Abstract
Objective
To determine the clinical significance of the presence of amniotic fluid (AF) ‘sludge’ among asymptomatic patients at high-risk for spontaneous preterm delivery.
Study design
This retrospective case-control study included 281 patients with (n=66) and without (n=215) AF ‘sludge’, who underwent transvaginal ultrasound between 13 and 29 completed weeks of gestation. Patients with threatened preterm labor, multiple gestation, fetal anomalies, placenta previa, and uterine contractions were excluded.
Results
The prevalence of AF ‘sludge’ in the study population was 23.5% (66/281). The rates of spontaneous preterm delivery at <28, <32, <35, and <37 weeks of gestation were 14.7% (29/197), 21.3% (46/216), 28.7% (62/216), and 42.1% (91/216), respectively. Patients with ‘sludge’ had: (1) a higher rate of spontaneous preterm delivery at <28 weeks [46.5% (20/43) vs. 5.8% (9/154), p<0.001], <32 weeks [55.6% (25/45) vs. 12.3% (21/171), p<0.001], and <35 weeks [62.2% (28/45) vs. 19.9% (34/171), p<0.001]; (2) a higher frequency of clinical chorioamnionitis [15.2% (10/66) vs. 5.1% (11/215), p=0.007], histologic chorioamnionitis [61.5% (40/65) vs. 28% (54/193), p<0.001] and funisitis [32.3% (21/65) vs. 19.2% (37/193), p=0.03]; (3) a higher frequency of preterm PROM [(39.4% (26/66) vs. 13.5% (29/215), p<0.001], lower gestational age at preterm PROM [24.7 weeks (22.3-28.1) vs. 32.3 weeks (27.7-34.8); p<0.001]; and (4) shorter ultrasound-to-delivery [‘sludge’ positive: 127 days (95% CI: 120-134) vs. ‘sludge’ negative: 161 days (95% CI: 153-169), p<0.001] and ultrasound-to-preterm PROM intervals [‘sludge’ positive: 23 days (95% CI: 7-39) vs. ‘sludge’ negative: 57 days (95% CI: 38-77), p=0.003] than those without ‘sludge’. AF ‘sludge’ was an independent explanatory variable for the occurrence of spontaneous preterm delivery at <28, <32, and <35 weeks, preterm PROM, MIAC, and histologic chorioamnionitis. Moreover, the combination of a cervical length <25 mm and ‘sludge’ confers an odds ratio of 14.8 and 9.9 for spontaneous preterm delivery at <28 and <32 weeks, respectively.
Conclusions
AF ‘sludge’ is an independent risk factor for spontaneous preterm delivery, preterm PROM, MIAC, and histologic chorioamnionitis in asymptomatic patients at high risk for spontaneous preterm delivery. Furthermore, the combination of ‘sludge’ and a short cervix confers a higher risk for spontaneous preterm delivery at <28 and <32 weeks than that of a short cervix alone.
Keywords: amniotic fluid ‘sludge’, preterm labor, transvaginal ultrasound, spontaneous preterm delivery, microbial invasion of the amniotic cavity, MIAC, chorioamnionitis, funisitis, preterm premature rupture of membranes, PPROM
INTRODUCTION
The sonographic finding of dense aggregates of particulate matter in the amniotic fluid close to the internal cervical os, known as amniotic fluid (AF) ‘sludge’, is associated with impending preterm delivery, microbial invasion of the amniotic cavity (MIAC), and histologic chorioamnionitis in patients with spontaneous preterm labor and intact membranes.1 Similar observations were recently reported among patients with a history of preterm delivery or threatened preterm labor.2
Intra-amniotic infection is sometimes chronic in nature.3-5 Indeed, accumulating evidence indicates that asymptomatic patients with a positive amniotic fluid culture at the time of mid-trimester amniocentesis have a higher risk of adverse pregnancy outcome including fetal loss and/or preterm delivery.3-5 To the extent that AF ‘sludge’ may represent clusters of bacteria and inflammatory cells,1 it is possible that the presence of AF ‘sludge’ may represent chronic intra-amniotic infection.
AF ‘sludge’ has been observed in some asymptomatic patients with risk factors for preterm delivery. However, the clinical implications of the presence of this ultrasonographic finding in these patients are unknown. The objective of this study was to determine the clinical significance of amniotic fluid ‘sludge’ in asymptomatic patients at high-risk for spontaneous preterm delivery.
METHODS
Study design
A retrospective case-control study was conducted by searching our clinical database and digital library of ultrasound images from consecutive patients at high-risk for spontaneous preterm delivery between March 2002 and November 2005. Inclusion criteria were the following: 1) Transvaginal ultrasound (TVUS) of the cervix between 13 and 29 completed weeks of gestation; 2) history of spontaneous preterm delivery; 3) prior mid-trimester loss; 4) short cervix, defined as a mid-trimester cervical length <25 mm on TVUS; 5) mullerian duct anomalies; and/or 6) history of cone biopsy. Exclusion criteria were: 1) threatened preterm labor in the index pregnancy; 2) multiple gestations; 3) fetal congenital anomalies; 4) placenta previa; and 5) presence of irregular uterine contractions.
