Abstract
Background and objectives: Vascular calcification and endothelial dysfunction contribute to the development of cardiovascular disease in patients with chronic kidney disease (CKD). Sevelamer, a non–calcium-based phosphate binder, has been shown to attenuate cardiovascular calcification in CKD patients, although the exact mechanism has not been clarified. This study was designed to investigate the effect of short-term sevelamer treatment on both serum fetuin-A concentrations and endothelial dysfunction seen in CKD patients.
Design, setting, participants, & measurements: Fifty nondiabetic stage 4 CKD patients whose phosphate levels were ≥5.5 mg/dl were enrolled in this 8-wk randomized prospective study. Thirty-six healthy volunteers served as matched controls. Patients were treated with either sevelamer (n = 25, 12 males) or calcium acetate (n = 25, 13 males). Fetuin-A, high-sensitivity C-reactive protein, Ca × PO4 product, flow-mediated dilation (FMD), insulin, and homeostasis model assessment (HOMA) were obtained at baseline and after the treatment period.
Results: As expected, CKD patients had significantly lower levels of fetuin-A and FMD, and significantly higher levels of intact parathyroid hormone, Ca × PO4 product, and high-sensitivity C-reactive protein than controls (P < 0.001 for all). The use of sevelamer led to a significant increase in the fetuin-A concentration with improvement in FMD, whereas no significant difference was observed in the calcium acetate group. In a multiple regression analysis, FMD levels were independently related to fetuin-A both before (β = 0.63, P < 0.001) and after (β = 0.38, P = 0.004) treatment.
Conclusions: This small, randomized, prospective study shows that short-term sevelamer treatment significantly increases fetuin-A levels and improves FMD in nondiabetic stage 4 CKD patients.
Cardiovascular disease (CVD) is prevalent in patients with chronic kidney disease (CKD) (1). Strong correlation between the derangement in mineral metabolism, such as hyperphosphatemia, hyperparathyroidism, as well as elevated calcium × phosphorus product (Ca × PO4) and mortality has been reported in hemodialysis (HD) patients (2). These derangements have also been shown to result in vascular calcification, an independent risk factor for cardiovascular mortality (3,4). Elevated Ca × PO4 product and higher doses of oral calcium ingestion significantly predicted coronary artery calcification (CAC) in patients with end-stage kidney disease (5). Moreover, London et al. (4) have shown significant association between the uses of calcium-based phosphate binders and arterial medial calcification in HD patients. Based on the deleterious effect of high calcium intake on vascular calcification, a calcium-free nonabsorbed phosphate binder, sevelamer hydrochloride, has been developed for the treatment of hyperphosphatemia in CKD patients (6). Studies have shown that sevelamer provides effective control in serum PO4 levels without inducing hypercalcemia (6,7). Additionally, these studies have also shown beneficial effects of sevelamer on the progression of vascular calcification, although the underlying mechanisms were not clarified (8). Recent studies on vascular calcification have evaluated a number of circulating systemic calcification inhibitors, such as fetuin-A, matrix-Gla protein, osteoprotegrin, etc. (9). Fetuin-A, the major circulating inhibitor of vascular calcification, has been shown to be lower in dialysis patients and to be associated with cardiovascular mortality (10).
Recent data have shown a relationship between vascular calcification and endothelial dysfunction (ED) in vascular disease. Nigam et al. (11) showed that there was a significant correlation between ED and large conduit vessel stiffness in patients with coronary artery disease (CAD). Additionally, in 201 healthy subjects, Budoff et al. (12) evaluated the relation between arterial distensibility, arterial reactivity, and CAC scores (electron beam computed tomography). They showed a significant relationship between brachial artery reactivity and CAC. In accordance, Huang et al. (13) have also reported a significant relationship between CAC and ED in patients with suspected CAD.
This study was designed to investigate whether the suggested beneficial effects of sevelamer on vascular calcification are related to changes in serum fetuin-A concentration in patients with CKD. On the basis of the recent evidence linking vascular calcification and ED, we also evaluated the effect of sevelamer treatment on ED.
Concise Methods
Subjects
CKD stage 4 patients >18 yr of age and willing to participate to the study were screened. Those who had serum PO4 >5.5 mg/dl were evaluated for the study. Patients with diabetes mellitus, hypercalcemia (serum Ca >11 mg/dl), and history of CAD, and smokers and those taking statins or renin-angiotensin blockers were excluded because of the putative effect of these factors on ED. Of 62 screened patients, 50 met the study criteria and were included in this study. The primary renal diseases were glomerulonephritis in 12 patients (24%), hypertension in 11 patients (22%), polycystic kidney disease in 6 patients (12%), reflux nephropathy in 4 patients (8%), and unknown in 17 patients (34%). Sixteen of the patients were on antihypertensive therapy (9 patients were treated with calcium-channel antagonists, 2 patients with β-blocker agents, and 5 patients with loop diuretics). The study also recruited a control group comprising 36 healthy, unrelated subjects matched for age, sex, and body mass index by advertisement in the hospital of Gülhane School of Medicine in Ankara, Turkey. The ethical committee of Gülhane School of Medicine approved the study, and written informed consent was obtained from all patients.
