Abstract
Background and objectives: The role of sodium bicarbonate in preventing contrast nephropathy needs to be evaluated in clinical settings.
Design, setting, participants, & measurements: We performed a retrospective cohort study at Mayo Clinic in Rochester, Minnesota, to assess the risk of contrast nephropathy associated with the use of sodium bicarbonate, N-acetylcysteine, and the combination of sodium bicarbonate with N-acetylcysteine from April 2004 to May 2005. Contrast nephropathy was defined as postexposure creatinine elevation of ≥25% or >0.5 mg/dl within 7 d of contrast exposure.
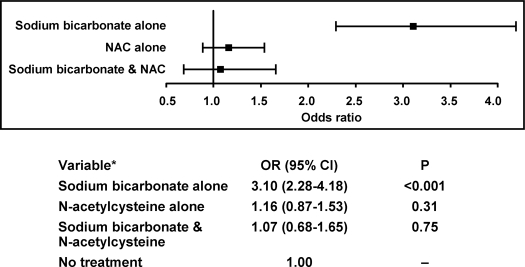
Results: A total of 11,516 contrast exposures in 7977 patients had creatinine values available for review before and after contrast exposure. More than 90% of exposures to agents prophylactic for contrast nephropathy were available for analysis. Sodium bicarbonate was used in 268 cases, N-acetylcysteine was used in 616 cases, and both agents were used in combination in 221 cases of contrast exposure. After adjustment for total volume of hydration, medications, age, gender, prior creatinine, contrast iodine load, prior exposure to contrast material, type of imaging study, heart failure, hypertension, renal failure, multiple myeloma, and diabetes mellitus, use of sodium bicarbonate alone was associated with an increased risk of contrast nephropathy compared with no treatment (odds ratio 3.10, 95% confidence interval 2.28 to 4.18; P < 0.001). N-acetylcysteine alone and in combination with sodium bicarbonate was not associated with any significant difference in the incidence of contrast nephropathy.
Conclusions: The use of intravenous sodium bicarbonate was associated with increased incidence of contrast nephropathy. Use of sodium bicarbonate to prevent contrast nephropathy should be evaluated further rather than adopted into clinical practice.
The increasing number of imaging procedures requiring intravenous contrast has triggered a parallel increase in the number of patients who are diagnosed with contrast nephropathy (CN), which is a leading cause of acute renal failure. The proportion of patients diagnosed with CN ranges from 3% to 16.5% and has been reported as high as 50% to 90% depending on population studied and the presence of coexisting diseases such as diabetes, choice of contrast agent, hydration, and medications (1–4). This is concerning because compromise of renal function accounts for significant increases in hospital morbidity, mortality, and length of stay, independent of comorbid disease (5,6).
Multiple strategies have been proposed to prevent CN (7). Randomized trials have suggested that N-acetylcysteine (NAC) (6,8–12) and sodium bicarbonate (13) may prevent CN, although there is controversy regarding routine usage (14–16). In addition to hydration, some randomized trials have shown that NAC prevents CN, whereas others have failed to demonstrate a benefit in CN prevention. In recent years, sodium bicarbonate has been proposed to prevent CN although the mechanism remains unclear (13). Some investigators have hypothesized that the combination of NAC and sodium bicarbonate may be superior to the use of either alone (11,13) because, as Merten and others have noted, NAC alone does not always prevent CN (13,17–19).
We hypothesized that in our actual practice cohort that sodium bicarbonate would be associated with a decreased risk of CN and that the combination of sodium bicarbonate and NAC would be associated with an incremental reduction of CN compared with the use of either agent alone.
Concise Methods
Study Population and Data Collection
After approval by the Mayo Foundation Institutional Review Board, all individuals who received intravenous contrast at Mayo Clinic Rochester from April 1, 2004, to May 30, 2005, were identified. Patients who underwent computerized tomography, noncardiac angiography, or plain film requiring intravenous contrast were identified by searching the radiology information system database that records all procedures performed in the Department of Radiology. Coronary angiography cases were obtained from the Mayo Clinic Catheterization Laboratory, which maintains a computerized registry of all coronary angiography procedures. Because previous reports suggested that CN may occur up to 1 wk after contrast exposure (1,2,7,20,21), we included cases of intravenous contrast administered to patients ≥17 yr old with at least one creatinine value available within 7 d pre- and postexposure. The most recent creatinine value before contrast exposure was used in the analyses unless otherwise stated. The highest creatinine value after the contrast exposure was used as the postexposure creatinine in the analyses. As described previously (13), patients with precontrast creatinine >8 mg/dl or history of dialysis were excluded. Cases where sodium bicarbonate was administered for continuous renal replacement therapy were excluded.
More than 96% of patients received low osmolar nonionic contrast. Contrast volume was collected for each administration. Contrast iodine load (grams) was calculated using the equation: iodine concentration (g/ml) × volume of contrast (ml). Contrast iodine load was available in 99% of cases.
Comorbid diseases were identified using a coding system maintained for record identification at Mayo Clinic as previously reported (22). In brief, the codes, which are not based on hospital billing codes, were assigned by coders primarily according to physician diagnoses for outpatient visits and from discharge diagnoses for hospitalizations and closely approximate the International Classification of Disease (Ninth Revision) codes. A diagnosis of renal disease included any history of renovascular disease, nephropathy, or hypertensive renal disease. Fluid administration was determined by finding the total of all fluids administered 1 d before and on the day of contrast exposure.