All patients provided written informed consent before participating in the study. The use of clinical and ultrasound data for research purposes was approved by the Institutional Review Boards of Wayne State University and the National Institute of Child Health and Human Development (NICHD/NIH/DHHS).
Definitions and study procedures
Gestational age was determined by the last menstrual period or by ultrasound in case the ultrasonographic determination of gestational age was not consistent with the menstrual dating by >2 weeks. Spontaneous preterm parturition was defined by the presence of regular uterine contractions and cervical changes that led to delivery before 37 completed weeks of gestation. Preterm PROM was diagnosed by sterile speculum examination confirming pooling of amniotic fluid in the vagina (with nitrazine and ferning tests when necessary) before 37 weeks of gestation and in the absence of labor. Vaginal bleeding of uterine origin was diagnosed by sterile speculum examination confirming the presence of blood coming through the external cervical os.
Amniotic fluid collection was performed by transabdominal amniocentesis under ultrasonographic guidance for clinical indications (e.g. chromosomal analysis) or to determine the microbiologic state of the amniotic cavity in patients with a transvaginal cervical length <25 mm, because of previous observations demonstrated an association between a short cervix and histologic chorioamnionitis,6 which subsequently have been also linked to sub-clinical intra-amniotic infection.7 Amniotic fluid was transported to the laboratory in a sterile capped plastic syringe and cultured for aerobic and anaerobic bacteria, as well as genital Mycoplasmas. White blood cell (WBC) count, glucose concentration, and Gram stain for microorganisms were performed in amniotic fluid shortly after collection using methods previously described.8-10
Microbial invasion of the amniotic cavity and intra-amniotic inflammation (IAI) were defined as a positive amniotic fluid culture for microorganisms and a WBC count >50 cells/mm3,10 respectively. Clinical chorioamnionitis was diagnosed according to the criteria proposed by Gibbs et al11 including maternal temperature of ≥37.8°C and two or more of the following criteria: 1) uterine tenderness; 2) malodorous vaginal discharge; 3) maternal leukocytosis (WBC ≥15,000 cells/mm3); 4) maternal tachycardia (>100 beats/min); and 5) fetal tachycardia (≥160 beats/min). The diagnosis of histologic chorioamnionitis was based on the presence of inflammatory cells in the chorionic plate and/or chorioamniotic membranes. Acute funisitis was diagnosed by the presence of neutrophils in the wall of the umbilical vessels and/or Wharton’s jelly using the criteria previously described.12
Composite severe neonatal morbidity was defined in the presence of one or more of the following neonatal complications: 1) sepsis or suspected sepsis; 2) assisted ventilation due to respiratory distress syndrome; 3) bronchopulmonary dysplasia; 4) intraventricular hemorrhage (IVH); and 5) necrotizing enterocolitis. Neonatal sepsis was diagnosed in the presence of a positive blood culture. Suspected neonatal sepsis was diagnosed in the absence of a positive blood culture, but in the presence of clinical, radiographic or laboratory parameters suspicious for infection, such as a WBC count <5,000 cells/mm3, polymorphonuclear leukocyte count of <1,800 cells/mm3, ratio of immature neutrophils to total neutrophils >0.2, and/or a positive aspirate for polymorphonuclear leukocytes >5 per high power field. The diagnosis of respiratory distress syndrome required the presence of respiratory grunting and retracting, increased oxygen requirement and diagnostic radiographic and laboratory findings in the absence of evidence for other causes of respiratory disease.13 Bronchopulmonary dysplasia was diagnosed if the neonate required oxygen and ventilatory therapy for more than 28 days during the first 2 months of life, had typical radiographic changes, and/or had dysplasia of the bronchopulmonary tree at autopsy.13 Intraventricular hemorrhage was diagnosed by ultrasonographic examination of the neonatal head. Necrotizing enterocolitis was diagnosed in the presence of abdominal distention and feeding intolerance for at least 24 hours (vomiting or increased gastric residual), with clear radiologic evidence of intramural air, perforation, meconium plug syndrome or definite surgical or autopsy findings of necrotizing enterocolitis.13
Sonographic assessment of the cervix
Transvaginal ultrasounds were conducted with commercially available two-dimensional (2D) and three-dimensional (3D) ultrasound systems (Acuson Sequoia, Siemens Medical Systems, Mountain View, CA and Voluson 730, GE Healthcare, Milwaukee, WI) equipped with endovaginal transducers with frequency ranges of 5-7.5 MHz and 5-9 MHz, respectively. All sonographic examinations of the cervix were performed by Registered Diagnostic Medical Sonographers using a technique previously described,14 and reviewed by a perinatologist. Amniotic fluid ‘sludge’ was defined as the presence of dense aggregates of particulate matter in proximity to the internal cervical os (Figure 1). Two experienced sonographers, blinded to clinical outcome, reviewed the 2D images and 3D volume datasets of the cervix. Amniotic fluid ‘sludge’ was considered present when identified by both sonographers.
