Abstract
Background and objectives: In hemodialysis patients, the hematological response to erythropoietin (epo) is variable and clinical factors that explain this variability are incompletely understood. We tested the hypothesis that the variability in hemoglobin (Hgb) response (epo sensitivity) is determined by key nutritional, inflammation, and oxidative stress markers.
Design, setting, participants, & measurements: Eighty-two consecutive patients on hemodialysis had 3 consecutive monthly predialysis evaluations of Hgb, total white blood cell (WBC) count, serum albumin, malondialdehyde (MDA), and monocyte chemoattractant protein-1 (MCP1). We analyzed the time course of Hgb in relationship to serum albumin, WBC, MDA, MCP1, epo and iron administration, and tests of iron sufficiency in a linear growth curve model.
Results: Subjects with higher Hgb had a fall in Hgb and vice versa, regressing to a mean Hgb (SD) of 11.8 g/dl (1.8 g/dl). Whereas the average slope of Hgb was flat, the SD of slopes was 0.63 g/dl, which explained 39% of the variance in Hgb. Nonuse of epo was associated with a mean Hgb change of −0.18 g/dl (95% confidence interval [CI] −0.26 to −0.10) per 10,000 IU epo/mo (P < 0.05). Epo use was associated with steeper rate of change at 0.04 g/dl per mo per 10,000 IU (95% CI 0.01 to 0.07) (P < 0.01). Hgb at baseline was 0.73 g/dl higher for each 1-g/dl increase in albumin, and the rate of change increased by 0.49 g/dl per mo for each 1-g/dl increase in albumin concentration. WBC, MDA, or MCP1 had no role in predicting the baseline Hgb or its change over time.
Conclusions: Serum albumin concentration is an important predictor of both baseline Hgb and epo sensitivity in chronic hemodialysis patients. Factors that improve serum albumin may also improve Hgb in hemodialysis patients.
Parenteral iron and erythropoietin (epo) are the cornerstones of management of anemia in patients on hemodialysis. Recently concerns were raised by randomized controlled trials that high epo doses and higher target hemoglobin (Hgb) may increase mortality in patients with chronic kidney disease (1,2). There is thus a heightened interest in factors that may influence epo-responsiveness (3,4). Myriad factors that influence response to epo include, among the most well-studied, iron sufficiency, infectious and inflammatory disorders, chronic blood loss, adequacy of dialysis, and hyperparathyroidism (5). Among these factors, the broad category of inflammatory disorders is thought to play a major role in predicting epo sensitivity. Although obvious infections and inflammatory states are easy to diagnose, events such as infections in thrombosed arteriovenous graft or vascular inflammation—the so-called subclinical inflammatory events—may elude ready detection.
Sensitive markers of inflammation are available and have been associated with responsiveness to epo; however, they are not part of the panel of tests that are routinely ordered on a monthly basis in dialysis patients (6). Total white blood cell (WBC) count and serum albumin concentration that are routinely measured by all dialysis facilities on a monthly basis have the ability to detect inflammation, albeit with less sensitivity. Although there is abundant evidence that hypoalbuminemia is associated with epo hyporesponsiveness, data are limited by the cross-sectional nature of these studies (7–9). Even when longitudinal designs are implemented, the analyses are limited to multiple cross-sectional associations missing the opportunity to analyze the dynamic nature or the time-dependent changes in Hgb and epo use. By allowing individuals patients to have their own Hgb trajectories, it is possible to evaluate the relationship of substantive predictors of Hgb trajectories. Furthermore, we can evaluate whether clinical and experimental biomarkers are similarly effective in predicting epo sensitivity or responsiveness in hemodialysis patients.
We hypothesized that baseline WBC count and serum albumin concentration and the changes in these clinical markers over time can predict the epo sensitivity in patients receiving hemodialysis. Furthermore, we hypothesized that experimental markers of oxidative stress and inflammation (i.e., malondialdehyde (MDA) and monocyte chemoattractant protein-1 (MCP1), respectively), and the changes in these markers over time can predict the epo sensitivity. We compared the clinical markers—WBC and albumin concentration—with experimental markers—plasma MDA and MCP1—in their utility in predicting epo sensitivity.