Study Design
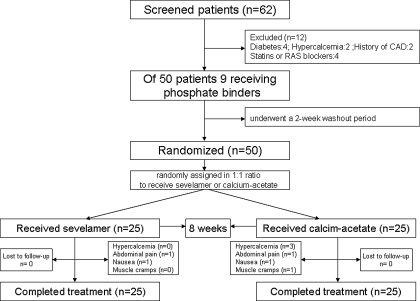
This was a randomized study conducted from 2005 through 2006 in Gülhane School of Medicine. The Outpatient Clinic of the Department of Nephrology is a tertiary referral center. At admission, most patients were untreated (including phosphate binders) or treated only with antihypertensive agents. After the first evaluation, 9 patients receiving phosphate binders underwent a 2-wk washout period. Patients who developed a phosphate level >5.5 mg/dl during this period were included in the study. Patients were randomly assigned in a 1:1 ratio to receive sevelamer (Renagel® capsule) or calcium acetate (PhosEx® tablet) (Figure 1). The treatment phase was 8 wk. During the study period, serum calcium and phosphorus concentration were measured every 2 wk and the dose of phosphate binders were titrated to achieve a serum phosphorus concentration <5.5 mg/dl. The starting dose for sevelamer was two capsules (800 mg) three times a day and for calcium acetate one tablet (1000 mg) three times a day. The average dose of sevelamer treatment was two tablets three times a day (4800 mg/d). The medications were given with meals and the doses were increased as needed. Patients were not given calcitriol during the study period. If hypercalcemia (serum Ca > 11 mg/dl) occurred during the study, phosphate binder doses were decreased according to a fixed algorithm. The primary end point was defined as an increase in serum fetuin-A levels and FMD levels after 8 wk of treatment. Secondary end points were a decrease in PO4 levels and Ca × PO4 product in serum.
Figure 1.
Flow chart of patients enrolled in trial.
Fasting blood samples were taken before and after the study to measure serum creatinine, serum albumin, hs-CRP, insulin, intact parathyroid hormone (iPTH), lipid profile, and serum fetuin-A concentration. Flow-mediated dilation (FMD) was evaluated before and after the study.
Blood Chemistry
All samples were obtained from patients and controls between 8:00 and 8:30 a.m. after 12 h of fasting for measurement of fasting plasma glucose (FPG), serum albumin, total cholesterol, triglyceride, HDL cholesterol, and LDL cholesterol. Total plasma cholesterol, triglyceride, and HDL cholesterol were measured by the enzymatic colorimetric method with an Olympus AU 600 auto analyzer using reagents from Olympus Diagnostics, GmbH (Hamburg, Germany). LDL cholesterol was calculated with use of Friedewald's formula (14). Hemoglobin levels were measured with an automated analyzer (Abbott Cell-Dyn 4.000, Abbott Park, IL).
The serum basal insulin value was determined by the coated tube method (DPC-USA). An insulin resistance score Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) was computed by the following formula(15):
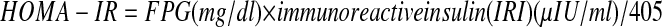 |
Serum total Ca was measured by the Cresolphtalein complexone method using Menagent Calcium 60-s kits (Menarini Diagnostics, Florence, Italy). Serum PO4 was measured by the ammonia molybdate complex method using Menagent Phosphofix kits (Menarini Diagnostics). iPTH was measured by IRMA, using kits (Immulite iPTH) from Diagnostic Product Corporation (Los Angeles, CA) with a sensitivity of 1 pg/ml.
Serum concentrations of fetuin-A (AHSG) were measured by using a human fetuin-A ELISA kit (BioVendor Laboratory Medicine, Inc., Brno, Czech Republic) in an ELISA plate reader (Synergy HT, Multidetection Multi-Plate Reader, Bio-tek Instruments, Inc., Winooski, VT). Interassay and intraassay coefficients of variations were <9%. For the measurement of hsCRP, serum samples were diluted with a ratio of 1/101 with the diluents solution. Calibrators, kit controls, and serum samples were all added on each microwell with an incubation period of 30 min. After three washing intervals, 100 μl enzyme conjugate (peroxidase-labeled anti-CRP) was added on each microwell for additional 15 min incubation in room temperature in dark. The reaction was stopped with a stop solution, and photometric measurement was performed at the 450-nm wavelength. The amount of serum samples was calculated as mg/L with a graphic that was made by noting the absorbance levels of the calibrators.