Medication administration was recorded using a computerized record system. Administration of intravenous sodium bicarbonate and or oral NAC was recorded if administered within 48 h of contrast exposure.
Because the administration of sodium bicarbonate for prevention of CN according to the Merten et al. protocol (13) is complex and variations of the protocol occur in clinical settings, we analyzed all administration to determine if patients received sodium bicarbonate as described by Merten et al. (i.e., per the “Merten et al. protocol group”: a solution of 150 mEq/L sodium bicarbonate administered on the day of exposure infused at an initial rate of 3 ± 0.5 ml/kg per h before contrast exposure and at 1 ± 0.25 ml/kg per h after contrast exposure), or if administration deviated from the Merten et al. protocol (the “deviated Merten et al. protocol group”).
Patients who received NAC were similarly divided into two groups: those who were given NAC on two consecutive days (per the “Tepel et al. protocol group”) (11) and those who were given NAC on one day only (the “deviated Tepel et al. protocol group”) (11).
β-Blockers, diuretics, nonsteroidal antiinflammatory agents, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aspirin therapies were determined from search of the electronic medical records up to 7 d before contrast administration.
Study End Point and Statistical Analyses
At the time of study design, before data were collected, we planned the following analyses. Similar to previous studies (13,21,23), the primary end point was CN, defined as an increase in serum creatinine of ≥25% or a creatinine increase of >0.5 mg/dl within 7 d of contrast administration. Each case of contrast administration was stratified according to CN prophylaxis treatment groups, specifically those who received sodium bicarbonate alone, NAC alone, both sodium bicarbonate and NAC, and neither sodium bicarbonate nor NAC (i.e., no treatment). Odds ratios (OR) were calculated using the SAS GENMOD procedure (SAS Institute Inc., Cary, NC). Each treatment group was modeled individually with reference to the no-treatment group. Results of the analyses were summarized as OR with 95% confidence intervals (CI). All analyses were performed by SAS version 9.1.3 (SAS Institute Inc.).
In the subsequent analyses, unless otherwise stated, the words “all covariates” are based on known and hypothesized predictors of CN (1,4,24) and include total volume of hydration, β-blocker, diuretic, nonsteroidal antiinflammatory drug, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker and aspirin therapies, age, gender, prior creatinine, contrast iodine load, prior exposure to contrast material, type of imaging study, heart failure, hypertension, renal failure, multiple myeloma, and diabetes mellitus.
Propensity scoring analysis performed as described previously (25,26) was done because patients who are given prophylaxis for CN may differ in important characteristics from those who do not receive prophylaxis even after adjustment for known and hypothesized predictors of CN.
Results
We identified 53,177 cases of contrast administration in 35,922 patients from the Radiology and Cardiac Catheterization Laboratory databases. Ninety-five cases were excluded because of pre-exposure creatinine values >8 mg/dl; 266 cases with history of dialysis were excluded, as were 29 cases where sodium bicarbonate was administered for continuous renal replacement therapy. Cases without pre- and postexposure creatinine assessments were excluded. Among the excluded cases the average age was 58 ± 17 yr, and 46% were male. A total of 1970 excluded cases (4.7% of all excluded cases) had prior creatinine values available; creatinine among these cases averaged 1.3 ± 0.9 mg/dl. Heart failure was present in 1392 (3.3%) cases, diabetes mellitus was present in 4151 (10.0%) cases, and hypertension was present in 12,777 (31.0%) cases.
After these exclusions, 11,516 cases of contrast exposure in 7977 patients had creatinine values available for review before and after contrast exposure. All cases using contrast prophylactic agents were identified before exclusion on the basis of available creatinine measurements. Of these, 616 of 662 administrations of NAC alone, 268 of 298 administrations of sodium bicarbonate alone, and 221 of 231 administrations of both sodium bicarbonate and NAC had pre- and postcontrast creatinine values available for analysis. Therefore, the case capture rates were 93% for NAC alone, 90% for sodium bicarbonate alone, and 96% for the concomitant administration of NAC and sodium bicarbonate. Sensitivity analysis vis-à-vis case capture rates was performed with adjustments for covariates and demonstrated no effect on the significance of the results. Neither sodium bicarbonate nor NAC (i.e., no treatment) were used in 10,411 episodes.
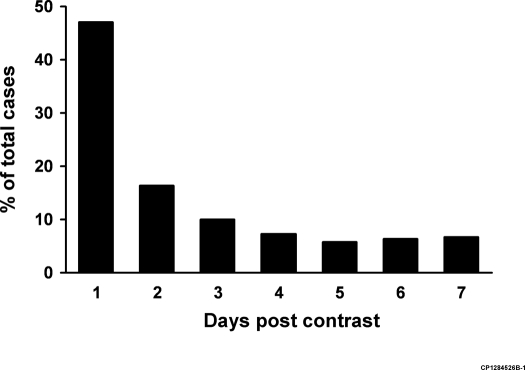
Table 1 shows baseline characteristics for all four treatment groups. On average the peak creatinine value was available 2.6 ± 2.0 d after contrast exposure with a median time of 2 d after contrast exposure (Figure 1).