Concise Methods
A prospective observational cohort study in one dialysis unit was conducted over 3 mo. Consecutive dialysis patients dialyzed three times weekly who were willing to provide informed consent were the subject of the study. Hgb was maintained between 11 and 12 g/dl, ferritin concentration was maintained between 100 and 800 ng/ml, and transferrin saturation was maintained between 20% and 50% using intravenous iron or epo, which followed the standard guidelines at the time of the study.
Each dose of iron or epo was captured by study personnel prospectively. Monthly epo dose that was administered subcutaneously in all patients was calculated for each patient. Laboratory data were prospectively collected and recorded. In addition, predialysis blood was drawn into tubes containing ethylenediamine tetraacetic acid once a month for 4 mo and plasma frozen until analysis. Malondialdehyde was analyzed by high-performance liquid chromatography (10) and MCP1 by ELISA (11) as previously reported. Of the 89 patients who consented to participate, 7 patients had <3 Hgb measurements; these patients were excluded and the remaining 82 patients are part of this report. The study was approved by the Institutional Review Board and all patients gave their written informed consent.
Statistical Analyses
Change in Hgb from baseline over the 3 mo of the study was analyzed with a growth curve model. This model allows individual patients to have their own Hgb trajectories, accounts for the correlation among repeated measurements in the same patient, and is unaffected by randomly missing data. We explored the relationships between Hgb change and predictors of this change.
In this model, the level 1 change describes how each subject changes over time (intraindividual change). More explicitly, a straight-line change model for the individuals was used to model the Hgb:
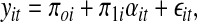 |
(1) |
where yit is the Hgb for the ith individual (i = 1, …, N) at the tth measurement occasion (t = 1, …, T), πoi, is the intercept for the ith individual, πli is the slope for the ith individual, ait represents the value of time for the ith individual at the tth measurement occasion, and ɛit is the error for the ith individual at the tth measurement occasion. ɛit was assumed to distribute normally and independently with a mean of zero and constant variance across time. The level 2 model describes how these change coefficients (i.e., πoi and πli) differ across people (interindividual change). The covariates of interest were used to model interindividual differences in intraindividual change. More explicitly, each of the change coefficients in Equation 1 are modeled as dependent variables in another equation:
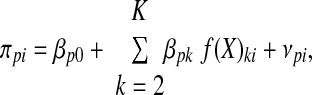 |
(2) |
where βp0 is the intercept for the pth change parameter, βpk is the regression coefficient for the kth covariate, f(X)ki is some function of the Xki (possibly the identity function where no transformation of X occurs) and vpi represents the unique effect for the ith individual for the pth change coefficient. An unstructured covariance matrix was used to allow for intercepts and slopes to be determined by the data (12). The unstructured covariance matrix allows the slopes and intercepts to vary independently from each other. Some of the second level covariates tested were postdialysis weight, epo dose/mo, iron dose per mo, serum iron, ferritin, total iron binding capacity, serum albumin concentration, WBC, MDA, and MCP1. Because ferritin and MCP1 had skewed distributions, the data were log transformed. A “final” model was created to incorporate the relevant predictors. Model fit was evaluated by the −2 × log likelihood information criterion. We used a taxonomy of models to describe the optimization of the model fit (12,13). All analyses were performed using Stata version 9.2 using standard procedures and significance was set at two-sided P value of <0.05 (StataCorp, College Station, TX).
