0 | 28.2 | 16.1 | 21.4 | 22.3 | <0.0001 |
| 1 to <3 | 31.5 | 27.2 | 35.9 | 27.5 | 32.3 | 31.7 | 30.8 | |
| 3 to <5 | 20.8 | 19.6 | 22.9 | 17.9 | 22.1 | 20.7 | 20.2 | |
| ≥5 | 27.2 | 34.5 | 27.3 | 26.5 | 29.6 | 26.3 | 26.7 | |
Monthly hemoglobin values were categorized as low (L, <11 g/dl), intermediate (I, 11 to 12.5 g/dl), and high (H, >12.5 g/dl); variability groups were classified on the basis of the lowest and highest categories seen in the 6-mo observation period. Values are percents.
χ2 test.
Proportional hazards results based on the first classification system (Figure 1) showed no increased risk of death for patients in the I-H (HR 1.02; 95% CI 0.95 to 1.11) and H-H (HR 1.06; 95% CI 0.93 to 1.21) categories, compared with patients consistently within the 11 to 12.5 g/dl range. Patients in the L-H and L-I categories had significantly increased mortality risks: HR 1.19, 95% CI 1.10 to 1.28 and HR 1.44, 95% CI 1.33 to 1.56, respectively. Patients in the consistently low category had the highest risk of death (HR 2.18; 95% CI 1.93 to 2.45).
Figure 1.
Hazard ratios for mortality based on the first classification system. Monthly hemoglobin values were categorized as low (L, <11 g/dl), intermediate (I, 11 to 12.5 g/dl) and high (H, >12.5 g/dl); variability groups were classified on the basis of the lowest and highest categories seen in the 6-mo observation period. Each P value tests the corresponding variability group hazard ratio compared with the reference group (consistently intermediate).
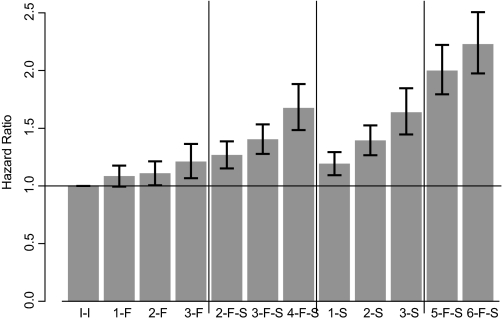
Figure 2 shows adjusted mortality results using the second classification method. Patients with hemoglobin values <11 g/dl in the first 3 mo of the 6-mo exposure assessment period and >11 g/dl in the second 3 mo generally had a lower mortality risk than patients with at least one hemoglobin value <11 g/dl in the second 3 mo. Also, the more low hemoglobin values in the second 3 mo of the exposure assessment period, the higher the risk of death.
Figure 2.
Hazard ratios for mortality based on the second classification system. Number of months with hemoglobin <11 g/dl and first or second half of the 6-mo exposure period. For example, 2-F represents 2 mo with hemoglobin <11 g/dl during the first 3 mo; 4-F-S represents 4 mo with hemoglobin <11 during both the first and the second 3-mo periods. I-I, consistently intermediate (11 to 12.5 g/dl), represents the reference group.
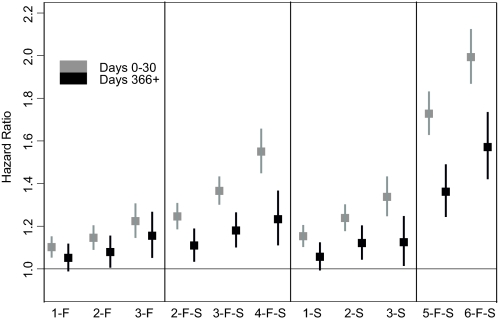
Interval Poisson modeling was performed to assess how HR estimates changed depending on the length of follow-up (Figure 3). Figure 3 displays two time periods, 0 to 30 d and >365 d. All hemoglobin patterns showed some decline in HR between these time periods. However, HR for hemoglobin values <11 g/dl, particularly late in the entry period, remained significantly elevated. All analyses were repeated using 12 g/dl as the cutoff instead of 12.5 g/dl, with similar overall results.
Figure 3.
Interval Poisson model examining the change in hazard ratios over follow-up time. Number of months with hemoglobin <11 g/dl and first or second half of the 6-mo exposure period, as explained in Figure 2. I-I, consistently intermediate (11 to 12.5 g/dl), represents the reference group.