Figure 1.
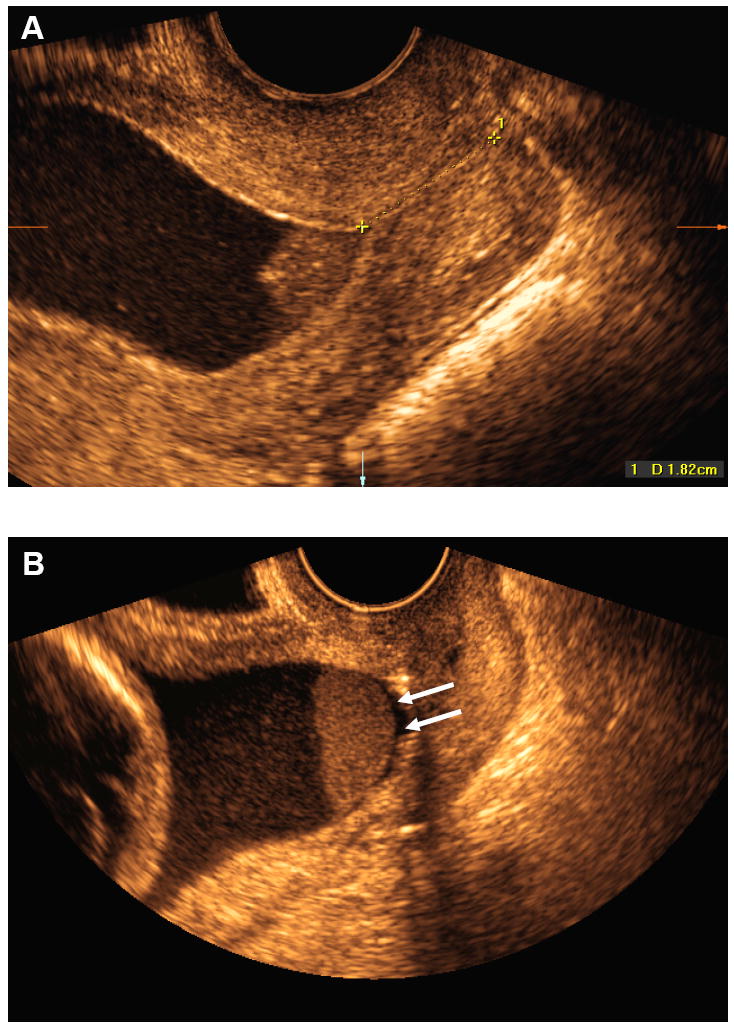
A) Transvaginal ultrasound image showing the presence of dense aggregates of particulate matter (amniotic fluid ‘sludge’) in the proximity of the internal cervical os; B) Similar image showing a slight detachment of the fetal membranes (white arrows) at the level of the internal cervical os. The ‘sludge’ contour is well defined, confirming that ‘sludge’ is located in the amniotic cavity.
Statistical analysis
Outcome variables included: 1) spontaneous preterm delivery at <28 weeks, <32 weeks and <35 weeks of gestation; 2) preterm PROM; 3) microbial invasion of the amniotic cavity; 4) intra-amniotic inflammation; 5) clinical chorioamnionitis; 6) histologic chorioamnionitis; 7) admission to the neonatal intensive care unit (NICU); 8) composite severe neonatal morbidity; 9) neonatal death; and 10) ultrasound-to-delivery and ultrasound-to-preterm PROM intervals. Patients with indicated preterm delivery (e.g. fetal distress, placental abruption, severe maternal condition, etc.) were excluded from the sub-analysis to determine the association between AF ‘sludge’ and spontaneous preterm delivery.
Comparisons among groups were performed using Chi-square or Fisher’s exact test for categorical variables, and Mann-Whitney U test for continuous variables. Diagnostic indices, predictive values, and likelihood ratios of the presence of cervical length <25 mm or AF ‘sludge’ were calculated for the identification of spontaneous preterm delivery at <28 weeks, <32 weeks, and <35 weeks of gestation in a subgroup of asymptomatic high-risk patients with ultrasound examination at 14-24 weeks, because this is the gestational age range at which cervical measurement is performed in most centers.15-20
Multivariable stepwise logistic regression analyses were performed to determine the relationship between the presence of AF ‘sludge’ and other potential explanatory variables (cervical length of <25 mm, gestational age at the time of ultrasound examination, prior preterm delivery, vaginal bleeding in the index pregnancy, and cervical cerclage) in the prediction of the study outcomes. For the analysis of neonatal outcomes, the gestational age at delivery was also included as a covariate. A Kaplan-Meier survival analysis was performed to assess the ultrasound-to-delivery and ultrasound-to-preterm PROM intervals according to the presence or absence of AF ‘sludge’. Patients who delivered preterm for maternal or fetal indications were included in the analysis with a censored time that was equal to the ultrasound-to-delivery interval. Cox regression analysis was performed to assess the ultrasound-to-delivery and ultrasound-to-preterm PROM intervals while controlling for the above mentioned covariates. A p value of <0.05 was considered statistically significant (SPSS 14.0, SPSS Inc., Chicago, IL, USA).