Results
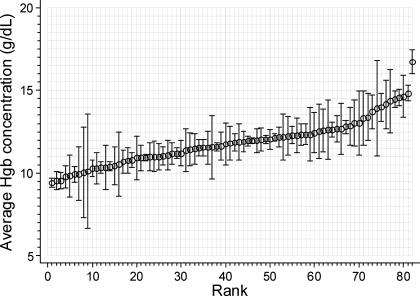
The baseline characteristics of the sample are shown in Table 1 and the relevant hematological tests averaged over 3 mo are shown in Table 2. Hgb level had slightly lower SD within subjects than between subjects, which yielded an intraclass correlation coefficient (ICC) of 0.61. The ICC was similar to that seen in serum albumin. ICC for serum ferritin and total iron binding capacity were high, but that of serum iron was low. WBC had higher ICC compared with MDA or MCP1. Figure 1 shows the variability in Hgb as a function of increasing mean Hgb. It appears that the variability follows a U-shaped relationship. Those with lower or higher levels of Hgb appear to have greater variability compared with those with average Hgb.
Table 1.
Baseline characteristics
| Clinical Characteristic | Result |
|---|---|
| n | 82 |
| Age (yr) | 53.3 ± 12.6 |
| Men | 42 (51%) |
| Race | |
| white | 27 (33%) |
| black | 52 (64%) |
| other | 2 (2%) |
| Predialysis weight (kg) | 77.0 ± 23.8 |
| Postdialysis weight (kg) | 75.6 ± 20.5 |
| Years of ESRD | 5.7 ± 5.9 |
| Etiology of ESRD | |
| diabetes mellitus | 21 (26%) |
| hypertension | 18 (22%) |
| others | 43 (52%) |
| Access type | |
| fistula | 22 (27%) |
| graft | 30 (37%) |
| catheter | 29 (36%) |
| Duration of dialysis (min) | 230 ± 35 |
| Blood volume processed (L) | 92.8 ± 16.7 |
| Urea reduction ratio (%) | 73.6 ± 9 |
Values are mean ± SD or number (percent of total).
Table 2.
Hematological characteristics
| Hematological and Laboratory Variables | Overall | Between SD | Within SD | ICC |
|---|---|---|---|---|
| Hemoglobin (g/dl) | 11.8 ± 1.8 | 1.4 | 1.13 | 0.61 |
| Serum iron | 75 ± 132 | 93 | 92 | 0.51 |
| Total iron binding capacity | 304 ± 56 | 51 | 27 | 0.78 |
| Serum ferritin | 615 ± 524 | 492 | 203 | 0.85 |
| Total leukocyte count (n × 1000/μl) | 7.6 ± 3.1 | 2.7 | 1.6 | 0.74 |
| Albumin (g/dl) | 3.8 ± 0.40 | 0.32 | 0.24 | 0.64 |
| MCP1 (ng/ml) | 97.6 ± 59.0 | 40.8 | 42.4 | 0.48 |
| Malondialdehyde (μmol/L) | 3.1 ± 2.2 | 1.4 | 1.8 | 0.38 |
Between SD, SD between patients; within SD, SD within individual patients; ICC, intraclass correlation coefficient or within-patient correlation; MCP1, monocyte chemoattractant protein 1. ICC directly measures the closeness of observations within patient relative to observations between patients.
Figure 1.
Rank-ordered variability in Hgb within individuals. Average Hgb concentration is plotted in rank order. The error bars indicate SD within individuals. A greater variability seems to occur at extremes of Hgb. Hgb, hemoglobin.
The average (±SD) epo dose was 40,718 ± 37,345 IU/mo, and the average (±SD) dose of iron was 191 ± 300 mg/mo. Although iron and epo use followed the guidelines, patients could switch categories over time.
Mean Hgb Level and Its Variability
Table 3 shows the development of the models of Hgb variation. Model 1, the so-called unconditional means model, which ignores the effect of time, found a mean Hgb level of 11.8 g/dl. The unconditional means model can be thought of average Hgb between patients calculated as the average of the within-patient between-month Hgb. Including the effect of time (Model 2), which allows the intercepts to vary between subjects but the slopes to remain constant, considerably improved the model fit and explained 27% of the variation in Hgb. However, when we allowed the slopes and intercepts to vary independently of each other (Model 3, an unstructured covariance model), the model fit was even further improved. Model 3 could account for 39% of the variation in Hgb. Incorporating epo dose (Model 4) and the interaction of epo dose with time (Model 5) improved model fit further. Note that Model 5 represents a model of epo sensitivity because the time-varying relationship of epo dose and Hgb response is accounted for. All subsequent models used Model 5 to explore the effects of predictors.