Estimated GFR (eGFR) was calculated according to the simplified version of the Modification of Diet in Renal Disease prediction equation formula [GFR = 186 × Pcr−1.154 × age−0.203 × 1.212 (if black) × 0.742 (if female)] was defined by Levey et al. (16).
Vascular Assessment
ED.
The determination of ED was performed according to Celermajer et al. (17). Measurements were made by a single observer using an ATL 5000 ultrasound system (Advanced Technology Laboratories, Inc., Bothell, WA) with a 12-Mhz probe. All vasoactive medications were withheld for 24 h before the procedure. The subjects remained at rest in the supine position for at least 15 min before the examination started. Each subject's arm was comfortably immobilized in the extended position to allow consistent recording of the brachial artery 2 to 4 cm above the antecubital fossa. Three adjacent measurements of end-diastolic brachial artery diameter were made from single two-dimensional frames. All ultrasound images were recorded on S-VHS videotape for subsequent blinded analysis. A pneumatic tourniquet was inflated to 200 mmHg with obliteration of the radial pulse. After 5 min the cuff was deflated. Flow measurements were made 60 seconds postdeflation. The maximum FMD diameters were calculated as the average of the three consecutive maximum diameter measurements. The FMD was then calculated as the percent change in diameter compared with baseline resting diameters. To assess the reproducibility of our technique, the same operator repeated brachial artery studies. The operator was blinded to the FMD result and medications remained unchanged between the initial and repeated scans.
Statistical Analyses
All statistical analyses were performed by using the SPSS 11.0 statistical package (SPSS, Inc., Chicago, IL). Non-normally distributed variables were expressed as median (range) and normally distributed variables were as mean±SD as appropriate. A P value <0.05 was considered to be statistically significant. One-sample Kolmogorov-Smirnov test was used for analysis distribution of data. One-way ANOVA, t test, and paired-sample t test were used to compare numeric data. Spearman rank correlation was used to determine correlations with continuous variables. Stepwise multivariate regression analysis was used to assess the predictors for FMD levels. Sample size was calculated by the Power and Sample Size V.2.0 program (Vanderbilt University, Department of Biostatistics, Free Software). The criteria for sample size calculation were as follows; 95% confidence intervals, 80% power, decrease in fetuin-A levels (0.1 g/L for sevalemer treatment group and 0.025 g/L for calcium acetate group, according to clinical experience). According to these criteria, 22 patients were to be recruited in each group. Considering the possibility of laboratory and other process mishaps, we decided to include 25 patients in each group.
Results
Basic Characteristics
Baseline clinical and laboratory characteristic as well as vascular measurements for the study population are shown in Table 1. There were no differences between CKD patients and controls with respect to age, sex, insulin levels, HOMA index, lipid profiles, and body mass index. Also, these variables were not significantly different between the sevelamer (n = 25) and calcium acetate (n = 25) groups. As expected, serum Ca, fetuin-A, and albumin concentrations and eGFR levels were lower and serum PO4, iPTH, Ca × PO4 product and hs-CRP levels were higher in CKD patients. FMD levels were lower in CKD patients than in controls. There were no significant basal differences in the sevelamer and calcium acetate groups’ treatment according to serum fetuin-A concentration and vascular measurements (Table 1).
Table 1.