RESULTS
Prevalence of outcome variables among asymptomatic patients at high-risk for preterm delivery
Two hundred eighty-one patients met the inclusion criteria. The prevalence of AF ‘sludge’ in asymptomatic patients at high–risk for spontaneous preterm delivery was 23.5% (66/281). A cervical length of <25 mm was present in 50.5% (142/281) of the patients. The prevalence of preterm PROM was 19.6% (55/281), and the rates of spontaneous preterm delivery at <28 weeks, <32 weeks, <35 weeks, and <37 weeks of gestation were 14.7% (29/197), 21.3% (46/216), 28.7% (62/216), and 42.1% (91/216), respectively. Clinical and histologic chorioamnionitis were diagnosed in 7.5% (21/281) and 36.4% (94/258) of the patients, respectively.
Demographic and clinical characteristics of the study population
The demographic and clinical characteristics of the study population are described in Table I and Table II, respectively. Patients with AF ‘sludge’ had significantly shorter cervical length at ultrasound, lower gestational age at delivery, and lower birth weight compared to patients without AF ‘sludge’ (Table II). A significantly higher proportion of patients with AF ‘sludge’ had a cervical length <25 mm, as well as vaginal bleeding and cervical cerclage in the index pregnancy than those without AF ‘sludge’ (Table II). In addition, the shorter the cervical length, the higher the frequency of AF ‘sludge’ (Table III).
Table I.
Demographic characteristics of asymptomatic patients at risk for spontaneous preterm delivery according to the presence or absence of AF ‘sludge’
| Demographic characteristics | No ‘sludge’ (n=215) | ‘sludge’ present (n=66) | p |
|---|---|---|---|
| Maternal age (years) | 26 (21 – 30) | 26.5 (23 – 32) | NS |
| Gravidity | 4 (2 – 5) | 4 (2 – 5) | NS |
| Prior preterm delivery | 46.5 (100/215) | 36.4 (24/66) | NS |
| Race | |||
| African-American | 89.8 (193/215) | 90.9 (60/66) | NS |
| Caucasian | 6 (13/215) | 4.5 (3/66) | NS |
| Other | 4.2 (9/215) | 4.5 (3/66) | NS |
| Risk factors for preterm delivery | |||
| History of preterm delivery | 34.4 (74/215) | 31.8 (21/66) | NS |
| History second trimester losses | 10.2 (22/215) | 9.1 (6/66) | NS |
| Short cervix | 43.3 (93/215) | 42.4 (28/66) | NS |
| More than one risk factor | 12.1 (26/215) | 16.7 (11/66) | NS |
| Smoking | 22.4 (43/192) | 17.5 (10/57) | NS |
Values are expressed as percentage (number) or median (interquartile range).
NS: not significant.
Table II.
Clinical characteristics of asymptomatic patients at risk for spontaneous preterm delivery according to the presence or absence of AF ‘sludge’
| Clinical characteristics | No ‘sludge’ (n=215) | ‘sludge’ present (n=66) | p |
|---|---|---|---|
| Gestational age at ultrasound (weeks) | 22.7 (18.9 – 26.1) | 21.6 (17.7 – 23.5) | 0.02 |
| Cervical length at ultrasound (mm) | 27 (17 – 35) | 15 (4 – 27) | <0.001 |
| Cervical length <15 mm | 16.3 (35/215) | 50 (33/66) | <0.001 |
| Cervical length <25 mm | 43.3 (93/215) | 74.2 (49/66) | <0.001 |
| Cervical length ≥30 mm | 44.7 (96/215) | 19.7 (13/66) | <0.001 |
| Gestational age at delivery (weeks) | 37.9 (35.3 – 39.3) | 30.7 (24.2 – 37.9) | <0.001 |
| Birth weight (g) | 2868 (2409 – 3221) | 1430 (600 – 2969) | <0.001 |
| Vaginal bleeding in the index pregnancy | 11.6 (25/215) | 24.2 (16/66) | 0.01 |
| Cerclage in the index pregnancy | 13 (28/215) | 31.8 (21/66) | <0.001 |
Values are expressed as percentage (number) or median (interquartile range).
Table III.
Frequency of AF ‘sludge’ according to cervical length
| AF ‘sludge’ present | |
|---|---|
| Cervical length <5 mm | 69 (20/29) |
| Cervical length <15 mm | 49 (33/68) |
| Cervical length <25 mm | 35 (49/142) |
| Cervical length >30 mm | 12 (12/99) |
Values are expressed as percentage (number).