Table 3.
Taxonomy of models for hemoglobin variation
| Parameter | Model Number
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Fixed Effect Parameters | Means Only | Straight Line (Covariance: Identity) | Straight Line (Covariance: Unstructured) | Straight Line (Covariance: Unstructured) | Straight Line (Covariance: Unstructured) |
| Intercept | 11.8 [11.5, 12.1]a | 11.8 [11.5, 12.1]a | 11.9 [11.6, 12.2]a | 12.5 [12.1, 12.9]a | 12.5 [12.1, 12.9]a |
| Epo dose | −0.15 [−0.22, − 0.08]a | −0.19 [−0.27, −0.11]a | |||
| Epo dose × timec | 0.03 [0.001, 0.06]b | ||||
| Random effect parameters | |||||
| SD Intercept | 1.22 | 1.29 | 1.63 | 1.51 | 1.52 |
| SD Time | NA | 0.45 | 0.63 | 0.59 | 0.58 |
| Corr (intercept, time) | NA | NA | −0.61 | −0.59 | −0.58 |
| SD Residual | 1.320 | 1.130 | 1.030 | 1.020 | 1.01 |
| Pseudo R2 | 0.267 | 0.391 | 0.403 | 0.415 | |
| LL | −593 | −587 | −578 | −569 | −566.8 |
| Model comparison | 2 vs. 1a | 3 vs. 2a | 4 vs. 3a | 5 vs 4b | |
P < 0.001;
P < 0.05.
Epo dose in 10,000 units/month.
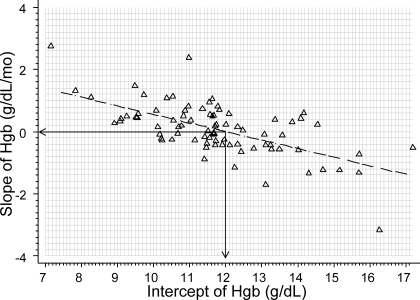
We found Hgb slope of 0.076 g/dl/mo that was clinically and statistically insignificant. It is not surprising that the intercepts of Hgb were significantly different between patients as were the slopes. More importantly, slope of Hgb change was inversely related to the intercept, which is regression to the mean (Figure 2). Figure 2 shows that the rise in Hgb over time was likely to be greater in those who had lower Hgb at baseline. In those with higher Hgb the slope of Hgb was likely to be negative. Thus, the Hgb was maintained at about 11.8 g/dl over the 3 mo of the study.
Figure 2.
Inverse relationship between individual Hgb trajectories and intercepts. Slopes and intercept for each patient was calculated by ordinary least-squares regression. The inverse relationship between the slopes and intercepts demonstrates the phenomenon of regression to the mean is supported by model 3 in Table 4 that indicates an inverse relationship between intercept and slopes.
Influence of Biomarkers
In a model with all parameters incorporated, we found that only a few predictors were statistically significant (Table 4). We also tested the interaction effect of epo × iron and found no statistical significance. In the final model we found that after accounting for epo dose the only factor that played an important role in explaining Hgb variation was serum albumin. Serum albumin concentration was related to the baseline Hgb concentration and its change over time. The mean Hgb concentration was 12.6 g/dl at an estimated albumin concentration of 4 g/dl and was 0.73 g/dl higher with each 1-g/dl increase in albumin (P = 0.01). The rate of change in Hgb was increased by 0.49 g/dl per mo for each 1-g/dl per mo increase in albumin concentration (P < 0.01). WBC count had no independent role in predicting the baseline Hgb or its change over time.
Table 4.