Table 1.
Baseline characteristicsa
| Characteristic | No Treatment (n = 10,411) | Sodium Bicarbonate + NAC (n = 221) | NAC Alone (n = 616) | Sodium Bicarbonate Alone (n = 268) |
|---|---|---|---|---|
| Age, mean (SD) | 60.2 (17.1) | 70.9 (12.4) | 68.6 (14.5) | 64.4 (16.8) |
| Male, n (%)b | 5458 (52) | 125 (57) | 363 (59) | 149 (56) |
| White, n (%)b | 8844 (85) | 189 (86) | 532 (86) | 231 (86) |
| Black, n (%) | 123 (1) | 3 (1) | 13 (2) | 3 (1) |
| Iodine load, mean (SD), g | 37.6 (21.3) | 34.1 (9.1) | 35.5 (14.5) | 34.5 (11.2) |
| Volume of contrast, mean (SD), ml | 122.6 (68.8) | 109.0 (28.1) | 113.1 (44.0) | 110.9 (35.5) |
| Average prior creatinine, mean (SD), mg/dl | 1.05 (0.41) | 1.38 (0.58) | 1.29 (0.42) | 1.20 (0.64) |
| Total hydration, mean (SD), mlc | 3218 (2580) | 2517 (2810) | 2038 (2429) | 3005 (3998) |
| Any prior contrast exposure, n (%) | 3096 (30) | 106 (48) | 223 (36) | 114 (43) |
| Type of study | ||||
| computerized tomography, n (%) | 9126 (88) | 166 (75) | 438 (71) | 223 (83) |
| noncardiac angiography, n (%) | 572 (5) | 8 (4) | 54 (9) | 19 (7) |
| coronary catheterization, n (%) | 584 (6) | 47 (21) | 124 (20) | 26 (10) |
| plain film, n (%) | 129 (1) | 0 (0) | 0 (0) | 0 (0) |
| Comorbid diseases | ||||
| diabetes mellitus, n (%) | 1617 (16) | 74 (33) | 193 (31) | 49 (18) |
| hypertension, n (%) | 4230 (41) | 137 (62) | 402 (65) | 146 (54) |
| congestive heart failure, n (%) | 898 (9) | 54 (24) | 130 (21) | 46 (17) |
| renal disease, n (%) | 128 (1) | 10 (5) | 26 (4) | 10 (4) |
| multiple myeloma, n (%) | 138 (1) | 6 (3) | 14 (2) | 4 (1) |
| Medications | ||||
| ACE inhibitors, n (%) | 1796 (17) | 71 (32) | 196 (32) | 73 (27) |
| ARBs, n (%) | 757 (7) | 35 (16) | 84 (14) | 21 (8) |
| acetylsalicylic acid, n (%) | 3190 (31) | 106 (48) | 261 (42) | 106 (40) |
| β-blockers, n (%) | 2166 (21) | 101 (46) | 193 (31) | 98 (37) |
| diuretics, n (%) | 2512 (24) | 95 (43) | 274 (44) | 87 (32) |
| NSAIDs, n (%) | 1526 (15) | 11 (5) | 46 (7) | 27 (10) |
No Treatment, neither sodium bicarbonate nor N-acetylcysteine; NAC, N-acetylcysteine; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; NSAIDs, nonsteroidal antiinflammatory drugs.
Race was available in 90%, 91%, 90%, and 91% of the no treatment, sodium bicarbonate + NAC, NAC alone, and sodium bicarbonate along groups, respectively.
Total volume of hydration in the 24 h before contrast administration was available for 100% of the cases that received N-acetylcysteine, sodium bicarbonate, or both agents. Hydration information was available in 3785 (36%) of the No Treatment group because of the vast majority of outpatients in this group compared to the other three groups.
Figure 1.
Days postcontrast that peak creatinine occurred.
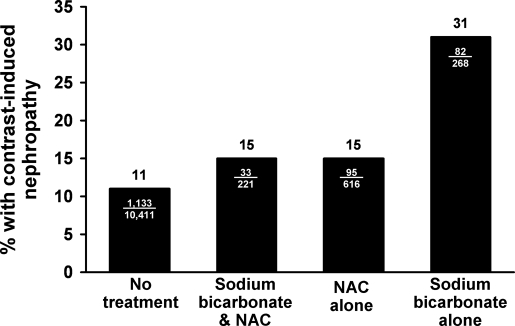
CN occurred in 1343 cases (11.7%) of all contrast administrations. The incidence of CN was increased compared with no treatment in all treatment groups among all patients, specifically: 11% in the no treatment group, 15% in the sodium bicarbonate with NAC group, 15% in the NAC alone group, and 31% in the sodium bicarbonate alone group (Figure 2).
Figure 2.
Contrast nephropathy according to treatment group. NAC, N-acetylcysteine.
Associations with CN
In this instance and for subsequent analyses, unless otherwise stated the words “all covariates” are based on known and hypothesized predictors of CN (1,4,24) and include total volume of hydration, β-blocker, diuretic, nonsteroidal antiinflammatory drug, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker and aspirin therapies, age, gender, prior creatinine, contrast iodine load, prior exposure to contrast material, type of imaging study, heart failure, hypertension, renal failure, multiple myeloma, and diabetes mellitus.