Discussion
Variability in hemoglobin levels over time is very common, with almost 90% of patients experiencing hemoglobin level changes during a 6-mo period, in part as a result of intercurrent illness, infections, bleeding complications, hospitalizations, and epoetin dosing changes (11). Excursions of hemoglobin values over and under the target range of 11 to 12 g/dl or the Medicare audit level of 12.5 g/dl were very common, approaching 25% of patient-months. Ebben et al. (11) and the current study defined stable hemoglobin over 6 mo as levels within the 11 to 12.5 g/dl range each month and showed that only 10% of patients met the definition. The association of hemoglobin variability patterns with concurrent comorbidity and hospitalizations has been shown previously (11); the current analysis reports a significant association between specific hemoglobin variability patterns and increased mortality risk.
The current study suggests that variability in and of itself may not have a strong association with mortality. Rather, the key factors seem to be the number and timing of hemoglobin values <11 g/dl during a specific exposure assessment period and the overall direction of hemoglobin values during that period. Specifically, if a patient's hemoglobin values start low but rise over time, as might be expected among incident hemodialysis patients or patients recently hospitalized, there is little association between this variability and mortality. Falling hemoglobin levels, however, are significantly associated with increased mortality risk. This analysis generally supports earlier reports showing an association between hemoglobin levels <11 g/dl and elevated mortality risk (2,7), although these studies investigated average hemoglobin values rather than hemoglobin level change or time with values <11 g/dl.
Additionally, this analysis examined the mortality risk associated with hemoglobin levels consistently above the target range or fluctuating near the upper end of the range, and we did not observe elevated mortality associations for either group. Despite the different patient populations, a comparison of these results with those of the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) (14) and Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta (CREATE) (15) studies is of particular interest. These randomized clinical trials among CKD patients not on dialysis raised concerns about targeting hemoglobin levels >13 g/dl. The CHOIR trial (14) found a statistically significant 34% increased risk of a composite end point including death and nonfatal cardiovascular events; in contrast, the CREATE trial (15) found a near null estimate for a similar composite end point. Compared with randomized clinical trials in which hemoglobin targets were assigned by randomization, patient hemoglobin levels in the current study reflect actual achieved levels. Consequently, significant differences in the demographic and comorbidity profiles of patients with different hemoglobin variability patterns may not have been fully addressed, despite attempts to control for these differences using statistical adjustment. This was an observational study likely to have important confounding-by-indication biases compared with randomized clinical trials such as CHOIR (14) or the Normal Hematocrit Study (8), and the magnitude of risk for the hemoglobin variability groups with higher hemoglobin values may be underestimated.
Importantly, the mortality risks associated with hemoglobin values <11 g/dl were considerably greater than those associated with values >12.5 g/dl, but these findings may still represent unmeasured confounding. On the basis of both safety and efficacy considerations, these data support the clinical target hemoglobin range of 11 to 12.5 g/dl and are not inconsistent with the current United States Food and Drug Administration label recommendations of a hemoglobin range of 10 to 12 g/dl. The upper value of 12.5 g/dl was chosen in the current study for two primary reasons. First, <1% of patient hemoglobin levels were consistently between 11 and 12 g/dl for all 6 mo, making multivariate analyses difficult to perform using such a small percent of the population as the reference group. Second, the value 12.5 g/dl corresponds with the Medicare audit value enacted in 1998, which triggers an audit only if a 90-d rolling average exceeds 12.5 g/dl to allow for transient excursions above 12.5 g/dl. Although this study does not address 12 versus 12.5 g/dl as a recommended upper hemoglobin level, it did include analyses using 12 g/dl as the cutoff instead of 12.5 g/dl; although standard errors changed considerably as a result of the small size of the reference group, the overall pattern of results was similar.
The limitations of this study should be carefully considered. Because EPO claims are the only source of hemoglobin level data available in the Medicare ESRD data, the impact of excluding patients with no claims during 1 or more months of the exposure assessment period cannot be fully assessed because a variability pattern could not be assigned to them. Medicare claims data reflect only a single reported hemoglobin level in a billing period; more granular data from weekly or bimonthly hemoglobin reports may provide greater refinement of the variability patterns and associated mortality. Another limitation is lack of lab data. We assessed the impact of adding urea reduction ratio to the models as a measure of dialysis adequacy and the impact of adding EPO dose during the 6-mo variability period. Adding urea reduction ratio resulted in no change to the findings, whereas adding EPO dose, which can be viewed as a measure of inflammatory status, attenuated the relative risks somewhat toward the null; however, the general patterns were still apparent. Assessment of the relationship of hemoglobin variability and outcomes is clearly very complex, because levels for 90% of patients are in flux at any point in time (11), which makes misclassification a major problem. Although the use of proportional hazards and interval Poisson models can adjust for important confounders, more complex modeling techniques that address time-varying confounding should also be considered. However, the current results may be important to report because safety of anemia treatment is a current and important issue.