Baseline clinical and laboratory characteristics of CKD 4 patients and controlsa
| Controls (n = 32) | Sevelamer (n = 25) | Calcium acetate (n = 25) | Pb | |
|---|---|---|---|---|
| Age (yr) | 43.9 ± 11.6 | 43.1 ± 12.6 | 43.6 ± 13.6 | NS |
| BMI (kg/m2) | 24.9 ± 2.1 | 24.9 ± 2.6 | 24.7 ± 2.4 | NS |
| Total cholesterol (mg/dl) | 182.7 ± 16.6 | 189.8 ± 18.8 | 190.1 ± 19.2 | NS |
| Triglycerides (mg/dl) | 134.6 ± 13.8 | 137.7 ± 12.4 | 128.7 ± 21.3 | NS |
| LDL cholesterol (mg/dl) | 114.0 ± 12.3 | 113.5 ± 16.0 | 117.8 ± 19.2 | NS |
| HDL cholesterol (mg/dl) | 41.4 ± 5.1 | 38.6 ± 5.9 | 38.9 ± 6.9 | NS |
| Hemoglobin (g/dl) | 14.0 (13.0 to 15.4) | 11.4 (10.5 to 13.5) | 11.0 (10.2 to 13.4) | <0.05 |
| SBP (mmHg) | 127 ± 7 | 132 ± 7 | 129 ± 8 | <0.05 |
| DBP (mmHg) | 81 ± 3 | 83 ± 5 | 82 ± 4 | NS |
| eGFR (ml/min) | 111 (95 to 126) | 25 (17 to 30) | 24 (15 to 29) | <0.001 |
| HOMA-IR | 1.24 ± 0.33 | 1.34 ± 0.34 | 1.31 ± 0.31 | NS |
| Serum Ca (mg/dl) | 9.1 ± 0.5 | 8.1 ± 0.4 | 8.0 ± 0.4 | <0.001 |
| Serum PO4 (mg/dl) | 3.6 ± 0.4 | 7.8 ± 0.6 | 7.8 ± 0.7 | <0.001 |
| Ca × PO4 product | 34 (23 to 41) | 62 (54 to 73) | 63 (46 to 71) | <0.001 |
| Serum albumin (g/dl) | 4.2 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.3 | <0.01 |
| iPTH (pg/ml) | 42 (21 to 65) | 155 (120 to 183) | 148 (148 to 186) | <0.001 |
| hs-CRP (mg/L) | 2 (1 to 4) | 15 (9 to 18) | 14 (10 to 24) | <0.001 |
| FMD (%) | 8.8 (7.3 to 12.4) | 6.0 (4.3 to 6.8) | 5.8 (4.7 to 7.0) | <0.001 |
| Fetuin-A (g/L) | 0.40 (0.34 to 0.46) | 0.27 (0.23 to 0.34) | 0.27 (0.24 to 0.31) | <0.001 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FMD, flow-mediated dilation; FPG, fasting plasma glucose; HOMA-IR, homeostasis model assessment-insulin resistance; eGFR, estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; iPTH, intact parathyroid hormone; NS, not significant. Data are means± SD or median.
P values determined by t test.
Effects of Sevelamer on Fetuin-A Concentrations and FMD Levels
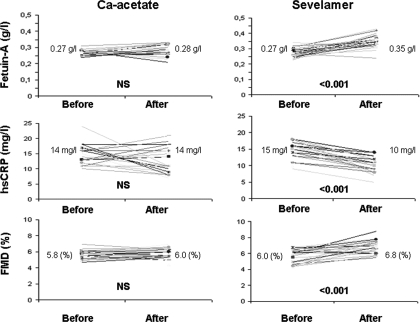
Whereas median serum fetuin-A concentration increased significantly in the sevelamer-treated group (0.27 g/L to 0.35 g/L, P < 0.001) no significant difference (0.27 g/L to 0.28 g/L) was observed in the calcium acetate–treated group (Table 2). Figure 2 shows the effects of sevelamer and calcium acetate treatment on serum fetuin-A, FMD, and hs-CRP levels. The mean change in serum fetuin-A concentration was 0.07 ± 0.04 g/L in the sevelamer-treated group. Thus, at the end of the 8-wk treatment period, there was a statistically significant (P < 0.001) difference in fetuin-A between the sevelamer- and calcium acetate–treated groups. In parallel, whereas FMD significantly improved in the sevelamer-treated group (5.7 ± 0.8% to 6.7 ± 0.8%, P < 0.001), no significant change was observed in the calcium acetate–treated group (5.7 ± 0.4% to 5.7 ± 0.6%) (Table 3).
Table 2.
Comparison of the effects of sevelamer and calcium acetate on the parametera
| Sevelamer (n = 25)
|
Calcium Acetate (n = 25)
|
|||
|---|---|---|---|---|
| BT | AT | BT | AT | |
| Fetuin-A (g/L) | 0.27 (0.23 to 0.34) | 0.35 (0.24 to 0.43)b | 0.27 (0.24 to 0.31) | 0.28 (0.21 to 0.33) |
| FMD (%) | 6.0 (4.3 to 6.8) | 6.8 (5.5 to 8.8)b | 5.8 (4.7 to 7.0) | 6.0 (4.4 to 7.0) |
| LDL cholesterol (mg/dl) | 113.5 ± 16.0 | 103.7 ± 17.0b | 117.8 ± 19.2 | 123.2 ± 15.8 |
| Serum albumin (g/dl) | 3.9 ± 0.3 | 4.0 ± 0.4 | 3.9 ± 0.3 | 3.8 ± 0.3 |
| hsCRP (mg/L) | 15 (9 to 18) | 10 (5 to 14)b | 14 (10 to 24) | 14 (7 to 21) |
| iPTH (pg/ml) | 155 (120 to 183) | 164 (117 to 232)b | 148 (148 to 186) | 176 (123 to 253)b |
| Serum Ca (mg/dl) | 8.1 ± 0.4 | 7.9 ± 0.5 | 8.0 ± 0.4 | 8.2 ± 0.3 |
| Serum PO4(mg/dl) | 7.8 ± 0.6 | 5.9 ± 0.9b | 7.8 ± 0.7 | 6.0 ± 0.7b |
| Ca × PO4 product | 62 (54 to 73) | 48.0 (29.6 to 59.5)b | 63 (46 to 71) | 48.0 (39.1 to 60.2)b |
Data are mean± SD and median. Paired samples t test.
