Abstract
Objective
To assess dose-response relations between severity of iron deficiency (ID) and infant social-emotional behavior.
Study design
Cohort of 9- to 10-month-old African-American infants (n = 77 with final iron status classification). Infants were provided oral iron for 3 months. Social-emotional outcomes included mother and examiner ratings at 9 and 12 months and quantitative behavioral coding from videotape at 12 months. General linear model analyses tested for linear effects of iron status group (ordered from worst to best – iron-deficient anemic (IDA), non-anemic iron-deficient (NA ID), iron-sufficient (IS)) and determined thresholds for effects.
Results
There were significant (p < .05) linear effects of poorer iron status for shyness (increasing, maternal rating), orientation-engagement and soothability (decreasing, examiner ratings), and the following quantitatively-coded behaviors: positive affect (decreasing) and latencies to engage with the examiner (increasing) and move away from the examiner (decreasing). The threshold for all but one effect was ID with or without anemia vs. IS.
Conclusions
Infant social-emotional behavior appears to be adversely affected by ID with or without anemia. ID without anemia is not detected by common screening procedures and is more widespread than IDA. Infant social-emotional behavior can profoundly influence the caregiving environment, with repercussions for overall development.
The prevalence of iron deficiency anemia (IDA) has markedly declined in US infants in the last 30 years. However, poor, minority, and immigrant infants and toddlers remain at increased risk for IDA and iron deficiency (ID) without anemia.1 In developing countries, 46-66% of children under 4 years of age are anemic, with half attributed to ID.2
More than 25 years ago, Oski and Honig reported improvements in mental development test scores 1 week after intramuscular iron therapy for IDA infants.3 Upon hearing these results, the first author (BL) hypothesized that only a change in behavior such as affect or attention could account for the results because major cognitive advances are unlikely to occur so quickly. Though findings of rapid improvement in mental test scores have not been replicated,4 social-emotional alterations are among the most consistent findings. Virtually every study that compared social-emotional behavior of IDA to non-anemic (NA) infants found them to be more wary, hesitant, solemn, unhappy, or closer to their mothers.5 Four of 6 randomized trials of supplemental iron that assessed this domain showed affective benefits of iron (e.g., more positive affect, social interaction, etc.).5 Notwithstanding the consistency of results, social-emotional effects have captured less attention than cognitive ones, but they could equally result from direct effects of ID on associated brain systems. Furthermore, there has been little infant research on ID without anemia, which is not detected by hemoglobin (HB) screening. There is reason for concern because brain iron may be reduced before the HB concentration falls to the level of anemia.5,6
This study, specifically designed to identify effects of ID with or without anemia, assessed infant social-emotional behavior and other related behaviors in an inner-city African-American sample. If dose-response relations were present (i.e., worse outcome with more severe ID), we expected that outcome for non-anemic iron-deficient (NA ID) infants would be intermediate between IDA and iron-sufficient (IS) infants. The study was also part of an integrated cross-species program project grant. A non-human primate model with measures and ages closely comparable to the human infant study used diet to induce periods of iron deprivation.7 To identify underlying neural mechanisms, a rodent model investigated the behavioral domain and related brain systems, again with experimental manipulation of dietary iron to produce ID and to support causal inferences.8,9
Methods
Subjects
The study was approved by the Wayne State University and University of Michigan Institutional Review Boards. Signed informed consent was obtained for the screening phase of the project and again for the neurobehavioral study. Infants and caregivers were recruited during routine 9-month visits to Children's Hospital of Michigan, which serves an economically-stressed inner-city community. Screening was based on a 10-minute questionnaire and a routine venous blood sample with extra blood (< 5 ml total) for additional iron assays for infants qualified by history. Less than 10% of those contacted declined screening. A total of 881 infants were screened between April 2002 and August 2005. Because African-Americans comprised > 90% of the clinic population, recruitment was restricted to those infants. Participation in the neurobehavioral study was further restricted to healthy, full-term singleton infants, born to mothers > 17 years, with birth weight > 5th percentile, without perinatal complications, emergency C-section, maternal diabetes in pregnancy, heavy alcohol use, or other incapacitating condition. The subjects were in foster care and had no chronic health problem or hospitalization more than once, or for > 5 days. Those who also met initial hematologic criteria (see below) were considered for the neurobehavioral study. The mothers or primary caregivers of 242 potentially qualifying infants were invited to participate; 31% declined, 20% could not be enrolled due to repeated missed appointments, and 2% did not meet entrance criteria on further review. Of 113 infants with neurodevelopmental testing, 77 met final iron status criteria (see below; also Figure 1 [available at www.jpeds.com] and ref.10 for details of subject enrollment and exclusion).
Figure 1.