AF ‘sludge’ and its association with spontaneous preterm delivery
A higher proportion of patients with AF ‘sludge’ had spontaneous preterm delivery at <28 weeks [46.5% (20/43) vs. 5.8% (9/154), p<0.001], <32 weeks [55.6% (25/45) vs. 12.3% (21/171), p<0.001], and <35 weeks [62.2% (28/45) vs. 19.9% (34/171), p<0.001] than those without AF ‘sludge’ (Table I in supplemental material). The frequency of spontaneous preterm delivery at <28 weeks and <32 weeks was higher in patients with AF ‘sludge’ than in those without it, regardless of cervical length (Figures 2 and 3).
Figure 2.
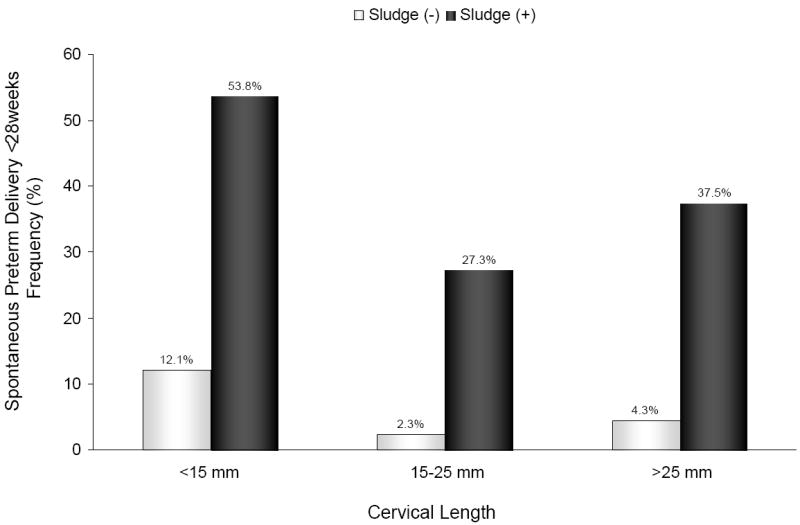
Frequency of spontaneous preterm delivery at <28 weeks of gestation according to cervical length and the presence or absence of AF ‘sludge’: [<15 mm: ‘sludge’ (+) 53.8% vs. ‘sludge’ (-) 12.1%; p=0.001]; [15-25 mm: ‘sludge’ (+) 27.3% vs. ‘sludge’ (-) 2.3%; p=0.02]; and [>25 mm: ‘sludge’ (+) 37.5% vs. ‘sludge’ (-) 4.3%; p<0.001].
Figure 3.
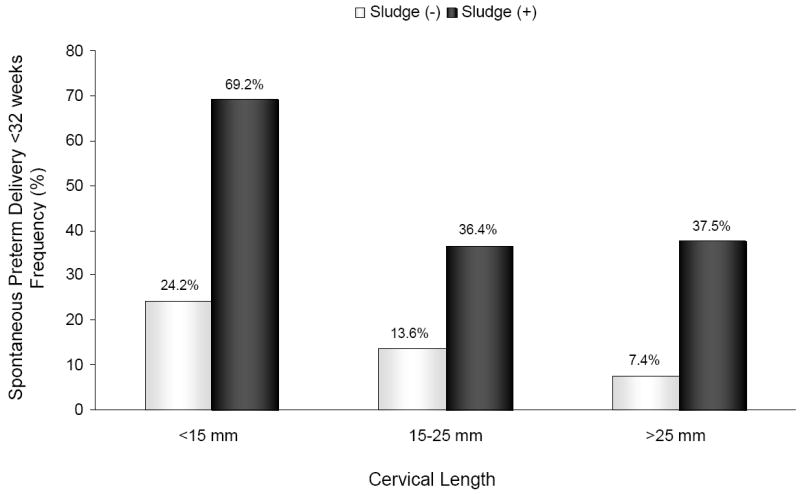
Frequency of spontaneous preterm delivery at <32 weeks of gestation according to cervical length and the presence or absence of AF ‘sludge’: [<15 mm: ‘sludge’ (+) 69.2% vs. ‘sludge’ (-) 24.2%; p<0.001]; [15-25 mm: ‘sludge’ (+) 36.4% vs. ‘sludge’ (-) 13.6%; p=0.1]; and [>25 mm: ‘sludge’ (+) 37.5% vs. ‘sludge’ (-) 7.4%; p=0.006].
Patients with ‘sludge’ had a shorter ultrasound-to-delivery interval than those without AF ‘sludge’ [AF ‘sludge’ positive, median: 127 days (95% CI: 120-134) vs. AF ‘sludge’ negative, median: 161 days (95% CI: 153-169); log rank test, p<0.001] (Figure 4). These results remained significant after adjusting for the presence of ‘sludge’, cervical length of <25 mm, prior preterm delivery, gestational age at ultrasound, vaginal bleeding, and cervical cerclage (hazard ratio: 2.96; 95% CI: 1.6-5.3).
Figure 4.