P < 0.001
Figure 2.
Fetuin-A, high-sensitivity C-reactive protein (hsCRP), and flow-mediated dilation (FMD) levels in calcium acetate and sevelamer groups before and after 8 wk of treatment.
Table 3.
Changes of important parameters after phosphate binding with sevelamer and calcium acetate over 8 wk
| Sevelamer | Calcium Acetate | P | |
|---|---|---|---|
| Fetuin-A (g/L) | 0.07 ± 0.04 | 0.01 ± 0.03 | <0.001 |
| FMD (%) | 1.1 ± 0.9 | 0.01 ± 0.55 | <0.001 |
| hs-CRP (mg/L) | −4 (−11 to 0) | −1 (−13 to 6) | 0.002 |
| iPTH (pg/ml) | 9.8 ± 23.2 | 33.2 ± 33.3 | 0.003 |
| Serum Ca (mg/dl) | −0.06 ± 0.58 | 0.17 ± 0.56 | NS |
| Serum PO4 (mg/dl) | −1.9 ± 0.8 | −1.8 ± 0.7 | NS |
| Ca × PO4 product | −15.6 ± 8.8 | −13.2 ± 7.7 | NS |
At the end of the study period there were no significant differences in serum albumin, Ca, or PO4 levels with respect to binder assignment (Table 2). However, although not statistically significant, serum Ca levels and Ca × PO4 product were lower in the sevelamer-treated group than in the calcium acetate–treated group. In both treatment groups iPTH levels increased during the study period.
Baseline median hs-CRP levels did not differ between sevelamer- and calcium acetate–treated groups. However, whereas sevelamer therapy significantly decreased median hs-CRP levels (15 mg/L to 10 mg/L, P < 0.001), calcium acetate–based therapy had no significant effects on median hs-CRP levels (14 mg/L to 14 mg/L). Whereas sevelamer treatment significantly decreased LDL-cholesterol levels (113.5 ± 16.0 mg/dl to 103.7 ± 17.0, P < 0.05), calcium acetate–based therapy had no such effect (117.8 ± 19.2 mg/dl to 123.2 ± 15.8 mg/dl, P = NS).
Correlations
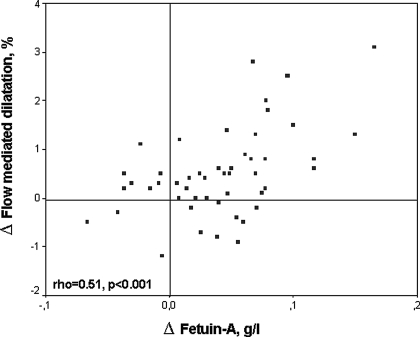
At baseline, FMD was positively correlated with serum fetuin-A levels and negatively correlated with hs-CRP levels. As expected, a negative correlation was observed between serum fetuin-A and hs-CRP levels (rho = −0.43, P = 0.002). The changes in FMD were positively correlated with basal serum fetuin-A levels (rho = 0.51, P < 0.001) (Figure 3). In parallel, the reduction in hs-CRP levels correlated with the changes in serum fetuin-A concentration (rho = 0.40, P = 0.005). A multiple regression model incorporating variables expected to influence FMD (sex, age, Ca × PO4 product, hs-CRP, and iPTH), as well as fetuin-A was performed both before and after treatment. The results showed that FMD levels were independently related to fetuin-A levels both before (P < 0.001) and after (P = 0.004) treatment (Table 4).
Figure 3.
Scatter plot shows the significant positive relationship between the change in fetuin-A and FMD.
Table 4.