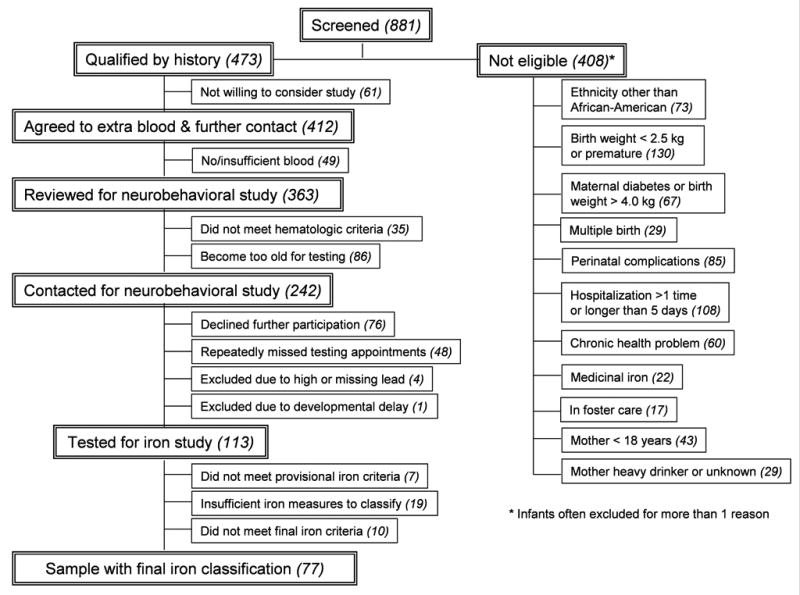
(online). Flow chart to sample with final iron status classification (ns). Of 7 tested infants who were later determined not to meet initial hematologic criteria, 6 with HB < 110 g/dL and normal MCV, RDW, and ZPP/H had been included early in the study, expecting that additional iron measures would show them to meet the criterion for ID; 1 with HB = 114 g/L and normal MCV, RDW, and ZPP/H was considered IS by mistake. Among infants with data for all iron measures, 10 did not meet criteria for final iron classification (7 anemic with only 1 abnormal iron measure, 3 neither NA ID nor IS – they had only 1 abnormal iron measure and HB between 110 and 115 g/L).
Iron status assessment
Initial venous blood tests included a complete blood count, lead, and zinc protoporphyrin/heme ratio (ZPP/H), performed at the Detroit Medical Center. Remaining blood was separated and sent frozen to John Beard, Pennsylvania State University, for determination of serum iron, total iron binding capacity, transferrin saturation, ferritin, transferrin receptor (TfR), and markers of inflammation. Details of assay techniques and quality control have previously been reported.11 To determine which infants qualified for the study hematologically, we used cutoffs from NHANES II,12 NHANES III,13 and CDC publications.14,15 Initial hematologic criteria were based on measures available for all infants within a few days: HB, mean corpuscular volume (MCV), red cell distribution width (RDW), lead, and ZPP/H (missing for only 4 infants). Infants with least one abnormal value among MCV < 74 fl,15 RDW > 14%,14 and ZPP/H > 69 μmol/mol heme (corresponding to free erythrocyte protoporphyrin > 80 μg/dl12) with or without anemia (HB < 110 g/L12-15) and those who were clearly NA (HB ≥ 115 g/L) with normal MCV, RDW, and ZPP/H received neurobehavioral testing. For final iron status classification, ID was defined as ≥ 2 abnormal iron measures, with transferrin saturation < 12%12 and ferritin < 12 μg/L as additional abnormalities. The latter was in between suggested cutoffs.13,14 IS was defined as HB ≥ 115 g/L and ≤ 1 abnormal iron measures. TfR was not used due to lack of an accepted cutoff for infants of this age. At least some of these additional iron measures were available for 87 infants (82%), of whom 77 met criteria for final classification (28 IDA, 28 NA ID, and 21 IS). Missing data were due to insufficient blood or technical problems.
Behavioral measures
The neurobehavioral study entailed infant assessments and interviews with primary caregivers (mother for all but 1 infant) performed at 9 and 12 months at the Child Development Research Laboratory, Department of Psychiatry and Behavioral Neurosciences, Wayne State University. Mothers completed the 20-item Emotionality, Activity, and Sociability Temperament Survey (EAS).16 Two infant examiners completed a 30-item Behavior Rating Scale (BRS)17 after the half-day assessment. Relevant composite factors at 9-12 months are Orientation/Engagement, Emotion Regulation, and Additional Items. Mothers and examiners completed the ratings without awareness of infant iron status. Of the 77 study infants at 9 months, 62 (81%) returned for behavioral assessments at 12 months.
Videotaped behavior was quantitatively coded at 12 months (n = 57; missing data were secondary to missing tapes due to technical problems). Two parts of a measure assessing symbolic play18 were coded using Observer® software (Noldus Information Technology, Wageningen, The Netherlands): 1) the opening 10-min free play period and 2) a 7- to 8-min period of elicited play. During free play, the infant was provided a standard set of toys on the floor while the mother was seated several feet away being interviewed. Infants could explore the immediate surroundings or seek their mothers but were guided back to the toys by the videographer. For elicited play, the examiner sat next to the infant on the floor, and using toys the baby had manipulated during free play, modeled a sequence of play acts such as giving a doll a drink from a cup. Infant behaviors (positive affect, activity, engagement, etc.) were quantitatively coded by a single coder, blind to iron status, who reached > 90% intra-tester reliability (tapes coded twice).