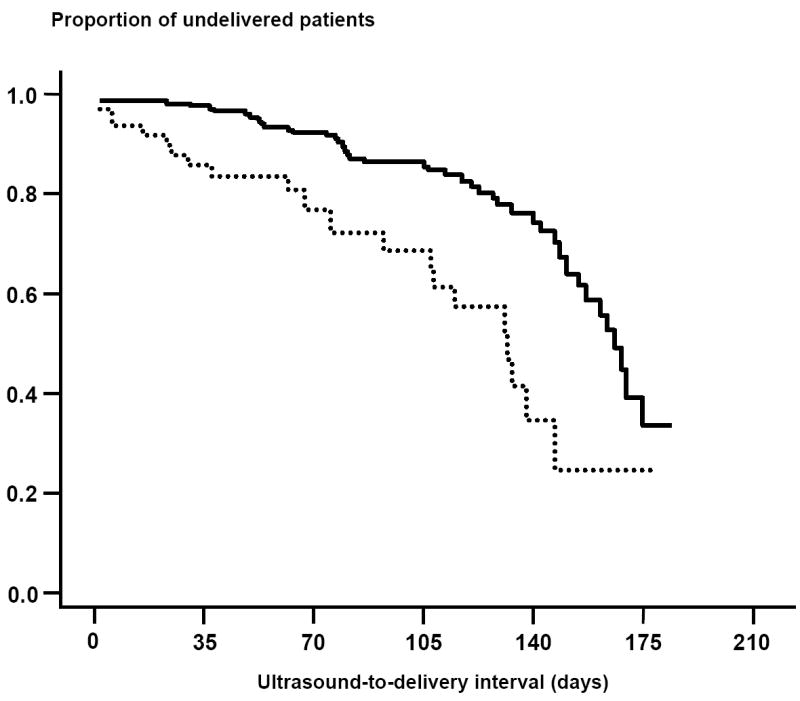
Kaplan-Meier survival analysis of the ultrasound-to-delivery interval (days) according to the presence or absence of AF ‘sludge’ in asymptomatic high-risk patients for preterm delivery. Patients with ‘sludge’ (dotted line) had a shorter ultrasound-to-delivery interval than those without AF ‘sludge’ (solid line). [AF ‘sludge’ positive, median: 127 (95% CI: 120-134) days vs. AF ‘sludge’ negative, median: 161 (95% CI: 153-169) days; log rank test, p<0.001].
Patients with AF ‘sludge’ also had a higher frequency of clinical chorioamnionitis [15.2% (10/66) vs. 5.1% (11/215), p=0.007], histologic chorioamnionitis [61.5% (40/65) vs. 28% (54/193), p<0.001], and funisitis [32.3% (21/65) vs. 19.2% (37/193), p=0.03] than those without AF ‘sludge’ (Table I in supplemental material).
AF ‘sludge’ and its association with preterm PROM
Patients with AF ‘sludge’ had a higher frequency of preterm PROM [(39.4% (26/66) vs. 13.5% (29/215), p<0.001], and a lower gestational age at preterm PROM [median: 24.7 weeks (22.3-28.1) vs. median: 32.3 weeks (27.7-34.8); p<0.001] than those without AF ‘sludge’ (Table I in supplemental material). Patients with ‘sludge’ had a shorter ultrasound-to-preterm PROM interval than those without AF ‘sludge’ [AF ‘sludge’ positive, median: 23 days (95% CI: 7-39) vs. AF ‘sludge’ negative, median: 57 days (95% CI: 38-77); log rank test, p=0.003] (Figure 1 in supplemental material). These results remained significant after adjusting for the presence of AF ‘sludge’ and other explanatory variables listed in the analysis of the ultrasound-to-delivery interval in women with intact membranes (hazard ratio: 2.76; 95% CI: 1.5-5.1).
A sub-analysis among patients with preterm PROM (n=55) indicated that those with AF ‘sludge’ had a shorter cervical length at ultrasound and earlier gestational age at delivery than those without AF ‘sludge’. In addition, those with AF ‘sludge’ have a higher proportion of histologic chorioamnionitis, proven or suspected neonatal sepsis, composite severe neonatal morbidity, and neonatal death than those without AF ‘sludge’ (Table II in supplemental material).
AF ‘sludge’ and its association with intra-amniotic infection/inflammation
Analysis restricted to 51 asymptomatic patients who underwent transabdominal amniocentesis within 14 days of transvaginal ultrasound because of a short cervix indicated that a significantly higher proportion of patients with AF ‘sludge’ had MIAC [21.7 % (5/23) vs. 0% (0/28), p=0.01], and intra-amniotic inflammation [27.3% (6/22) vs. 3.6% (1/28), p=0.03] than those without AF ‘sludge’. No significant differences were found in gestational age, cervical length, and cervical dilatation at the time of amniocentesis between patients with and without AF ‘sludge’ (Table III in supplemental material). Microorganisms isolated from the AF of patients with ‘sludge’ included Ureaplasma urealyticum (n=3), Staphylococcus aureus (n=1), and Fusobacterium nucleatum (n=1).