After adjustment for all covariates, sodium bicarbonate alone remained associated with an increase in CN of more than three-fold compared with no treatment (OR 3.10, 95% CI 2.28 to 4.18; P < 0.001). After adjustment for all covariates, NAC alone and in combination with sodium bicarbonate was not significantly associated with CN (Figure 3). Analysis using only one case per patient (the most recent case) revealed similar results.
Figure 3.
Association of treatment groups with contrast nephropathy adjusted for multiple risk factors. *Adjusted for total volume of hydration, β-blocker, diuretic, nonsteroidal antiinflammatory drugs, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and aspirin therapies, age, gender, prior creatinine, contrast iodine load, prior exposure to contrast material, type of imaging study, heart failure, hypertension, renal failure, multiple myeloma, and diabetes mellitus.
Use of sodium bicarbonate alone remained significantly associated with CN when compared with the NAC alone group after adjustment for all covariates (OR 2.73, 95% CI 1.86 to 3.97; P < 0.001). Because these results were surprising given our a priori hypotheses, we undertook a series of subgroup and secondary analyses in an attempt to further clarify the results.
Subgroup Analyses
Elevated Pretreatment Creatinine Values and Reduced Glomerular Filtration Rate.
In the study by Merten et al. (13), only cases where the preexposure creatinine was ≥1.1 mg/ml were included. We assessed our data similarly. Among these 5073 cases (4328 received no treatment,160 were treated with both sodium bicarbonate and NAC, 441 were treated with NAC only, and 144 were treated with sodium bicarbonate only), after adjusting for all covariates there was a significant increase in CN associated with sodium bicarbonate alone (OR 2.34, 95% CI 1.46 to 3.70; P < 0.001), but no significant association with NAC alone (OR 1.02, 95% CI 0.69 to 1.49; P = 0.92) or in combination with sodium bicarbonate (OR 1.19, 95% CI 0.69 to 2.07; P = 0.52).
GFR was estimated using the four-variable Modification of Diet and Renal Disease (MDRD) equation (27–29). This equation uses race (black or not) as a variable. Race was available in 90%, 91%, 90%, and 91% of the no treatment, sodium bicarbonate and NAC, NAC alone, and sodium bicarbonate alone groups, respectively. Sensitivity analysis vis-à-vis race in the MDRD equation revealed no effect on the significance of the results. Estimated GFR ranges corresponding to stages of chronic kidney disease were based on previously published guidelines (29). The distribution of cases in each stage of chronic kidney disease is shown in Table 2.
Table 2.
Assessments related to glomerular filtration rate stratified by treatment group
| Characteristic | No Treatment (n = 9,375) | Sodium Bicarbonate + NAC (n = 202) | NAC Alone (n = 553) | Sodium Bicarbonate Alone (n = 244) | P Value of Sodium Bicarbonate versus NAC |
|---|---|---|---|---|---|
| GFR,b mean (SD), ml/min per 1.73 m2 | 73.8 (23.3) | 55.4 (21.5) | 58.6 (22.6) | 68.5 (29.4) | <0.001 |
| Stages of kidney diseasea | |||||
| Stage 1, n (% total) | 1831 (20) | 10 (5) | 49 (9) | 46 (19) | |
| n CN (% stage) | 293 (16) | 2 (20) | 14 (29) | 23 (50) | 0.03 |
| Stage 2, n (% total) | 4995 (53) | 62 (31) | 152 (27) | 94 (39) | |
| n CN (% stage) | 425 (9) | 10 (16) | 22 (14) | 22 (23) | 0.05 |
| Stage 3, n (% total) | 2417 (26) | 120 (59) | 334 (60) | 90 (37) | |
| n CN (% stage) | 250 (10) | 16 (13) | 44 (13) | 21 (23) | 0.03 |
| Stage 4, n (% total) | 95 (1) | 8 (4) | 17 (3) | 10 (4) | |
| n CN (% stage) | 39 (41) | 1 (13) | 3 (18) | 5 (50) | 0.16 |
| Stage 5, n (% total) | 37 (0.4) | 2 (1) | 1 (0.2) | 4 (2) | |
| n CN (% stage) | 14 (38) | 0 (0) | 0 (0) | 2 (50) | 0.40 |
| CN related to decreased GFR | |||||
| ≥60 ml/min per 1.73 m2; OR (95% CI)c | 1.00 | 1.26 (0.59 to 2.45) | 1.58 (1.02 to 2.39) | 3.62 (2.38 to 5.41) | — |
| <60 ml/min per 1.73 m2; OR (95% CI)c | 1.00 | 1.27 (0.64 to 2.39) | 0.98 (0.62 to 1.54) | 2.64 (1.49 to 4.59) | — |
Stages 1 to 5 correspond to glomerular filtration rates of ≥90, 60 to 89, 30 to 59, 15 to 29, and <15 ml/min per 1.73 m2, respectively (9). CN, contrast nephropathy; OR, odds ratio.