Maternal interviews covered infant health, family background, social support, life events, home support for child development, and maternal depression, anxiety, and verbal competence.
Iron treatment
All infants were given 3 months of liquid iron sulfate with a uniform dose (22 mg) that provided about 2-3 mg/kg/day of elemental iron. For IS infants, we wanted to prevent ID as many started unmodified cow milk. Project personnel were not able to supervise iron administration in person due to lack of neighborhood safety. Despite good retention for the 12-month neurobehavioral assessment, only 58% returned to the pediatric clinic for a 12-month blood sample. In light of these missing and uncertain data, the study cannot confirm hematologic response to iron or assess the ability of iron therapy to improve infant behavior.
Data analysis
The independent sample t test and X2 test assessed group differences in background and iron measures. Preliminary analyses considered the issue of lower HB cutoffs sometimes recommended for African-Americans. There were no significant differences for any background characteristic or behavioral outcome for infants with HB ≤ 105 g/L vs. between 105 g/L and 110 g/L, and plots of the results showed that they closely resembled each other. Generalized linear model analyses were used to test for linear effects of iron status group on behavioral outcomes and determine whether the threshold for effects was IDA or ID (with or without anemia). The following pre-planned contrasts were analyzed: a) IDA vs. no anemia (NA ID + IS) and b) ID (IDA + NA ID) vs. IS. Background factors (Table I) even weakly correlated (p <.1) with a given behavioral outcome were covaried; those that were not statistically significant were deleted until the most parsimonious model was obtained. Statistical tests of significance (SAS 9.1, SAS Institute, Inc., Carey NC) were 2-tailed (alpha = 0.05).
Table I. Background characteristics by iron group1.
| Iron group | Iron-deficient anemic | Non-anemic iron-deficient | Iron-sufficient |
|---|---|---|---|
| (IDA) | (NA ID) | (IS) | |
| N2 | 28 | 28 | 21 |
| Infant | |||
| Age at 9-mo test | 9.6 ± 0.4 | 9.8 ± 0.3 | 9.8 ± 0.3 |
| Sex, % male (n) | 46 (13) | 64 (18) | 62 (13) |
| Birth weight, kg | 3.28 ± 0.3 | 3.2 ± 0.4 | 3.32 ± 0.4 |
| Gestational age, weeks | 39.5 ± 0.8 | 39.9 ± 1.3 | 39.8 ± 0.9 |
| Breast-fed, % yes (n) | 32 (9) | 46 (13) | 43 (9) |
| Mother and family | |||
| Maternal marital status (married), % (n) | 4 (1) | 7 (2) | 14 (3) |
| Maternal age, years | 23.9 ± 5.6 | 24.5 ± 5.4 | 24.9 ± 6.5 |
| Maternal education, years | 12.3 ± 1.6 | 12.2 ± 1.2 | 12.4 ± 1.5 |
| Maternal depressive symptoms3 | 6.1 ± 5 | 7 ± 5 | 5 ± 4.7 |
| Maternal anxiety (Trait)4 | 32.8 ± 9.1 | 36.1 ± 10.4 | 35.4 ± 8 |
| Maternal alcohol intake (oz absolute alcohol/day) | 0.04 ± 0.13 | 0.01 ± 0.06 | 0.01 ± 0.04 |
| Socioeconomic status5 | 28.1 ± 9.4 | 28.8 ± 5 | 27.8 ± 10.2 |
| HOME score6 | 32.4 ± 5.2 | 32.1 ± 5.9 | 30 ± 6.2 |
| Life events7 | 6.4 ± 4.5 | 5.9 ± 3.9 | 5.6 ± 5.3 |
| Social support8 | 3.5 ± 0.3 | 3.3 ± 0.5 | 3.3 ± 0.5 |
Values are means ± SD or % (n) for categorical variables. There were no statistically significant group differences using the independent t test for continuous variables and X2 for categorical variables.
N varies slightly due to occasional missing data for some measures.
Beck Depression Inventory30
Spielberger State-Trait Anxiety Scale31
Hollingshead Scale for Socioeconomic Status32
Home Observation for Measurement of the Environment-Revised33
Life Experiences Survey34
Social Support (Crnic's adaptation of a scale by Henderson)35
Results
Background and nutrition
There were no statistically significant group differences in background characteristics (Table I), but the IDA group tended to have poorer growth (Table II). Iron status differed across groups, as expected at 9 months but also at 12 months (Table II). Nonetheless, there was some indication of response to iron: increase in HB in IDA infants (p = .01) and absence of significant differences between NA ID and IS groups on iron measures.