Full model and final model for hemoglobin variation
| Parameter | Model with All Parameters
|
Final Model
|
||||
|---|---|---|---|---|---|---|
| Coef. | 95% CI | P | Coef. | 95% CI | P | |
| Epo dose (per 10,000 units/month) | −0.18 | −0.28 to −0.09 | <0.0001 | −0.18 | −0.26 to −0.10 | <0.0001 |
| Epo dose × time | 0.05 | 0.01 to 0.10 | 0.01 | 0.04 | 0.01 to 0.07 | <0.01 |
| Serum albumin (g/dl) | 0.78 | 0.15 to 1.41 | 0.02 | 0.73 | 0.18 to 1.28 | 0.01 |
| Albumin × time | 0.41 | 0.03 to 0.79 | 0.03 | 0.49 | 0.14 to 0.84 | <0.01 |
| WBC count (n/1000/ml) | 0.12 | 0.01 to 0.23 | 0.03 | |||
| Iron dose (per 100 mg/month) | −0.04 | −0.13 to 0.04 | 0.3 | |||
| Iron dose × time | 0 | −0.00 to 0.00 | 0.1 | |||
| Post dialysis weight (kg) | −0.01 | −0.03 to 0.00 | 0.1 | |||
| WBC count × time | −0.03 | −0.08 to 0.02 | 0.2 | |||
| Plasma malonaldehyde (mmol/L) | 0.02 | −0.09 to 0.13 | 0.7 | |||
| Malonaldehyde × time | 0.01 | −0.04 to 0.07 | 0.6 | |||
| log Plasma MCP1 (ng/ml) | 0.07 | −0.31 to 0.45 | 0.7 | |||
| log Plasma MCP1 × time | −0.14 | −0.32 to 0.05 | 0.2 | |||
| Serum iron | 0 | −0.00 to 0.00 | 0.5 | |||
| Serum iron × time | 0 | −0.00 to 0.00 | 0.7 | |||
| Log serum ferritin | −0.31 | −0.69 to 0.06 | 0.1 | |||
| Log ferritin × time | 0.1 | −0.02 to 0.22 | 0.1 | |||
| Total iron binding capacity | 0 | −0.01 to 0.01 | 0.9 | |||
| Total iron binding capacity × time | 0 | −0.00 to 0.00 | 0.9 | |||
| Constant | 14.82 | 11.40 to 18.23 | <0.0001 | 12.6 | 12.2 to 13.0 | <0.0001 |
| Random Effects | ||||||
| SD Intercept | 0.6 | 1.53 | ||||
| SD Time | 1.63 | 0.59 | ||||
| Corr (intercept, time) | −0.49 | −0.46 | ||||
| SD Residual | 0.76 | 0.84 | ||||
| LL | −442 | −519.8 | ||||
Albumin is centered at 4 g/dl and white blood cell (WBC) count at 4000/μl.
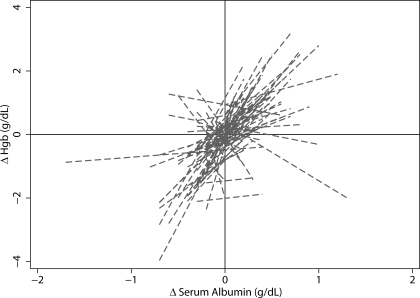
Figure 3 plots the relationship of change in Hgb from month to month with the change in serum albumin from month to month. Each line represents an individual patient. In general, a direct relationship is seen for most patients as is evident from positive slopes.
Figure 3.
Direct relationship between change in albumin and change in Hgb. Individual slopes of the relationship between change in albumin and change in Hgb were calculated by ordinary least-squares regression. A positive slope in most patients illustrates the positive coefficient on the albumin × time interaction term in the full model.
Discussion
This study found a significant interindividual variation in Hgb levels at baseline and over time. Furthermore, the rate of change in Hgb was inversely related to baseline Hgb. The latter phenomenon of regression to the mean to a set point of about 12 g/dl probably reflects appropriate management of anemia to control Hgb within a narrow range in the dialysis unit. The major finding of our study is that albumin is an important predictor of baseline Hgb and epo sensitivity in a representative sample of chronic hemodialysis patients. Important negative findings in our study are that no independent relationships between WBC count, MDA, and MCP-1 levels and epo sensitivity were found.