GFR was estimated using the four-variable MDRD equation (9,17,27). This equation uses race (black or not) as a variable. Race was available in 90%, 91%, 90%, and 91% of the no treatment, sodium bicarbonate + NAC, NAC alone, and sodium bicarbonate along groups, respectively. It is for this reason that the number of patients (n) in each group differs from Table 1. Sensitivity analysis vis-à-vis race in the MDRD equation revealed no effect on the significance of the results.
Adjusted for total volume of hydration, β -blocker, diuretic, NSAIDs, ACE inhibitors/ARBs and aspirin therapies, age, gender, contrast iodine load, prior exposure to contrast material, type of imaging study, heart failure, hypertension, renal failure, multiple myeloma, and diabetes mellitus.
Similar to recent reports, we assessed the difference in CN incidence between treatment groups on the basis of whether the patient had reduced GFR defined as GFR <60 ml/min per 1.73 m2 (6). The associations with CN on the basis of stages of chronic kidney disease and reduced GFR classification were consistent with our a priori analysis (Table 2).
Manner of Administration of Sodium Bicarbonate and NAC.
Sodium bicarbonate was administered according to the Merten et al. protocol (13) in 43 (16%) cases in which it was used. NAC was administered according to the Tepel protocol (11) on two consecutive days in 230 (36%) cases in which it was used. Table 3 shows the association of CN with the manner of administration of sodium bicarbonate and NAC. Regardless of how it was administered, sodium bicarbonate was associated with an increase in CN.
Table 3.
Association of CN relative to no treatment according to manner of administration of each treatment adjusted for multiple risk factorsa
| Variable | N | OR (95% CI) | P Value |
|---|---|---|---|
| Sodium bicarbonate alone | |||
| Sodium bicarbonate administered per the Merten et al. protocol (23) | 43 | 2.26 (1.04 to 4.53) | 0.03 |
| Sodium bicarbonate administration deviated from the Merten et al. protocol (23) | 225 | 3.23 (2.33 to 4.46) | <0.001 |
| NAC alone | |||
| NAC administered over 2 d per Tepel protocol (34) | 230 | 0.81 (0.49 to 1.28) | 0.38 |
| NAC administration deviated from the Tepel protocol (34) | 386 | 1.38 (0.99 to 1.91) | 0.05 |
| Sodium bicarbonate + NAC | |||
| Sodium bicarbonate administered per the Merten et al. protocol & NAC administered over 2 d | 20 | 0.97 (0.21 to 3.32) | 0.96 |
| Sodium bicarbonate administration deviated from the Merten et al. protocol & NAC deviated administration | 90 | 1.30 (0.68 to 2.31) | 0.40 |
| Sodium bicarbonate administered per the Merten et al. protocol & NAC deviated administration | 61 | 1.35 (0.62 to 2.67) | 0.42 |
| Sodium bicarbonate administration deviated from the Merten et al. protocol & NAC administered over 2 d | 50 | 0.40 (0.09 to 1.27) | 0.17 |
| No Treatment | 1.00 | — |
Adjusted for total volume of hydration, β -blocker, diuretic, NSAIDs, ACE inhibitors/ARBs and aspirin therapies, age, gender, prior creatinine, contrast iodine load, prior exposure to contrast material, type of imaging study, heart failure, hypertension, renal failure, multiple myeloma, and diabetes mellitus.
Assessment within 48 h of Contrast Exposure.
Despite the fact that creatinine may peak 3 to 7 d after contrast exposure (1,2,7,20,21) (Figure 1), some studies have used creatinine elevations within the first 48 h after contrast exposure to define CN (4,13). We assessed our data as follows: in 10,422 cases (no treatment group = 9320 cases; bicarbonate alone = 268; NAC alone = 613; sodium bicarbonate and NAC = 221) a creatinine value was available within 2 d after contrast exposure. In cases where multiple values were available, the highest was used. After adjustment for all covariates significant increases in CN compared with the no treatment group were noted for sodium bicarbonate (OR 6.78, 95% CI 4.98 to 9.18; P < 0.001), NAC alone (OR 1.54, 95% CI 1.13 to 2.09; P = 0.006), and for sodium bicarbonate used in conjunction with NAC (OR 1.76, 95% CI 1.10 to 2.74; P = 0.01). Use of sodium bicarbonate alone remained significantly associated with CN when compared with the NAC alone group after adjustment for all covariates (OR 4.16, 95% CI 2.79 to 6.20; P < 0.001). We present the raw data for the incidence of CN within 2 d of exposure as well as several other definitions of CN in Table 4.
Table 4.
Incidence of CN for each prophylactic treatment stratified by definitions of CN
| Definition of Contrast Nephropathy
|
||||
|---|---|---|---|---|
| Creatinine Increase >0.5 mg/dl or ≥25% within 7 d | Creatinine Increase >0.5 mg/dl within 7 d | Creatinine Increase ≥25% within 7 d | Creatinine Increase >0.5 mg/dl or ≥25% within 2 da | |
| No treatment | 11% | 3% | 11% | 7% |
| Sodium bicarbonate + NAC | 15% | 8% | 15% | 12% |
| NAC alone | 15% | 8% | 15% | 15% |
| Sodium bicarbonate alone | 31% | 16% | 30% | 35% |
Maximum postcontrast creatinine was available within 2 d of contrast exposure in 10,422 cases.