Analysis of association between FMD and some different parameters by univariate and multivariate linear regression both before and after treatment
| Univariate β (P) | Multivariate β (P) | |
|---|---|---|
| Before treatment | ||
| Fetuin A (g/L) | 0.65 (<0.001) | 0.63 (<0.001) |
| Ca × PO4 product | −0.12 (NS) | NS |
| iPTH (pg/ml) | −0.02 (NS) | NS |
| hs-CRP (mg/L) | −0.17 (NS) | NS |
| Age (yr) | 0.03 (NS) | NS |
| Sex | 0.07 (NS) | NS |
| After treatment | ||
| Fetuin A (g/L) | 0.48 (0.001) | 0.38 (0.004) |
| Ca × PO4 product | −0.10 (NS) | NS |
| iPTH (pg/ml) | −0.30 (0.04) | NS |
| hs-CRP (mg/L) | −0.39 (0.005) | NS |
| Age (yr) | 0.24 (NS) | NS |
| Sex | 0.03 (NS) | NS |
Whereas three patients experienced hypercalcemic episodes during treatment with calcium acetate, no patient experienced hypercalcemia with sevelamer. Some patients experienced abdominal pain (n = 1 for calcium acetate; n = 1 for sevelamer), nausea (n = 1 for calcium acetate; n = 1 for sevelamer) and muscle cramps (n = 1 for calcium acetate; n = 0 for sevelamer) during the study period. No patients withdrew from the study as a result of adverse events.
Discussion
This randomized controlled study shows that short-term (8 wk) sevelamer treatment significantly increases serum fetuin-A concentration in CKD stage 4 patients. In contrast, calcium acetate had no significant effect on fetuin-A levels during the observation period. This study also shows that ED improves in parallel with the increase in serum fetuin-A concentration in sevelamer-treated CKD patients. On the basis of the present observation one could speculate that an increment in fetuin-A during sevelamer treatment may be one underlying mechanism by which this drug attenuates progression of vascular calcification (18) and improves outcome (19) in CKD patients.
Fetuin-A, a circulating glycoprotein synthesized by liver cells, has been shown to be one of the major calcification inhibitors. Notably, fetuin-A also has several other properties, such as inhibition of TGF-β and impact on insulin sensitivity (20). Fetuin-A has been suggested to be responsible for approximately 50% of the precipitation inhibitory effect of serum PO4 (20). Recently, Westenfeld et al. (21) showed that fetuin-A deficiency, CKD, and a high-PO4 diet synergistically acts in the pathogenesis of extraosseous calcification in a mouse model. Thus, fetuin-A knockout mice have been shown to develop severe vascular and other tissue calcifications (22). Because vascular calcification is a common event even in young CKD patients (23) and is one of the predictors of cardiovascular mortality (3), several studies have examined the role of serum fetuin-A levels in various CKD populations. Our study shows that fetuin-A levels are significantly reduced in nondiabetic CKD stage 4 patients, which confirms previous studies in CKD stage 5 patients (10,24–26). In contrast, in 970 CAD patients with mild CKD, Ix et al. (27) reported that serum fetuin-A concentration was not reduced. In a cross-sectional study, Mehrotra et al. (28) reported that the significance of correlation between fetuin-A and GFR was lost after adjusting the data for serum albumin in nondialyzed patients with diabetic nephropathy. On the basis of these observations, it could be suggested that serum fetuin-A levels decline only late during the course of progression of patients with CKD.
The fact that low levels of fetuin-A are associated with vascular calcification (29) and mortality (10) in prevalent HD patients has resulted in a lot of interest in this calcification inhibitor. Subsequent studies show that a reduction in serum fetuin-A levels is associated with both all-cause and cardiovascular mortality in CKD stage 5 patients starting dialysis treatment (24) as well as prevalent continuous ambulatory peritoneal dialysis patients (25). These findings were recently corroborated by Hermans et al. (26) who demonstrated in prospective multicenter cohort study of 987 Dutch dialysis patients that low fetuin-A is a general predictor of mortality.
Vascular calcification is a common finding both in dialysis patients (30) and CKD patients not yet on dialysis therapy (31,32). Given the strong association between the development of vascular calcification and cardiovascular mortality in patients with CKD (3), elucidating this process seems to be very important. Chertow et al. (8) have shown that, compared with Ca-based PO4 binders, sevelamer attenuated the progression of coronary and aortic calcification in 200 HD patients. In accordance, Braun et al. (33) reported that HD patients on Ca-based therapy showed greater progression in coronary and aortic calcification than patients on sevelamer therapy. However, the exact mechanism(s) by which sevelamer attenuates the calcification process have not been elucidated. Although normalization of Ca and PO4 metabolism may be a major contributor, other beneficial effects of sevelamer, such as lipid reduction (34) and uric acid reduction (35), may also be of potential benefit. In addition, sevelamer may have beneficial effects on uremic toxin absorption, such as p-cresol (36). The mechanism(s) by which sevelamer therapy increased serum fetuin-A concentration in this study is not evident. However, because fetuin-A is a negative acute-phase reactant (37) and because we observed that sevelamer therapy was associated with a reduction in hs-CRP levels, a nonspecific antiinflammatory effect of sevelamer therapy may explain the observed increase in fetuin-A. Indeed, Ferramosca et al. (38) demonstrated that sevelamer treatment had antiinflammatory and potential antiatherogenic effects in HD patients. In addition, Phan et al. (39) showed that sevelamer prevented uremia-enhanced atherosclerosis in apolipoprotein E–deficient mice. Given the observed reduction in LDL cholesterol levels, it could also be speculated that favorable effects on uremic dyslipidemia may also contribute to less inflammation in sevelamer-treated patients. Finally, given the structure of the sevelamer molecule, nonspecific binding in the gut of one or several factors that inhibit fetuin-A synthesis could also theoretically contribute to the observed finding.