Several studies have demonstrated that iron is required to maintain effective anemia repair in patients who receive epo (14). Somewhat surprising, we did not find an independent effect of intravenous iron administration on Hgb. This may be caused by several reasons. Whereas epo was used 80% of the time in 82% of the patients, iron was used only 40% of the time in 60% of the patients. This discrepancy in epo and iron use may be detrimental to effective anemia repair. Continuous iron delivery, such as through the dialysate, has resulted in reduction of epo needs and effective Hgb synthesis (15,16). Recent data suggest that oral iron may be effective in hemodialysis patients (17). We did not record the use of oral iron in our study. The small size of the study may have missed an effect of iron even if one was present.
Our data support the poor value of the so-called iron deficiency markers in predicting Hgb response. Although the reproducibility of transferrin and ferritin was high within individuals, these tests were poor markers of Hgb change. Multiple studies have found a similar poor relationship between markers of iron deficiency and Hgb response (18,19). In fact, in a recent study comparing intravenous iron administration to no additional iron in epo-treated patients, an increase in Hgb in hemodialysis patients occurred despite substantially elevated serum ferritin levels, and the utility of markers of iron stores to guide iron management in hemodialysis patients was found to be of little value (20,21).
The lack of association of markers of oxidative stress and inflammation with Hgb response was surprising. There are several explanations. First, there is a large variability in these markers from month to month; in fact the intraclass correlation coefficient for both MCP1 and MDA were the lowest of any laboratory measurement. Second, the generation of MCP1 and MDA may be transient and countered by protective cytokines. Finally, an increase in C-reactive protein and a fall in serum albumin is thought to more accurately reflect an inflammatory response of moderate severity (22). In fact, an increase in C-reactive protein is strongly associated with blunted epo response (20).
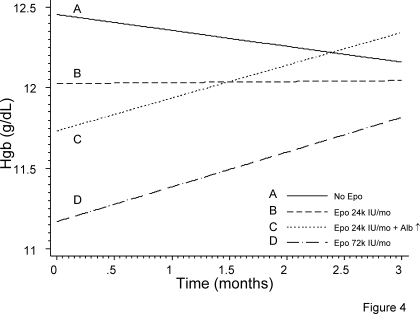
To appreciate the relative importance of epo and serum albumin on anemia management in the day-to-day practice, we have considered four different scenarios. Figure 4 shows the modeled relationship of Hgb to understand the relationship of epo, albumin and change in albumin on anemia management. When no epo is used (line A), our model shows that patients in general will have a small decline in Hgb over several months. An average monthly dose of 24,000 IU epo given subcutaneously will maintain a stable Hgb level (line B). In both of the situations mentioned above we have kept the albumin concentration constant at the average level of 3.8 g/dl seen in our sample. Line C demonstrates the trajectory of Hgb in a patient who is on a stable dose of 24,000 IU/mo epo but has serum albumin concentration of 3.4 g/dl, which increases 1 SD above the mean to 4.2 g/dl. Although low serum albumin is more likely associated with anemia, increase in serum albumin concentration is also associated with rapid improvement in anemia. Such a rate of anemia correction can only be realized by tripling the dose of epo as shown in slope D.
Figure 4.
Modeled relationship of Hgb trajectories, epo dose, and serum albumin concentration. No epo use is associated with Hgb decline (slope A). An average of 24,000 units in a patient with average and stable albumin is required to maintain Hgb (slope B). When serum albumin concentration is 1 SD below average, anemia is likely. Increase in serum albumin to SD above average is associated with rapid Hgb response (slope C). This slope is similar to that achieved by tripling the dose in the average patient (slope D).