Exclusion of Cases with Precontrast Creatinine Rises.
If creatinine values were rising before contrast exposure, the postexposure creatinine could be elevated regardless of contrast exposure and potentially bias the assessment of CN. In 10,140 cases, more than one precontrast exposure creatinine was available within 7 d before contrast administration; 9363 cases remained after those with a creatinine change ≥0.5 mg/dl before contrast exposure were excluded (no treatment group = 8546 cases; sodium bicarbonate alone = 186 cases; NAC alone = 472 cases; sodium bicarbonate and NAC = 159 cases). In this subgroup, after adjustment for all covariates sodium bicarbonate alone remained associated with an increase in CN compared with no treatment (OR 2.41, 95% CI 1.56 to 3.63; P < 0.001). NAC alone was also significantly associated with CN (OR 1.58, 95% CI 1.10 to 2.23; P = 0.01). However, sodium bicarbonate used in conjunction with NAC was not significantly associated with CN compared with no treatment after adjustments (OR 1.63, 95% CI 0.91 to 2.75; P = 0.08)
Secondary Analysis: Propensity Scoring
Propensity scoring methods have been proposed to allow adjustment for undefined patient characteristics associated with the treatment groups that may bias the results (25,26). We examined our data using this methodology, but we found that the results were similar to our primary logistic regression model. Specifically compared with the no treatment group, OR for CN associated with each treatment group were as follows: sodium bicarbonate alone (OR 3.20, 95% CI 2.43 to 4.19; P < 0.001), NAC alone (OR 1.13, 95% CI 0.94 to 1.50; P = 0.13), and NAC in combination with sodium bicarbonate (OR 1.02, 95% CI 0.68 to 1.48; P = 0.92).
Discussion
In this large actual practice cohort, the use of sodium bicarbonate was associated with a significant increase in CN compared with both no prophylaxis and with the use of NAC. The use of NAC alone or in combination with sodium bicarbonate was not associated with a significant change in incidence of CN compared with no prophylaxis in our a priori defined analysis. Our results verify that CN is common with incidences >10% regardless of prophylactic measures or patient characteristics. We also found that contrast prevention measures were used in the manner described in randomized controlled trials <40% of the time, but this did not affect the associations with CN.
Increased Risk of CN with Sodium Bicarbonate
Given the seemingly strong protective benefit of sodium bicarbonate in the study by Merten et al. (13), the increased incidence of CN in our study is surprising. The discrepancy from the Merten et al. study could be caused by the exclusion of multiple patients in the study by Merten et al., particularly the exclusion of 13% of patients in each study arm because of lack of follow-up tests or protocol violations after randomization and the exclusion of patients with known risk factors for CN (e.g., hypertension, emergency catheterizations, and recent previous contrast administrations). For the patients who weren't excluded from the study by Merten et al., cardiac catheterization procedures comprised >80% of the analyzed cases in the randomized portion (13). In addition, the study by Merten et al. showed a marginal statistical benefit in the bicarbonate group. Furthermore, the study by Merten et al. was a small trial that was terminated early after a small number of events, which, on the basis of a recent report, suggests that the results should be viewed with skepticism (30).
In our real-world population, many patients who received sodium bicarbonate had multiple comorbidities and often were imaged with computed tomography (83%). It is notable that the group of patients that received sodium bicarbonate in our study had a more favorable profile of baseline characteristics than the NAC group (Tables 1 and 2). Specifically, the patients in the sodium bicarbonate alone group were younger, had lower average creatinine levels and higher estimated glomerular filtration rates, were less likely to have diabetes or hypertension, had higher mean volumes of precontrast hydration, and fewer received angiotensin receptor blockers and diuretics compared with the NAC alone group. Despite these baseline characteristics, which would be expected to reduce the risk of CN, these patients were more likely to develop CN than those who received NAC. Furthermore, the magnitude and direction of this effect was consistent in all secondary and subgroup analyses.
The mechanism by which sodium bicarbonate might increase the incidence of CN is unknown. However, we can speculate that it may relate to the possible increase of reactive oxygen species (ROS). Bicarbonate in the presence of ROS enhances the generation of ROS such as peroxymonocarbonate (HCO4−), a very potent ROS, as described by Richardson et al. (34). We suggest that bicarbonate may increase the risk to develop CN by its prooxidant properties, particularly in states of ROS activation such as diabetes mellitus, and may have detrimental effects on renal blood flow in the presence of contrast agents. The proposed antioxidant properties of NAC may counteract the prooxidant effects of sodium bicarbonate and may explain why the use of sodium bicarbonate in conjunction with NAC was not associated with increases in CN.
Although the editorial that accompanied the original report of the efficacy of sodium bicarbonate for CN prophylaxis recommended its immediate adoption into clinical practice (14), we recommend that the data be verified in larger randomized controlled trials in multiple patient groups and practice settings before introduction into widespread clinical practice.
Reduced Risk of CN with NAC?