Table II. Growth and hematology 1.
| IDA | NA ID | IS | p | |||
|---|---|---|---|---|---|---|
| 9 months (n) | 28 | 28 | 21 | IDA v. NA ID | IDA v. IS | NA ID v. IS |
| Weight-for-age z-score | -0.54 ± 0.9 | -0.02 ± 1.5 | 0.28 ± 0.9 | 0.09 | 0.01 | ns |
| Height-for-age z-score | -0.51 ± 0.9 | -0.48 ± 1.1 | -0.31 ± 1.2 | ns | ns | ns |
| Weight-for-height z-score | 0.26 ± 0.9 | 0.81 ± 1.4 | 1.08 ± 1.1 | 0.08 | 0.02 | ns |
| Hemoglobin (g/L) | 101.9 ± 5.3 | 119 ± 5.3 | 123.2 ± 5.1 | < 0.001 | < 0.001 | 0.01 |
| MCV (fl) | 72.1 ± 5 | 73.8 ± 4.3 | 78.8 ± 3.3 | ns | < 0.001 | < 0.001 |
| RDW (%) | 14.8 ± 1.4 | 14.2 ± 0.9 | 12.9 ± 0.8 | 0.07 | < 0.001 | < 0.001 |
| ZPP/H | 119 ± 38.2 | 96.4 ± 51.9 | 70 ± 23.4 | 0.04 | < 0.001 | 0.03 |
| Transferrin saturation (%) | 20.2 ± 9 | 26.3 ± 11.6 | 24.8 ± 8.9 | 0.06 | ns | ns |
| Ferritin (μg/L)2 | 34.2 ± 29.3 | 34.6 ± 32.3 | 32.1 ± 21.5 | ns | ns | ns |
| TfR (mg/L) | 7.9 ± 5.5 | 5.7 ± 2.9 | 5.2 ± 1.9 | 0.05 | 0.02 | ns |
| Lead (μg/dL) | 2.4 ± 1.5 | 2.7 ± 2.5 | 2.5 ± 1.4 | ns | ns | ns |
| 12 months (n) | 24 | 21 | 17 | p | ||
| Weight-for-age z-score | -0.61 ± 1.5 | 0.12 ± 1.2 | 0.2 ± 1.1 | 0.06 | 0.05 | ns |
| Height-for-age z-score | -0.37 ± 0.8 | -0.3 ± 1 | 0.2 ± 1 | ns | 0.06 | 0.1 |
| Weight-for-height z-score | 0.01 ± 2.2 | 0.91 ± 1.1 | 0.65 ± 1.2 | 0.07 | ns | ns |
| Hemoglobin (g/L) | 110.5 ± 9.8 | 119.9 ± 7.3 | 117.3 ± 9.8 | 0.005 | 0.03 | ns |
| MCV (fl) | 72.4 ± 4.2 | 74.1 ± 3.5 | 76.7 ± 5.4 | ns | 0.01 | ns |
| RDW (%) | 14.9 ± 1.9 | 14 ± 1.1 | 13.1 ± 1 | 0.08 | < 0.001 | 0.1 |
| ZPP/H | 104.5 ± 44.4 | 77.6 ± 20.2 | 62.6 ± 16.8 | 0.02 | < 0.001 | ns |
| Transferrin saturation (%) | 26.4 ± 13.5 | 28.7 ± 12.8 | 25.5 ± 7.6 | ns | ns | ns |
| Ferritin (μg/L) | 37.2 ± 37.9 | 33.7 ± 19 | 34.5 ± 16.8 | ns | ns | ns |
| TfR (mg/L) | 7.1 ± 2.5 | 5.3 ± 1 | 6 ± 2.1 | 0.02 | ns | ns |
| Lead (μg/dL) | 3.4 ± 2.7 | 2.8 ± 2.2 | 3.9 ± 2 | ns | ns | ns |
Values are means ± SD.
One ferritin value of 243 μg/L was omitted due to evidence of inflammation (high C-reactive protein and high alpha1-acid glycoprotein [AGP]).
Behavioral outcomes
Measures showing statistically significant linear effects of iron status group (IDA least optimal, NA ID in the middle, IS most optimal) are presented in Figure 2. These include the EAS Shyness scale at 9 months (maternal ratings of response to strangers, ease of making friends, and sociability), BRS Orientation/Engagement factor at 9 and 12 months (examiner ratings of positive affect, energy, interest in test materials, initiative, exploration, persistence, enthusiasm, fearfulness, and social engagement), and BRS Additional Items at 12 months (explained by examiner rating of soothability during cognitive testing). In categorical analysis, 43% of IDA infants were considered not optimal in Orientation/Engagement (plus 18% questionable) and 18% of NA ID infants not optimal (plus 36% questionable) at 9 months, compared to 20% not optimal and 15% questionable in IS infants (Mantel-Haenszel X2 = 4.12, p = .04). Turning to quantitative behavioral coding from videotape at 12 months, there were no group differences during free play. However, in the elicited play segment there were linear effects for positive affect (decreasing with poorer iron status), latency to become engaged with the examiner (increasing), and latency to move away from the examiner (decreasing) (Figure 2). To describe one such effect in a different way, 48% of IDA infants never showed positive affect, compared to 32% and 24% for NA ID and IS groups, respectively. Table III (available at www.jpeds.com) shows values for all outcomes by iron status group, with significant covariates.
Figure 2.