Another important observation in this study was the small but significant improvement in ED in sevelamer-treated patients. Notably, FMD increased in parallel with increased fetuin-A concentration, and in a multiple regression analysis fetuin-A was independently associated with FMD. In accordance, we have previously shown that the improvement in FMD after successful kidney transplantation was associated with increased fetuin-A levels (40). Taken together, because this study suggests that sevelamer has a beneficial effect on endothelial function, further mechanistic studies are needed to confirm this finding and elucidate potential pathophysiological mechanism(s).
The results of our study must be considered with the following caveats. First, the number of patients was limited and studies in larger populations are needed to confirm this finding. Second, because the observation period was short, additional studies with longer observation time are needed. Third, because diabetic patients and smokers were not included in this study, it should be emphasized that this is a selected group of CKD patient with a low degree of comorbidities. Thus, these findings cannot by generalized to the whole CKD patient population. Fourth, endothelial-independent function as measured by response to glyceryl trinitrate as alterations in calcification was not evaluated. Fifth, we should have measured pH or serum bicarbonate levels because metabolic acidosis could be exacerbated by sevelamer, which in turn could lead to increased nitric oxide production and improved endothelial function. Finally, it should be acknowledged that the observed effects on both inflammatory markers and endothelial function were modest and its relevance for cardiovascular morbidity and mortality is unknown.
In conclusion, this 8-wk, prospective, randomized trial shows that treatment with sevelamer (in contrast to calcium acetate) was associated with less inflammation, increased levels of the circulating inhibitor of vascular calcification fetuin-A, and an improvement of endothelial function in nondiabetic CKD stage 4 patients. Further studies are needed to investigate whether these effects, at least in part, could contribute to less vascular calcification and better outcomes in sevelamer-treated CKD patients.
Disclosures
B.L. is an employee of Baxter Healthcare, Inc. P.S. is a member of the scientific advisory board of Gambro AB.
Acknowledgments
M.I.Y. and J.J.C. were supported in performing the present study by Fellowships from the EDTA-ERA. Baxter Novum is the result of an unconditional grant to the Karolinska Institutet from Baxter Healthcare, Inc. The name of the registry is “Sevelamer, Fetuin-A and Endothelial Dysfunction in CKD,” and the clinical trial number is NCT00486772 (http://www.clinicaltrial.gov).
Published online ahead of print. Publication date available at www.cjasn.org.
K.C. and M.I.Y. contributed equally to this study.
References
- 1.Levey AS, Eknoyan G: Cardiovascular disease in chronic renal disease. Nephrol Dial Transplant 14: 828–833, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 4.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Burke SK, Dillon MA, Slatopolsky E: Long-term effects of sevelamer hydrochloride on the calcium × phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant 14: 2907–2914, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Bleyer AJ, Burke SK, Dillon M, Garrett B, Kant KS, Lynch D, Rahman SN, Schoenfeld P, Teitelbaum I, Zeig S, Slatopolsky E: A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis 33: 694–701, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Chertow GM, Burke SK, Raggi P: Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Schlieper G, Westenfeld R, Brandenburg V, Ketteler M: Inhibitors of calcification in blood and urine. Semin Dial 20: 113–121, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Bohm R, Metzger T, Wanner C, Jahnen-Dechent W, Floege J: Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: A cross-sectional study. Lancet 361: 827–833, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Nigam A, Mitchell GF, Lambert J, Tardif JC: Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol 92: 395–399, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Budoff MJ, Flores F, Tsai J, Frandsen T, Yamamoto H, Takasu J: Measures of brachial artery distensibility in relation to coronary calcification. Am J Hypertens 16: 350–355, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Huang PH, Chen LC, Leu HB, Ding PY, Chen JW, Wu TC, Lin SJ: Enhanced coronary calcification determined by electron beam CT is strongly related to endothelial dysfunction in patients with suspected coronary artery disease. Chest 128: 810–815, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE: Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Chertow GM: Slowing the progression of vascular calcification in hemodialysis. J Am Soc Nephrol 14: S310–S314, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM: Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 71: 438–441, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Ketteler