Consistent with previous studies that reported no benefit of NAC in preventing CN (17–19), the use of NAC alone or in combination with sodium bicarbonate was not associated with significant change in the incidence of CN compared with no prophylaxis in our a priori defined analysis. Our study is an examination of multiple patients in a real-world practice setting. It is possible that NAC may be beneficial in selected groups of patients or in higher doses (6). It should be noted that in cases where administration of NAC deviated from the Tepel et al. protocol there was a trend toward increased incidence of CN. In those cases where NAC was administered per the Tepel et al. protocol, there was a trend toward decreased incidence of CN (Table 3). However, neither trend was significant, nor can unaccounted confounding be ruled out in either case. Furthermore, as opposed to our sodium bicarbonate analysis, compared with other studies our numbers of cases where NAC was used was relatively small. Regardless of the possible confounding and small numbers, it may be wise for the clinician to pay special attention to the manner of administration of NAC given the potential risks suggested by our study.
Bicarbonate Used in Conjunction with NAC
Since the publication of the original study by Merten et al. (13), several other studies have assessed the use of sodium bicarbonate in conjunction with NAC. Results have been mixed between benefit (31,33) and no effect (32). Our results are similar to the latter study with regard to the neutral effect of sodium bicarbonate in conjunction with NAC as compared with NAC alone. However, of these subsequent studies, only our study analyzes the use of sodium bicarbonate alone, without NAC, for CN prophylaxis and raises concerns as noted above.
Limitations and Strengths
This is a retrospective cohort study. It is possible that the covariates in our study have not fully captured the degree of risk for these patients, which results in residual confounding. For example, the group that received sodium bicarbonate had fewer comorbid diseases compared with the NAC group. Furthermore, only 16% of patients who received sodium bicarbonate received it according to the Merten et al. protocol, which is a small group. Although the risk of CN was still elevated in this small group (OR 2.26; P = 0.03) the CI was wider (1.04 to 4.53) because of the small sample size. Finally, this is a single-center study. The study of the effect of sodium bicarbonate on CN should be repeated with other populations in multiple practice settings in retrospective and prospective study designs.
On the other hand, this study is the largest of its kind with >11,000 contrast administrations over an entire year in a real-world practice setting at a major medical center analyzed with adjustment for multiple covariates known to be associated with the development of CN. Furthermore, propensity scoring methods used to adjust for potential selection bias were consistent with our a priori defined logistic regression model, as were all other subgroup analyses. Additionally, the case capture rates for this cohort study were very high, further strengthening the inferences from these data.
The a priori defined analysis of these data yield conservative estimates of association. For example, the subgroup analysis of CN using creatinine values available within 48 h found an OR for CN in the sodium bicarbonate alone group even higher than that found using our a priori analysis (OR 6.78, 95% CI 4.98 to 9.18; P < 0.001 versus OR 3.10, 95% CI 2.78 to 4.18; P < 0.001, respectively). Because the case capture rates were so high, only three cases from the treatment groups (all in the NAC group) used in the a priori analysis were not available for the assessment of CN at 48 h. Thus, the lower OR in the a priori analysis reflects the increase number of cases of CN in the no-treatment group that are available for comparison when assessed over 7 d rather than 2 d. These results and the results of all the other subanalyses are consistent; i.e., sodium bicarbonate used alone without NAC was associated with increased risk of CN regardless of analysis or stratification.
Conclusions
Intravenous sodium bicarbonate may increase the incidence of CN. The clinical use of sodium bicarbonate for renal protection should be reconsidered until further investigation can elucidate its proper use.
Disclosures
None.
Acknowledgments
The authors would like to sincerely thank Dale Kuisle, Edward Asmann, and Barbara Abbott for help with data collection. This study was supported in part by a grant from the Mayo Small Grants Program administered by the Division of General Internal Medicine Research Committee of the Mayo Clinic (Rochester, MN), which reviewed the design of the study before data collection but did not otherwise participate in the conduct of the study; collection, management, analysis, nor interpretation of the data; nor preparation, review, nor approval of the manuscript.