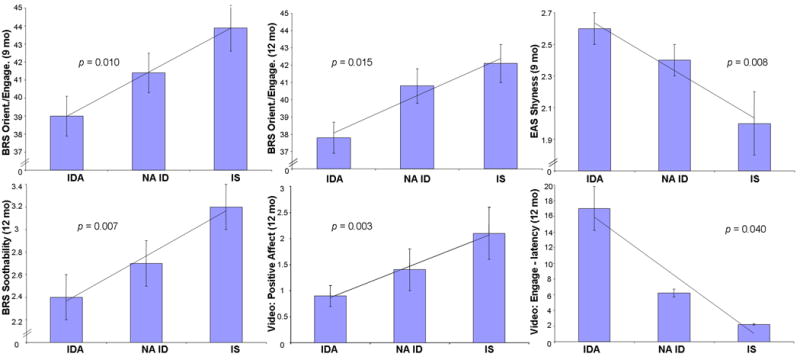
Behavioral outcomes showing statistically significant linear effects of iron status group (IDA least optimal, NA ID intermediate, IS most optimal). For EAS Shyness and latency to become engaged with the examiner, higher values indicate more hesitance/wariness.
Table III. Behavioral outcomes at 9 and 12 months 1.
| Assessment | IDA | NA ID | IS | p2 | Significant covariates 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EAS Temperament Survey (mother) | ||||||||||||
| EAS Shyness | 9 months | 2.6 ± 0.1 | 2.4 ± 0.1 | 2 ± 0.2 | 0.01 | WHZ, maternal education | ||||||
| 12 months | 2.6 ± 0.2 | 2.5 ± 0.2 | 2.4 ± 0.2 | 0.42 | Age (–) | |||||||
| EAS Activity | 9 months | 4.3 ± 0.1 | 4.4 ± 0.1 | 4.4 ± 0.1 | 0.54 | --- | ||||||
| 12 months | 4.4 ± 0.1 | 4.5 ± 0.1 | 4 ± 0.1 | 0.06 | --- | |||||||
| EAS Sociability | 9 months | 3.6 ± 0.1 | 3.8 ± 0.1 | 3.8 ± 0.1 | 0.61 | --- | ||||||
| 12 months | 3.4 ± 0.1 | 3.5 ± 0.1 | 3.3 ± 0.2 | 0.37 | --- | |||||||
| EAS Emotionality | 9 months | 2.8 ± 0.2 | 2.6 ± 0.2 | 2.5 ± 0.2 | 0.33 | HOME (–) | ||||||
| 12 months | 2.9 ± 0.2 | 2.6 ± 0.2 | 2.5 ± 0.2 | 0.24 | Maternal anxiety | |||||||
| Behavior Rating Scale (examiners) | ||||||||||||
| BRS Emotion Regulation | 9 months | 32 ± 0.6 | 31.8 ± 0.6 | 33.3 ± 0.7 | 0.1 | --- | ||||||
| 12 months | 36 ± 0.8 | 35.5 ± 0.9 | 37.2 ± 0.9 | 0.21 | Age | |||||||
| BRS Orientation/Engagement | 9 months | 39 ± 1.1 | 41.4 ± 1.1 | 43.9 ± 1.3 | 0.01 | Social support (–) | ||||||
| 12 months | 37.8 ± 0.9 | 40.8 ± 1 | 42.1 ± 1.1 | 0.01 | Age (–) | |||||||
| BRS Soothability | 9 months | 3 ± 0.2 | 2.8 ± 0.2 | 3.4 ± 0.2 | 0.12 | --- | ||||||
| 12 months | 2.4 ± 0.2 | 2.7 ± 0.2 | 3.2 ± 0.2 | 0.01 | Age (–), birth weight | |||||||
| Video: Elicited play | ||||||||||||
| Positive affect | 12 months | 0.9 ± 0.2 | 1.4 ± 0.3 | 2.1 ± 0.6 | 0.003 | --- | ||||||
| Positive affect – latency | 12 months | 164.5 ± 45.4 | 196.7 ± 37.5 | 188.7 ± 31.8 | 0.0003 | --- | ||||||
| Engage with examiner – latency | 12 months | 16.5 ± 7 | 5.6 ± 3 | 1.4 ± 0.7 | 0.04 | --- | ||||||
| Duration (% time) near examiner | 12 months | 68 ± 5.1 | 72.6 ± 5.3 | 82.6 ± 5.6 | 0.06 | WAZ (–), WHZ | ||||||
| Move away from examiner – latency | 12 months | 102.3 ± 30.1 | 78.6 ± 19 | 223.3 ± 48.1 | 0.78 | --- | ||||||
| Duration (% time) away from examiner | 12 months | 15.4 ± 2.9 | 17.6 ± 3.3 | 9.7 ± 2.9 | 0.11 | HAZ | ||||||
| Move near mother – latency | 12 months | 120.9 ± 38.9 | 107.7 ± 36 | 235.3 ± 53 | 0.23 | --- | ||||||
| Duration (% time) near mother | 12 months | 18.4 ± 5.1 | 8.8 ± 2.8 | 7.4 ± 3.1 | 0.13 | --- | ||||||
Values are adjusted means ± SE (latencies in seconds; positive affect as number of times). For EAS Shyness and Activity, higher scores are less optimal. For BRS Emotion Regulation, Orientation/Engagement, and Soothability, lower scores are worse. For maternal and examiner ratings, ns = 77 at 9 months and 62 at 12 months; for quantitative behavioral coding at 12 months, n = 57. Durations are shown as % time because there was a suggestive trend (p <.1) indicating that ID + NA ID groups had shorter elicited play segments than IS.