AF ‘sludge’ and its association with neonatal outcomes
The prevalence of admission to NICU, composite severe neonatal morbidity, and neonatal death in the study population was 20.7% (58/280), 22.8% (58/254), and 2.7% (7/255), respectively. A significantly higher proportion of neonates born to patients with AF ‘sludge’ was admitted to NICU, had a composite severe neonatal morbidity, and died in the neonatal period than those born to women without AF ‘sludge’ (Table IV).
Table IV.
Neonatal outcomes according to the presence or absence of AF ‘sludge’ in asymptomatic patients at risk for spontaneous preterm delivery
| Outcome variables | No ‘sludge’ (n=215) | ‘sludge’ present (n=66) | p |
|---|---|---|---|
| Admission to NICU | 17.3 (37/214) | 31.8 (21/66) | 0.01 |
| Sepsis or suspected sepsis | 5.8 (12/206) | 27.1 (13/48) | <0.001 |
| Mechanical ventilation | 13.6 (28/206) | 39.6 (19/48) | <0.001 |
| Bronchopulmonary dysplasia | 1.9 (4/215) | 12.1 (8/66) | 0.001 |
| Necrotizing enterocolitis | 0 (0/206) | 8.9 (4/45) | 0.001 |
| Intraventricular hemorrhage | 3.9 (8/206) | 19.1 (9/47) | 0.001 |
| Composite severe neonatal morbidity | 17 (35/206) | 47.9 (23/48) | <0.001 |
| Neonatal death | 0.5 (1/206) | 12.2 (6/49) | <0.001 |
Values are expressed as percentage (number).
NICU: neonatal intensive care unit.
Composite severe neonatal morbidity was defined as one or more of the following neonatal complications: sepsis or suspected sepsis, use of assisted ventilation due to respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular hemorrhage, or necrotizing enterocolitis.
Subgroup of patients examined between 14-24 weeks
The prevalence of AF ‘sludge’ in this subgroup was 29.9% (52/174). A subanalysis performed at this gestational age interval showed similar results to the ones described for the entire population (Table V). The diagnostic indices, predictive values, and likelihood ratios of the presence of cervical length <25 mm or AF ‘sludge’ for the identification of patients with spontaneous preterm delivery at <28 weeks, <32 weeks, and <35 weeks are displayed in Table VI. Table VII displays the odds ratios for the occurrence of spontaneous preterm delivery at <28 weeks, <32 weeks, and <35 weeks in the presence of a cervical length of <25 mm, AF ‘sludge’ positive, or both. Of note, the combination of a cervical length of <25 mm and AF ‘sludge’ confers an odds ratio of 14.8 and 9.9 for spontaneous preterm delivery at <28 and <32 weeks, respectively.
Table V.
Outcome variables according to the presence or absence of AF ‘sludge’ in asymptomatic patients at risk for spontaneous preterm delivery between 14-24 weeks of gestation
| Outcome variables | No ‘sludge’ (n=122) | ‘sludge’ present (n=52) | p |
|---|---|---|---|
| Cervical length <25 mm | 36.9 (45/122) | 73.1 (38/52) | <0.001 |
| Cervical length <15 mm | 17.2 (21/122) | 48.1 (25/52) | <0.001 |
| Gestational age at delivery (weeks) | 37.9 (35.1 – 39.4) | 26.9 (22.7 – 37.9) | <0.001 |
| Spontaneous preterm delivery(*) | |||
| <28 weeks | 9.4 (9/96) | 54.3 (19/35) | <0.001 |
| <32 weeks | 14.6 (14/96) | 60 (21/35) | <0.001 |
| <35 weeks | 19.8 (19/96) | 65.7 (23/35) | <0.001 |
| Preterm PROM | 13.9 (17/122) | 42.3 (22/52) | <0.001 |
| Gestational age at preterm PROM (weeks) | 30 (27 – 33.6) | 24 (21 – 27.1) | 0.006 |
| Clinical chorioamnionitis | 4.9 (6/122) | 15.4 (8/52) | 0.02 |
| Histologic chorioamnionitis | 30.3 (33/109) | 62.7 (32/51) | <0.001 |
| Funisitis | 17.4 (19/109) | 29.4 (15/51) | NS |
| Admission to NICU | 15.7 (19/121) | 36.5 (19/52) | 0.002 |
| Sepsis or suspected sepsis | 7 (8/115) | 32.4 (12/37) | <0.001 |
| Composite severe neonatal morbidity | 17.4 (20/115) | 51.4 (19/37) | <0.001 |
| Neonatal death | 0.9 (1/115) | 13.2 (5/38) | 0.004 |
Values are expressed as percentage (number) or median (interquartile range).
PROM: prelabor rupture of membranes; NICU: neonatal intensive care unit; NS: not significant.
Patients with indicated preterm delivery were excluded from the analysis of the association between the presence of AF ‘sludge’ and spontaneous preterm delivery.
