Abstract
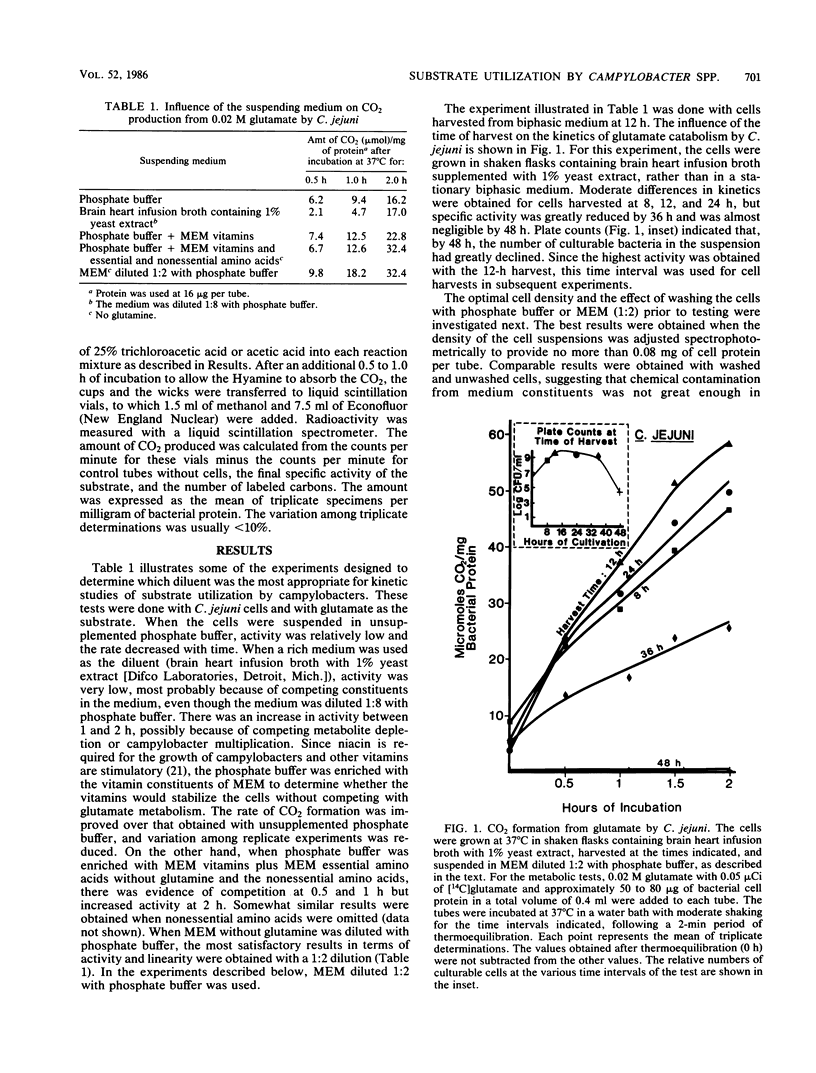
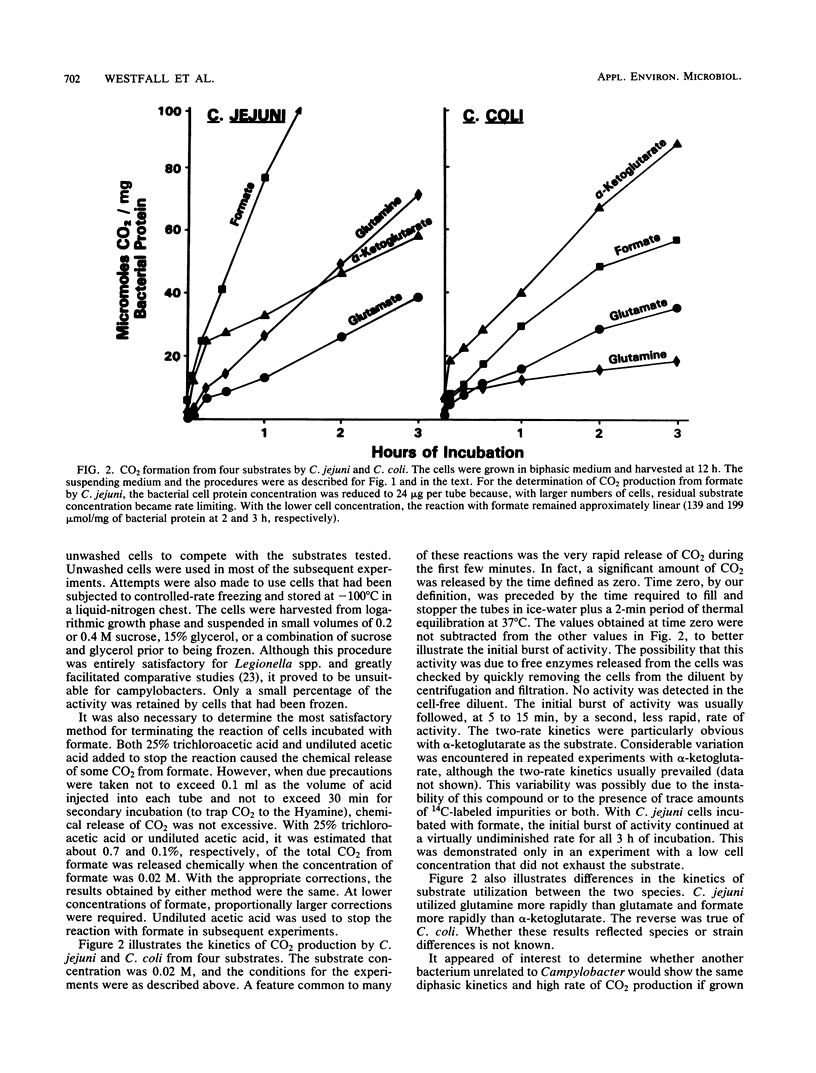
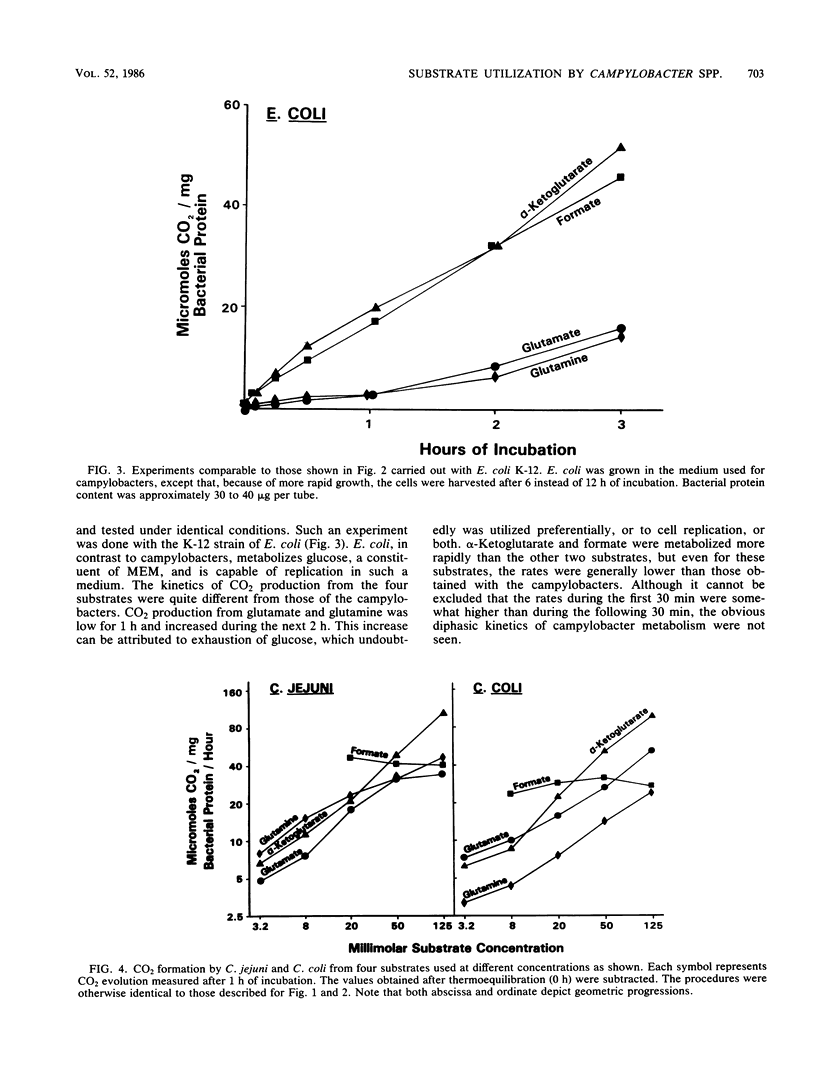
An attempt was made to elucidate in Campylobacter spp. some of the physiologic characteristics that are reflected in the kinetics of CO2 formation from four 14C-labeled substrates. Campylobacter jejuni and C. coli were grown in a biphasic medium, and highly motile spiral cells were harvested at 12 h. Of the media evaluated for use in the metabolic tests, minimal essential medium without glutamine, diluted with an equal volume of potassium sodium phosphate buffer (pH 7.2), provided the greatest stability and least competition with the substrates to be tested. The cells were incubated with 0.02 M glutamate, glutamine, alpha-ketoglutarate, or formate, or with concentrations of these substrates ranging from 0.0032 to 0.125 M. All four substrates were metabolized very rapidly by both species. A feature of many of these reactions, particularly obvious with alpha-ketoglutarate, was an immediate burst of CO2 production followed by CO2 evolution at a more moderate rate. These diphasic kinetics of substrate utilization were not seen in comparable experiments with Escherichia coli grown and tested under identical conditions. With C. jejuni, CO2 production from formate proceeded rapidly for the entire period of incubation. The rate of metabolism of glutamate, glutamine, and alpha-ketoglutarate by both species was greatly enhanced by increased substrate concentration. The approach to the study of the metabolism of campylobacters here described may be useful in detecting subtle changes in the physiology of cells as they are maintained past their logarithmic growth phase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER J. K. Energy sources utilized by Vibrio fetus. J Bacteriol. 1957 Aug;74(2):168–170. doi: 10.1128/jb.74.2.168-170.