Significance level for the linear effect of iron status group (ordered from worst to best in general linear model analyses).
Significant covariates inversely associated with more optimal infant behavior are indicated by (–). Effects of other covariates are positive.
Threshold for behavioral effects
The preplanned comparisons were a) IDA vs. all NA infants and b) ID with or without anemia vs. IS infants. The threshold for all but one effect was ID with or without anemia (Table IV). To illustrate one threshold effect: the proportion of infants who moved away from the examiner within 60 seconds was 56% and 55% of the IDA and NA ID groups, respectively, compared to 19% of IS (p = .01 for threshold of ID vs. IS).
Table 4. Behavioral outcomes showing a threshold of ID with or without anemia vs. IS 1.
| ID
(IDA + NA ID) |
IS | p | Significant covariates | |
|---|---|---|---|---|
| EAS Shyness (mother, 9 months) | 2.5 ± 0.1 | 2 ± 0.2 | 0.01 | WAZ, maternal education |
| EAS Activity (mother, 12 months) | 4.4 ± 0.1 | 4 ± 0.1 | 0.04 | --- |
| BRS Emotion Regulation (examiner, 9 months) | 31.9 ± 0.4 | 33.3 ± 0.7 | 0.09 | --- |
| BRS Orientation/Engagement (examiner, 9 months) | 40.3 ± 0.8 | 43.6 ± 1.3 | 0.03 | Social support |
| BRS Orientation/Engagement (examiner, 12 months) | 39.2 ± 0.7 | 42.1 ± 1.1 | 0.04 | Age |
| BRS Soothability (examiner, 9 months) | 2.9 ± 0.1 | 3.4 ± 0.2 | 0.09 | --- |
| BRS Soothability (examiner, 12 months) | 2.6 ± 0.1 | 3.2 ± 0.2 | 0.01 | Age, birth weight |
| Positive affect (elicited play, 12 months) | 1.1 ± 0.2 | 2.1 ± 0.6 | 0.01 | --- |
| Engage with examiner – latency (elicited play, 12 months) | 11.2 ± 3.8 | 1.8 ± 1.3 | 0.02 | --- |
| Move away from examiner – latency (elicited play, 12 months) | 91.3 ± 18.4 | 220.2 ± 44.1 | 0.01 | --- |
| Duration (% time) near examiner (elicited play, 12 months)2 | 70.3 ± 4 | 82.4 ± 4.3 | 0.04 | WAZ, WHZ |
Values are adjusted means ± SE (latencies in seconds; positive affect as number of times.) For EAS Shyness and Activity, higher scores are less optimal. For BRS Emotion Regulation, Orientation/Engagement, and Soothability, lower scores are worse. For maternal and examiner ratings, ns = 77 at 9 months and 62 at 12 months; for quantitative behavioral coding at 12 months, n = 57.
Duration is shown as % time because there was a suggestive trend (p <.1) indicating that ID + NA ID groups had shorter elicited play segments than IS.
Discusssion
This study adds to the accumulating evidence of social-emotional effects of early ID by its findings of dose-response relations between severity of ID and outcome. Linear effects indicated increasing shyness, decreasing orientation/engagement, and decreasing soothability with poorer iron status (maternal or examiner ratings) and when an examiner attempted to engage the infants in imitative play, decreasing positive affect and engagement (quantitative coding). That the threshold for effects was ID with or without anemia echoes Honig and Oski's early report of increased solemnity in NA ID infants19 and a recent preventive trial in which infants who did not receive supplemental iron were less likely to show positive affect or interact socially.20 Another new study observed a negative linear relation between cord-blood iron status across the full range and negative emotionality and a positive one for alertness and soothability.21 Taken together, these studies point to altered infant social-emotional behavior and affect with ID, with or without anemia.
The behavioral differences observed at 12 months might be interpreted as showing lack of improvement with iron. However, the present study could not determine the effects of iron therapy on infant behavior due to uncertainty about iron administration and a low proportion of infants with blood work at 12 months. Despite the unresolved question of iron therapy, other evidence points to lack of iron as the cause of the observed behavioral alterations: 1) Similar affective-social differences have been reported in studies of IDA infants in a wide variety of settings, circumstances, and study designs.5 2) Four of 6 recent randomized controlled trials of iron supplementation in infancy that assessed social-emotional behavior found benefits of iron.5 3) Animal models with experimental manipulation of iron status by diet show related affective-social differences. For instance, the effects of iron deprivation in our monkey project were most striking in the social-emotional domain.7 Post-natally iron-deprived monkeys were hyperemotional and “tense”, even though no animal ever had IDA.