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.jasn.org/
References
- 1.McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW: Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am J Med 103: 368–375, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT: Hospital-acquired renal insufficiency: A prospective study. Am J Med 74: 243–248, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Iakovou I, Dangas G, Mehran R, Lansky AJ, Ashby DT, Fahy M, Mintz GS, Kent KM, Pichard AD, Satler LF, Stone GW, Leon MB: Impact of gender on the incidence and outcome of contrast-induced nephropathy after percutaneous coronary intervention. J Invasive Cardiol 15: 18–22, 2003 [PubMed] [Google Scholar]
- 4.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr: Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105: 2259–2264, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Levy EM, Viscoli CM, Horwitz RI: The effect of acute renal failure on mortality. A cohort analysis. JAMA 275: 1489–1494, 1996 [PubMed] [Google Scholar]
- 6.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, De Metrio M, Galli S, Fabbiocchi F, Montorsi P, Veglia F, Bartorelli AL: N-Acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med 354: 2773–2782, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Kandzari DE, Rebeiz AG, Wang A, Sketch MH Jr: Contrast nephropathy: An evidence-based approach to prevention. Am J Cardiovasc Drugs 3: 395–405, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Sandoval LJ, Kosowsky BD, Losordo DW: Acetylcysteine to prevent angiography-related renal tissue injury (the APART trial). Am J Cardiol 89: 356–358, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Kay J, Chow WH, Chan TM, Lo SK, Kwok OH, Yip A, Fan K, Lee CH, Lam WF: Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: A randomized controlled trial. JAMA; 289: 553–558, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Briguori C, Manganelli F, Scarpato P, Elia PP, Golia B, Riviezzo G, Lepore S, Librera M, Villari B, Colombo A, Ricciardelli B: Acetylcysteine and contrast agent-associated nephrotoxicity. J Am Coll Cardiol 40: 298–303, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W: Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 343: 180–184, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Shyu KG, Cheng JJ, Kuan P: Acetylcysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J Am Coll Cardiol 40: 1383–1388, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Merten GJ, Burgess WP, Gray LV, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, Bersin RM, Van Moore A, Simonton CA 3rd, Rittase RA, Norton HJ, Kennedy TP: Prevention of contrast-induced nephropathy with sodium bicarbonate: A randomized controlled trial. JAMA 291: 2328–2334, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Chertow GM: Prevention of radiocontrast nephropathy: Back to basics. JAMA 291: 2376–2377, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Barrett BJ, Parfrey PS: Clinical practice: Preventing nephropathy induced by contrast medium. N Engl J Med 354: 379–386, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Bagshaw SM, McAlister FA, Manns BJ, Ghali WA: Acetylcysteine in the prevention of contrast-induced nephropathy: A case study in the pitfalls of the evolution of evidence. Arch Intern Med 166: 161–166, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Allaqaband S, Tumuluri R, Malik AM, Gupta A, Volkert P, Shalev Y, Bajwa TK: Prospective randomized study of N-acetylcysteine, fenoldopam, and saline for prevention of radiocontrast-induced nephropathy. Catheter Cardiovasc Interv 57: 279–283, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Boccalandro F, Amhad M, Smalling RW, Sdringola S: Oral acetylcysteine does not protect renal function from moderate to high doses of intravenous radiographic contrast. Catheter Cardiovasc Interv 58: 336–341, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Durham JD, Caputo C, Dokko J, Zaharakis T, Pahlavan M, Keltz J, Dutka P, Marzo K, Maesaka JK, Fishbane S: A randomized controlled trial of N-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int 62: 2202–2207, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Pannu N, Wiebe N, Tonelli M; Alberta Kidney Disease Network: Prophylaxis strategies for contrast-induced nephropathy. JAMA 295: 2765–2779, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Maeder M, Klein M, Fehr T, Rickli H: Contrast nephropathy: Review focusing on prevention. J Am Coll Cardiol 44: 1763–1771, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ: Trends in heart failure incidence and survival in a community-based population. JAMA 292: 344–350, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Birck R, Krzossok S, Markowetz F, Schnulle P, van der Woude FJ, Braun C: Acetylcysteine for prevention of contrast nephropathy: Meta-analysis. Lancet 362: 598–603, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Gami AS, Garovic VD: Contrast nephropathy after coronary angiography. Mayo Clin Proc 79: 211–219, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Rubin DB: Estimating causal effects from large data sets using propensity scores. Ann Intern Med 127: 757–763, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Hoffman RM, Barry MJ, Stanford JL, Hamilton AS, Hunt WC, Collins MM: Health outcomes in older men with localized prostate cancer: Results from the Prostate Cancer Outcomes Study. Am J Med 119: 418–425, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Poggio ED, Wang X, Weinstein DM, Issa N, Dennis VW, Braun WE, Hall PM: Assessing glomerular filtration rate by estimation equations in kidney transplant recipients. Am J Transplant 6: 100–108, 2006 [DOI] [PubMed] [Google Scholar]
- 29.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 30.Montori VM, Devereaux PJ, Adhikari NK, Burns KE, Eggert CH, Briel M, Lacchetti C, Leung TW, Darling E, Bryant DM, Bucher HC, Schünemann HJ, Meade MO, Cook DJ, Erwin PJ, Sood A, Sood R, Lo B, Thompson CA, Zhou Q, Mills E, Guyatt GH: Randomized trials stopped early for benefit. JAMA 294: 2203–2209, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Recio-Mayoral A, Chaparro M, Prado B, Cózar R, Méndez I, Banerjee D, Kaski JC, Cubero J, Cruz JM: The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: The RENO Study. J Am Coll Cardiol 49: 1283–1288, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Schmidt P, Pang D, Nykamp D, Knowlton G, Jia H: N-acetylcysteine and sodium bicarbonate versus N-acetylcysteine and standard hydration for the prevention of radiocontrast-induced nephropathy following coronary angiography. Ann Pharmacother 41: 46–50, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Briguori C, Airoldi F, D'Andrea D, Bonizzoni E, Morici N, Focaccio A, Michev I, Montorfano M, Carlino M, Cosgrave J, Ricciardelli B, Colombo A: Renal Insufficiency following Contrast Media Administration Trial (REMEDIAL): A randomized comparison of 3 preventive strategies. Circulation 115: 1211–1217, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Richardson DE, Regino CA, Yao H, Johnson JV: Methionine oxidation by peroxymonocarbonate, a reactive oxygen species formed from CO2/bicarbonate and hydrogen peroxide. Free Radic Biol Med 35: 1538–1550, 2003 [DOI] [PubMed] [Google Scholar]