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlone G. M., Lascelles J. Aerobic and anaerobic respiratory systems in Campylobacter fetus subsp. jejuni grown in atmospheres containing hydrogen. J Bacteriol. 1982 Oct;152(1):306–314. doi: 10.1128/jb.152.1.306-314.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. P., Roman D. J. Response of Campylobacter jejuni to sodium chloride. Appl Environ Microbiol. 1982 Mar;43(3):561–565. doi: 10.1128/aem.43.3.561-565.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T. G., Hoffman P. S. Hydrogenase activity in catalase-positive strains of Campylobacter spp. J Clin Microbiol. 1983 Oct;18(4):825–829. doi: 10.1128/jcm.18.4.825-829.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. S., Goodman T. G. Respiratory physiology and energy conservation efficiency of Campylobacter jejuni. J Bacteriol. 1982 Apr;150(1):319–326. doi: 10.1128/jb.150.1.319-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänninen M. L. The effect of NaCl on Campylobacter jejuni/coli. Acta Vet Scand. 1981;22(3-4):578–588. doi: 10.1186/BF03548681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert G. A., Hollis D. G., Weaver R. E., Lambert M. A., Blaser M. J., Moss C. W. 30 years of campylobacters: biochemical characteristics and a biotyping proposal for Campylobacter jejuni. J Clin Microbiol. 1982 Jun;15(6):1065–1073. doi: 10.1128/jcm.15.6.1065-1073.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIGGINS E. M., PLASTRIDGE W. N. Some metabolic activities of Vibrio fetus of bovine origin. J Bacteriol. 1958 Feb;75(2):205–208. doi: 10.1128/jb.75.2.205-208.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECCE J. G. Some biochemical characteristics of Vibrio fetus and other related Vibrios isolated from animals. J Bacteriol. 1958 Sep;76(3):312–316. doi: 10.1128/jb.76.3.312-316.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell B. R., Walker R. I., Coolbaugh J. C. Campylobacter fetus ss. Jejuni, a newly recognized enteric pathogen: morphology and intestinal colonization. Scan Electron Microsc. 1981;4:125–131. [PubMed] [Google Scholar]