We have speculated that affective-social alterations relate to effects of ID on dopamine function,20,22 which are consistently reported in rodent models.8 Dopamine plays a major role not only in systems of behavioral activation and inhibition but also in positive affect and the degree to which individuals experience inherent reward.23,24 We also considered that behavioral alterations might be especially apparent in circumstances of novelty, unfamiliarity, or stress.25,26 In the present study, there was little difference in free play behavior, but several differences became apparent when an examiner sought to engage the infant in elicited play. However, there had been little previous work specifically on related brain/behavior systems in ID animal models to support our speculations or to clarify the neural mechanisms. Our program project's rodent study systematically investigated the behavioral domain and related brain systems in an experimental paradigm of dietary iron restriction. Behaviors that depend on striatal dopamine function were delayed or disrupted, with alterations into adulthood despite iron repletion and normalization of brain iron.9 Other persistent consequences, such as less exploration and more hesitancy in a novel environment, were also consistent with altered dopaminergic function.8
These results contribute to our growing conviction that altered affect and response to novelty are among the core deficits in early ID. Altered affect or activity have become a fundamental component of conceptual frameworks for understanding poorer overall developmental outcome.25,27 Such alterations, combined with delayed or mistimed sensory input, and cognitive and motor delay or dysfunction,5,28 may adversely affect the infant's interactions with the physical and social environment, thereby compromising development even further. If an ID infant is unable to elicit or benefit from nurturant interactions with caregivers, the child may have fewer enriching experiences that foster optimal development. Over time, direct effects of ID on the developing brain and indirect effects via limited environmental input may, in combination, contribute to poorer long-term behavioral and developmental outcome.29
Limitations
The study is clearly limited in sample size. In addition, ID could not be confirmed by the “gold standard” of an increase in HB, because iron therapy could not be personally supervised and post-treatment hematology data were unavailable for more than 40% of the infants. This is problematic because indications of ID/IDA were generally milder than in previous developmental studies and other factors may alter iron measures (especially HB and MCV) in African-Americans.14 However, all NA ID and IDA infants met the criterion of ≥ 2 abnormal iron measures and biomarkers did not suggest inflammation as the explanation.11 Available data showed a HB increase in the IDA group and normalization of iron measures in the NA ID group. The results may not generalize to other than inner-city African-American infants and require replication in larger samples in different populations.
In sum, this study demonstrated linear effects of iron status on social-emotional behavior in young infants. The threshold of effects in this sample was ID with or without anemia v. IS. Combined with other human infant studies and a new nonhuman primate model, the results suggest that the social-emotional domain is adversely affected by lack of iron. This finding is worrisome because ID is not detected by common screening procedures and is more widespread than IDA. Combined with results in a developing rodent model, the observed behavior pattern of shyness/hesitance, reduced engagement, and altered response to the unfamiliar is consistent with early disruption of the dopamine system. Infant affect, activity, and social behavior can profoundly influence the caregiving environment, with repercussions for the child's overall development.
Acknowledgments
We are grateful to the study families; Rosa Angulo-Barroso, Matthew Burden, Douglas Fuller, Joseph L. Jacobson, Niko Kaciroti, Margo Laskowski, Tal Shafir, Jing Su, Renee Sun, and Jigna Zatakia for their contributions to study design, recruitment, infant assessment, or data management/analysis; Janis Estrella for video coding; and William Neeley (Director, Detroit Medical Center University Laboratories), John Beard (Pennsylvania State University), and the laboratory staff at both institutions for performing the hematologic and biochemical assays. All investigators in the Brain and Behavior in Early Iron Deficiency Program Project contributed to our thinking about social-emotional effects, especially altered response to the unfamiliar.
Support: NIH (P01 HD39386, Brain and Behavior in Early Iron Deficiency, Betsy Lozoff, Principal Investigator) and the Joseph Young, Sr., Fund, in Michigan (Sandra W. Jacobson, Principal Investigator).