M: Fetuin-A and extraosseous calcification in uremia. Curr Opin Nephrol Hypertens 14: 337–342, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Westenfeld R, Schafer C, Smeets R, Brandenburg VM, Floege J, Ketteler M, Jahnen-Dechent W: Fetuin-A (AHSG) prevents extraosseous calcification induced by uraemia and phosphate challenge in mice. Nephrol Dial Transplant 22: 1537–1546, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W: The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 112: 357–366, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman WG: Vascular calcification in chronic renal failure. Lancet 358: 1115–1116, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Stenvinkel P, Wang K, Qureshi AR, Axelsson J, Pecoits-Filho R, Gao P, Barany P, Lindholm B, Jogestrand T, Heimburger O, Holmes C, Schalling M, Nordfors L: Low fetuin-A levels are associated with cardiovascular death: Impact of variations in the gene encoding fetuin. Kidney Int 67: 2383–2392, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Wang AY, Woo J, Lam CW, Wang M, Chan IH, Gao P, Lui SF, Li PK, Sanderson JE: Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant 20: 1676–1685, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Hermans MM, Brandenburg V, Ketteler M, Kooman JP, van der Sande FM, Boeschoten EW, Leunissen KM, Krediet RT, Dekker FW: Association of serum fetuin-A levels with mortality in dialysis patients. Kidney Int 72: 202–207, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley MA: Fetuin-A and kidney function in persons with coronary artery disease: Data from the Heart and Soul Study. Nephrol Dial Transplant 21: 2144–2151, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehrotra R, Westenfeld R, Christenson P, Budoff M, Ipp E, Takasu J, Gupta A, Norris K, Ketteler M, Adler S: Serum fetuin-A in nondialyzed patients with diabetic nephropathy: Relationship with coronary artery calcification. Kidney Int 67: 1070–1077, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Moe SM, Reslerova M, Ketteler M, O’Neill K, Duan D, Koczman J, Westenfeld R, Jahnen-Dechent W, Chen NX: Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int 67: 2295–2304, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Kalpakian MA, Mehrotra R: Vascular calcification and disordered mineral metabolism in dialysis patients. Semin Dial 20: 139–143, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Kramer H, Toto R, Peshock R, Cooper R, Victor R: Association between chronic kidney disease and coronary artery calcification: The Dallas Heart Study. J Am Soc Nephrol 16: 507–513, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Tomiyama C, Higa A, Dalboni MA, Cendoroglo M, Draibe SA, Cuppari L, Carvalho AB, Neto EM, Canziani ME: The impact of traditional and non-traditional risk factors on coronary calcification in pre-dialysis patients. Nephrol Dial Transplant 21: 2464–2471, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Braun J, Asmus HG, Holzer H, Brunkhorst R, Krause R, Schulz W, Neumayer HH, Raggi P, Bommer J: Long-term comparison of a calcium-free phosphate binder and calcium carbonate–phosphorus metabolism and cardiovascular calcification. Clin Nephrol 62: 104–115, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Hervas JG, Prados D, Cerezo S: Treatment of hyperphosphatemia with sevelamer hydrochloride in hemodialysis patients: A comparison with calcium acetate. Kidney Int Suppl 85: S69–S72, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Garg JP, Chasan-Taber S, Blair A, Plone M, Bommer J, Raggi P, Chertow GM: Effects of sevelamer and calcium-based phosphate binders on uric acid concentrations in patients undergoing hemodialysis: A randomized clinical trial. Arthritis Rheum 52: 290–295, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Ketteler M: Kidney failure and the gut: p-Cresol and the dangers from within. Kidney Int 69: 952–953, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Lebreton JP, Joisel F, Raoult JP, Lannuzel B, Rogez JP, Humbert G: Serum concentration of human alpha 2 HS glycoprotein during the inflammatory process: Evidence that alpha 2 HS glycoprotein is a negative acute-phase reactant. J Clin Invest 64: 1118–1129, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferramosca E, Burke S, Chasan-Taber S, Ratti C, Chertow GM, Raggi P: Potential antiatherogenic and anti-inflammatory properties of sevelamer in maintenance hemodialysis patients. Am Heart J 149: 820–825, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Phan O, Ivanovski O, Nguyen-Khoa T, Mothu N, Angulo J, Westenfeld R, Ketteler M, Meert N, Maizel J, Nikolov IG, Vanholder R, Lacour B, Drueke TB, Massy ZA: Sevelamer prevents uremia-enhanced atherosclerosis progression in apolipoprotein E-deficient mice. Circulation 112: 2875–2882, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Caglar K, Yilmaz MI, Saglam M, Cakir E, Kilic S, Eyileten T, Sonmez A, Oguz Y, Oner K, Ors F, Vural A, Yenicesu M: Endothelial dysfunction and fetuin A levels before and after kidney transplantation. Transplantation 83: 392–397, 2007 [DOI] [PubMed] [Google Scholar]