Why was albumin such a strong marker of Hgb at baseline and time-dependent change in Hgb? Serum albumin is an excellent marker of overall health of dialysis patients. In those patients where serum albumin concentration is high, Hgb also appears to be high and vice versa. Albumin increase over time was associated with an increase in Hgb. Albumin increase over time may reflect improving infection, inflammation, oxidative stress, nutrition, and overall health (23). Furthermore, albumin is likely a surrogate for some common stimuli that suppress both epo and albumin gene synthesis. For example, malnutrition and inflammation can cause hypoalbuminemia and also suppress erythropoeisis (24). Furthermore, the relationship of albumin to hemoglobin is not limited to dialysis patients; albumin concentration is strongly associated with anemia in patients admitted to the hospital with acute coronary syndromes (25). Finally, drugs that reduce inflammation, such as statins, are associated with reduced epo requirements (26). Accordingly, it should be little surprise that time-dependent change in albumin is associated with commensurate changes in Hgb concentration.
Could parallel changes in Hgb and albumin simply reflect volume status? The average albumin in our study was 3.8 g/dl. For the purposes of the analyses we centered the albumin at 4.0 g/dl. On the basis of our statistical model, 1 g/dl increase in albumin (25% increase) would be expected to raise Hgb by 0.49 g/dl from a baseline of 12.6 g/dl. The 95% CI of this change would be 0.14 to 0.84 g/dl. If the relationship between albumin and Hgb was simply the result of volume, Hgb would be expected to increase 25% from baseline or by 3.15 g/dl. This is clearly outside the confidence interval (0.14 to 0.84 g/dl) reported here. All samples were drawn predialysis, therefore minimizing the impact of large weight changes such as from before to after dialysis. Therefore, the volume hypothesis appears implausible.
The strengths of this study are the prospective collection of the data and the use of a mixed modeling approach to account for missing data. A limitation of our study is the single-center nature of the study with a relatively small number of patients. Thus, we are unable to detect small effect sizes such as those that may be present in WBC, MDA, and MCP-1. We only collected data on the total leukocyte count, instead of lymphocyte or neutrophil count that may have improved our ability to detect changes in nutrition and inflammation, respectively. Unmeasured factors such as destruction of young circulating red blood cells—neocytolysis—may also influence Hgb levels (27). We did not measure markers of plasma volume, which would be able to more definitively answer the question whether the Hgb–albumin relationship is simply based on volume.
In conclusion, our study provides evidence for serum albumin concentration to be an effective marker of epo responsiveness. In studies designed to measure epo responsiveness, inclusion of serum albumin concentration should serve an effective way to control for confounding by factors such as infection, inflammation, and malnutrition. Thus, investigators designing clinical trials on epo responsiveness may stratify patients on the basis of this biomarker. Because serum albumin is routinely measured at monthly intervals, it provides clinicians with a bedside tool to determine whether the fall in Hgb is caused by infection, inflammation, or other factors. For example, if no changes in serum albumin were seen despite a fall in Hgb, it may signal the need to investigate for blood loss or call for repletion of iron stores. In epo-treated patients, falling Hgb increases mortality risk and rising Hgb reduces mortality risk (28). Poor responders to epo who continue to receive high doses of epo have increased mortality (29). These observations may be accounted for by improvement or deterioration in patient's health. Thus, anemia per se may not be a cardiovascular risk factor. Confounding by overall patient health may help explain observations where more complete correction of anemia does not lead to improved outcomes in patients with CKD (1,2). Finally, understanding modifiable risk factors that may impact serum albumin concentration may improve our ability to alter the natural history of outcomes, which remains dismal in dialysis patients.
Disclosures
None.