Abbreviations
- IDA
iron-deficiency anemia/iron-deficient anemic
- ID
iron-deficiency/iron-deficient
- NA
non-anemic
- IS
iron-sufficient
- HB
hemoglobin
- MCV
mean corpuscular volume
- RDW
red cell distribution width
- ZPP/H
zinc protoporphyrin/heme ratio
- TfR
transferrin receptor
- EAS
Emotionality Activity, and Sociability Temperament Survey
- BRS
Bayley Behavior Rating Scale
- HOME
Home Observation for Measurement of the Environment-Revised
- HAZ
height-for-age z-score
- WHZ
weight-for-height z-score
- WAZ
weight-for-age z-score
- NHANES
National Health and Nutrition Examination Survey
- CDC
Centers for Disease Control and Prevention
Footnotes
Edited by RW and WFB
No author has a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brotanek JM, Halterman J, Auinger P, Flores G, Weitzman M. Iron deficiency, prolonged bottle-feeding, and racial/ethnic disparities in young children. Arch Pediatr Adolesc Med. 2005;159:1038–1042. doi: 10.1001/archpedi.159.11.1038. [DOI] [PubMed] [Google Scholar]
- 2.Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anaemia. In: Ezzati M, Lopez AD, Rodgers A, et al., editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva: World Health Organization; 2004. [Google Scholar]
- 3.Oski FA, Honig AS. The effects of therapy on the developmental scores of iron-deficient infants. J Pediatr. 1978;92:21–25. doi: 10.1016/s0022-3476(78)80063-8. [DOI] [PubMed] [Google Scholar]
- 4.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:649S–668S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 5.Lozoff B. Iron deficiency and child development. Food Nutr Bull, as part of proceedings of WHO Consultation on Prevention and Control of Iron Deficiency in Children from Various Environments. 2006 in press. [Google Scholar]
- 6.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golub MS, Hogrefe CE, Germann SL, Capitano JL, Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicol Teratol. 2006;28:3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beard JL, Felt B, Schallert T, Burhans M, Connor JR, Georgieff MK. Moderate iron deficiency in infancy: Biology and behavior in young rats. Behav Brain Res. 2006;170:224–232. doi: 10.1016/j.bbr.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, Connor JR, Georgieff MK, Lozoff B. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;171:261–270. doi: 10.1016/j.bbr.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burden MJ, Westerlund A, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, Angelilli ML, Jacobson JL. An event-related potential study of attention and recognition memory in infants with iron deficiency anemia. Pediatrics. doi: 10.1542/peds.2006-2525. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozoff B, Angelilli ML, Zatakia J, Jacobson SW, Calatroni A, Beard JL. Iron status of inner-city African-American infants. Am J Hematol. 2007;82:112–121. doi: 10.1002/ajh.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Life Sciences Research Office. Assessment of the Iron Nutrition Status of the US Population Based on Data Collected in the Second National Health and Nutrition Survey, 1976-1980. Bethesda: Federation of American Societies for Experimental Biology; 1984. [Google Scholar]
- 13.Looker AC, Dallman P, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR. 1998;47:1–29. [PubMed] [Google Scholar]
- 15.Centers for Disease Control. Healthy People - 2000 National Health Promotion and Disease Prevention Objectives Final Review. Hyattsville, MD: Department of Health and Human Services; 2001. [Google Scholar]
- 16.Buss AH, Plomin R. Temperament: Early Developing Personality Traits. Hillsdale, NJ: Erlbaum; 1984. [Google Scholar]
- 17.Bayley N. Bayley Scales of Infant Development. San Antonio: The Psychological Corporation; 1993. [Google Scholar]
- 18.Belsky J, Garduque L, Hrncir E. Assessing performance, competence, and executive capacity in infant play: Relations to home environment and security of attachment. Dev Psyc. 1984;20:406–417. [Google Scholar]
- 19.Honig AS, Oski FA. Solemnity: A clinical risk index for iron deficient infants. Early Child Dev Care. 1984;16:69–84. [Google Scholar]
- 20.Lozoff B, De Andraca I, Castillo M, Smith J, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–854. [PubMed] [Google Scholar]
- 21.Wachs TD, Pollitt E, Cuerto S, Jacoby E, Creed-Kanishiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Dev Psychobiol. 2005;46:141–153. doi: 10.1002/dev.20049. [DOI] [PubMed] [Google Scholar]
- 22.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 23.Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour - review of data from preclinical research. Acta Psychiatr Scand. 2005;111:14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 24.Wild B, Rodden FA, Grodd W, Ruch W. Neural correlates of laughter and humour. Brain. 2003;126:2121–2138. doi: 10.1093/brain/awg226. [DOI] [PubMed] [Google Scholar]
- 25.Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron deficiency anemia. Child Dev. 1998;69:24–36. [PubMed] [Google Scholar]
- 26.Angulo-Kinzler RM, Peirano P, Lin E, Algarin C, Garrido M, Lozoff B. Twenty-four-hour motor activity in human infants with and without iron deficiency anemia. Early Hum Dev. 2002;70:85–101. doi: 10.1016/s0378-3782(02)00092-0. [DOI] [PubMed] [Google Scholar]
- 27.Brown JL, Pollitt E. Malnutrition, poverty and intellectual development. Sci Am. 1996;274:38–43. doi: 10.1038/scientificamerican0296-38. [DOI] [PubMed] [Google Scholar]
- 28.Algarin C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: Long-lasting effects on auditory and visual systems functioning. Pediatr Res. 2003;53:217–223. doi: 10.1203/01.PDR.0000047657.23156.55. [DOI] [PubMed] [Google Scholar]
- 29.Lozoff B, Black M. Impact of micronutrient deficiencies on behavior and development. In: Pettifor J, Zlotkin SH, editors. Nutrition-Micronutrient Deficiencies during the Weaning Period and the First Years of Life. Basel: Karger; 2003. [Google Scholar]
- 30.Beck AT, Steer RA, Brown GK. Beck Depression inventory -- II. San Antonio: The Psych Corp.; 1996. [Google Scholar]
- 31.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 32.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 33.Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment. Little Rock: University of Arkansas; 1984. Revised Edition. [Google Scholar]
- 34.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46:932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 35.Crnic KA, Greenberg MT, Ragozin AS, Robinson NM, Basham RB. Effects of stress and social support on mothers and premature and full-term infants. Child Dev. 1983;54:209–217. [PubMed] [Google Scholar]