This study does not attempt to separate out causes of variation in hemoglobin levels. Some proportion of the variability, related, for example, to EPO and iron dosing, is under the control of the physician and dialysis unit. The remaining variation may be the result of hospitalizations, infections, intercurrent illnesses, and other factors not under the control of the healthcare system. These events would themselves be associated with increased risk of future mortality, and thus such nonactionable variability may be simply a marker for patient illness.
This study concludes that hemoglobin variability is very common with many variability patterns (728 patterns based on a single reported monthly hemoglobin level) over a 6-mo period. The assessment of these patterns by Bayesian modeling identified those patterns significantly associated with mortality. This study was unable to determine risk associated with time >12 versus 12.5 g/dl; however, patients whose hemoglobin levels were consistently within the target range of 11 to 12.5 g/dl experienced the lowest mortality. The longer the amount of time with a hemoglobin level <11 g/dl, the greater the risk of death; additionally, the timing of the low hemoglobin value within the 6-mo period was strongly associated with an increased risk of death. These results, combined with accumulating clinical trial evidence suggesting possible adverse outcomes associated with high hemoglobin levels, suggest that the challenge to providers is to reduce the variation in hemoglobin levels over time on both the high and low ends of the range. Finally, as an observational study, this study cannot prove causality but only demonstrate the associations. However, carrying out a hemoglobin variability clinical trial would be difficult, and this study suggests that patients who experienced the fewest number of months with hemoglobin levels below the 11 to 12.5 g/dl range experienced the lowest mortality.
Disclosures
James P. Ebben and Eric D. Weinhandl have no conflicts of interest. David T. Gilbertson, Robert N. Foley, and Allan J. Collins have received consulting fees from Amgen. Brian D. Bradbury works in the Department of Epidemiology at Amgen, Inc.
Acknowledgments
This study was supported by a research contract from Amgen Inc., Thousand Oaks, CA. The contract provides for the investigators of the Minneapolis Medical Research Foundation to have the final determination of the content of this publication. The data used for this study were independently acquired from the Centers for Medicare & Medicaid Services under data use agreements with the Minneapolis Medical Research Foundation, and the authors complied with all Privacy Act requirements for confidentiality of the beneficiary-specific information. Standard fees for access to the data were paid to the Centers for Medicare & Medicaid Services. James P. Ebben and Eric D. Weinhandl were directly responsible for the dataset construction, analytical methods, SAS code, production of tables and figures, and review of the interpretation of the results. David T. Gilbertson, Robert N. Foley, and Allan J. Collins were involved in the overall study design, review of the results, and draft of the manuscript. Brian D. Bradbury works in the Department of Epidemiology at Amgen; he was involved in the overall study design, review of the results, and review of the manuscript. The authors thank Chronic Disease Research Group colleagues Nan Booth, for manuscript preparation and editing, and Stephan Dunning, for detailed project management.
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Anemia of Chronic Kidney Disease,” on pages 3–6.
References
- 1.Collins AJ, Li S, Ebben J, Ma JX, Manning W: Hematocrit levels and associated Medicare expenditures. Am J Kidney Dis 36: 282–293, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Ma JZ, Ebben J, Xia H, Collins AJ: Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 10: 610–619, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Madore F, Lowrie EG, Brugnara C, Lew NL, Lazarus JM, Bridges K, Owen WF: Anemia in hemodialysis patients: Variables affecting this outcome predictor. J Am Soc Nephrol 8: 1921–1929, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus JM: The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int 63: 1908–1914, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, Greenland S, Kalantar-Zadeh K: Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol 17: 1181–1191, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Robinson BM, Joffe MM, Berns JS, Pisoni RL, Port FK, Feldman HI: Anemia and mortality in hemodialysis patients: Accounting for morbidity and treatment variables updated over time. Kidney Int 68: 2323–2330, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Xia H, Ebben J, Ma JZ, Collins AJ: Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol 10: 1309–1316, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 9.National Kidney Foundation: DOQI Clinical Practice Guidelines for the Treatment of Anemia of Chronic Renal Failure. Am J Kidney Dis 30[Suppl 3]: S194–S240, 1997 [PubMed] [Google Scholar]
- 10.Fishbane S, Berns JS: Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int 68: 1337–1343, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Ebben JP, Gilbertson DT, Foley RN, Collins AJ: Hemoglobin level variability: Associations with comorbidity, intercurrent events, and hospitalizations. Clin J Am Soc Nephrol 1: 1205–1210, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Lacson E Jr, Ofsthun N, Lazarus JM: Effect of variability in anemia management on hemoglobin outcomes in ESRD. Am J Kidney Dis 41: 111–124, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Carlin BP, Louis TA. Bayes and Empirical Bayes Methods for Data Analysis. New York, Chapman and Hall, 1996
- 14.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D; CHOIR Investigators: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 16;355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A; CREATE Investigators: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 16: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]