Acknowledgments
The technical assistance of Jiao Liang BS is gratefully acknowledged.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Fishbane S, Berns JS: Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int 68: 1337–1343, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Berns JS, Elzein H, Lynn RI, Fishbane S, Meisels IS, DeOreo PB: Hemoglobin variability in epoetin-treated hemodialysis patients. Kidney Int 64: 1514–1521, 2003 [DOI] [PubMed] [Google Scholar]
- 5.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis 47: S11–S145, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Barany P, Divino Filho JC, Bergstrom J: High C-reactive protein is a strong predictor of resistance to erythropoietin in hemodialysis patients. Am J Kidney Dis 29: 565–568, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Gunnell J, Yeun JY, Depner TA, Kaysen GA: Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis 33: 63–72, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Locatelli F, Andrulli S, Memoli B, Maffei C, Del VL, Aterini S, De SW, Mandalari A, Brunori G, Amato M, Cianciaruso B, Zoccali C: Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol Dial Transplant 21: 991–998, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Portoles J, Lopez-Gomez JM, Aljama P: Anemia management and treatment response in patients on hemodialysis: The MAR study. J Nephrol 19: 352–360, 2006 [PubMed] [Google Scholar]
- 10.Agarwal R, Chase SD: Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci 775: 121–126, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R: Proinflammatory effects of iron sucrose in chronic kidney disease. Kidney Int 69: 1259–1263, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Singer JD: Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behavior Stat 24: 323–355, 1998 [Google Scholar]
- 13.Singer JD, Willett JB: Applied longitudinal data analysis modeling change and event occurrence. Oxford, Oxford University Press, 2003
- 14.Fishbane S: Iron supplementation in renal anemia. Semin Nephrol 26: 319–324, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Besarab A, Amin N, Ahsan M, Vogel SE, Zazuwa G, Frinak S, Zazra JJ, Anandan JV, Gupta A: Optimization of epoetin therapy with intravenous iron therapy in hemodialysis patients. J Am Soc Nephrol 11: 530–538, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Amin NB, Besarab A, Vogel SE, Divine GW, Yee J, Anandan JV: Dialysate iron therapy: Infusion of soluble ferric pyrophosphate via the dialysate during hemodialysis. Kidney Int 55: 1891–1898, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Lenga I, Lok C, Marticorena R, Hunter J, Dacouris N, Goldstein M: Role of oral iron in the management of long-term hemodialysis patients. Clin J Am Soc Nephrol 2: 688–693, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Tessitore N, Solero GP, Lippi G, Bassi A, Faccini GB, Bedogna V, Gammaro L, Brocco G, Restivo G, Bernich P, Lupo A, Maschio G: The role of iron status markers in predicting response to intravenous iron in haemodialysis patients on maintenance erythropoietin. Nephrol Dial Transplant 16: 1416–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Fishbane S, Galgano C, Langley RC Jr, Canfield W, Maesaka JK: Reticulocyte hemoglobin content in the evaluation of iron status of hemodialysis patients. Kidney Int 52: 217–222, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Singh AK, Coyne DW, Shapiro W, Rizkala AR: Predictors of the response to treatment in anemic hemodialysis patients with high serum ferritin and low transferrin saturation. Kidney Int 71: 1163–1171, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, Rizkala AR: Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: Results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol 18: 975–984, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Gabay C, Kushner I: Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340: 448–454, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Steinman TI: Serum albumin: Its significance in patients with ESRD. Semin Dial 13: 404–408, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Yeun JY, Kaysen GA: Factors influencing serum albumin in dialysis patients. Am J Kidney Dis 32: S118–S125, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Bindra K, Berry C, Rogers J, Stewart N, Watts M, Christie J, Cobbe SM, Eteiba H: Abnormal haemoglobin levels in acute coronary syndromes. QJM 99: 851–862, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Sirken G, Kung SC, Raja R: Decreased erythropoietin requirements in maintenance hemodialysis patients with statin therapy. ASAIO J 49: 422–425, 2003 [PubMed] [Google Scholar]
- 27.Alfrey CP, Fishbane S: Implications of neocytolysis for optimal management of anaemia in chronic kidney disease. Nephron Clin Pract 106: c149–c156, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, Greenland S, Kalantar-Zadeh K: Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol 17: 1181–1191, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Thamer M, Stefanik K, Kaufman J, Cotter DJ: Epoetin requirements predict mortality in hemodialysis patients. Am J Kidney Dis 44: 866–876, 2004 [PubMed] [Google Scholar]