- NG L. K., Sherburne R., Taylor D. E., Stiles M. E. Morphological forms and viability of Campylobacter species studied by electron microscopy. J Bacteriol. 1985 Oct;164(1):338–343. doi: 10.1128/jb.164.1.338-343.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niekus H. G., van Doorn E., Stouthamer A. H. Oxygen consumption by Campylobacter sputorum subspecies Bubulus with formate as substrate. Arch Microbiol. 1980 Sep;127(2):137–143. doi: 10.1007/BF00428017. [DOI] [PubMed] [Google Scholar]
- Rollins D. M., Colwell R. R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986 Sep;52(3):531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins D. M., Coolbaugh J. C., Walker R. I., Weiss E. Biphasic culture system for rapid Campylobacter cultivation. Appl Environ Microbiol. 1983 Jan;45(1):284–289. doi: 10.1128/aem.45.1.284-289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMIBERT R. M. Nutrition of Vibrio fetus. J Bacteriol. 1963 Feb;85:394–398. doi: 10.1128/jb.85.2.394-398.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B., Benjamin J. '1001' Campylobacters: cultural characteristics of intestinal campylobacters from man and animals. J Hyg (Lond) 1980 Dec;85(3):427–442. doi: 10.1017/s0022172400063506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smibert R. M. The genus Campylobacter. Annu Rev Microbiol. 1978;32:673–709. doi: 10.1146/annurev.mi.32.100178.003325. [DOI] [PubMed] [Google Scholar]
- Weiss E., Westfall H. N. Substrate utilization by Legionella cells after cryopreservation in phosphate buffer. Appl Environ Microbiol. 1984 Aug;48(2):380–385. doi: 10.1128/aem.48.2.380-385.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