Composite severe neonatal morbidity was defined as one or more of the following neonatal complications: sepsis or suspected sepsis, use of assisted ventilation due to respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular hemorrhage, or necrotizing enterocolitis.
Table VI.
Diagnostic indices, predictive values, and likelihood ratios of the presence of cervical length of <25 mm or AF ‘sludge’ positive for the identification of patients with spontaneous preterm delivery at <28 weeks, <32 weeks, and <35 weeks of gestation in asymptomatic high-risk patients at 14-24 weeks ultrasound
| CL <25 mm | Prevalence | Sensitivity | Specificity | PPV | NPV | LR (+) (95% CI) | LR (-) (95% CI) |
|---|---|---|---|---|---|---|---|
| Delivery <28 weeks | 21.4%
(28/131) |
75%
(21/28) |
56%
(58/103) |
32%
(21/66) |
89%
(58/65) |
1.7
(1.3-2.3) |
0.4
(0.3-0.6) |
| Delivery <32 weeks | 26.7%
(35/131) |
74%
(26/35) |
58%
(56/96) |
39%
(26/66) |
86%
(56/65) |
1.8
(1.3-2.4) |
0.4
(0.3-0.6) |
| Delivery <35 weeks | 32.1%
(42/131) |
74%
(31/42) |
61%
(54/89) |
47%
(31/66) |
83%
(54/65) |
1.9
(1.4-2.6) |
0.4
(0.3-0.6) |
|
| |||||||
| AF ‘sludge’ (+) | Prevalence | Sensitivity | Specificity | PPV | NPV | LR (+) (95% CI) | LR (-) (95% CI) |
|
| |||||||
| Delivery <28 weeks | 21.4%
(28/131) |
68%
(19/28) |
85%
(87/103) |
54%
(19/35) |
91%
(87/96) |
4.4
(2.6-7.3) |
0.4
(0.2-0.6) |
| Delivery <32 weeks | 26.7%
(35/131) |
60%
(21/35) |
85%
(82/96) |
60%
(21/35) |
85%
(82/96) |
4.1
(2.4-7.2) |
0.5
(0.3-0.8) |
| Delivery <35 weeks | 32.1%
(42/131) |
55%
(23/42) |
87%
(77/89) |
66%
(23/35) |
80%
(77/96) |
4.1
(2.2-7.4) |
0.5
(0.3-0.9) |
CL: cervical length; PPV: positive predictive value; NPV: negative predictive value; LR: likelihood ratio; CI: confidence interval.
Table VII.
Stepwise logistic regression analysis of the presence of cervical length <25 mm, amniotic fluid ‘sludge’ positive, or both as explanatory variables for the occurrence of spontaneous preterm delivery at <28 weeks, <32 weeks, and <35 weeks of gestation in asymptomatic high-risk patients at 14-24 weeks ultrasound
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
|---|---|---|---|
| CL <25 mm | AF ‘sludge’ positive | CL <25 mm + AF ‘sludge’ positive | |
| Delivery <28 weeks | 6.8 (1.2 – 39.5) | 9.1 (2.9 – 28.6) | 14.8 (3.9 – 56.5) |
| Delivery <32 weeks | 6.1 (1.4 – 27.5) | 6.2 (2.2 – 17.3) | 9.9 (3 – 32.4) |
| Delivery <35 weeks | 7.2 (1.8 – 28.3) | 5.3 (2 – 14) | 6.9 (2.3 – 20.5) |
CL: cervical length; OR: odds ratio; CI: confidence interval.
Explanatory variables: sludge, cervical length <25 mm, gestational age at ultrasound, prior preterm delivery, vaginal bleeding, and cervical cerclage.
AF ‘sludge’ as an independent risk factor for the occurrence of outcome variables
Multivariable stepwise logistic regression analysis showed that the presence of AF ‘sludge’ was an independent explanatory variable for the occurrence of spontaneous preterm delivery at <28 weeks, <32 weeks, and <35 weeks of gestation, preterm PROM, MIAC, and histologic chorioamnionitis, but not for adverse neonatal outcomes. As expected, the most important factor associated with adverse neonatal outcomes was gestational age at delivery (Table VIII). Of note, cervical cerclage was not an explanatory variable for the occurrence of all outcomes.
Table VIII.
Stepwise logistic regression analysis of the presence of amniotic fluid ‘sludge’ as an explanatory variable for the occurrence of outcome variables
| Outcome variable | OR | 95% CI | p |
|---|---|---|---|
| Spontaneous preterm delivery | |||
| <28 weeks | 10.4 | 3.5 – 30.9 | <0.001 |
| <32 weeks | 5.4 | 2.4 – 12.5 | <0.001 |
| <35 weeks | 4.1 | 1.9 – 9.0 | <0.001 |
| Preterm PROM | 3.1 | 1.6 – 6.1 | 0.001 |
| MIAC | 10.9 | 1.2 – 102.2 | 0.04 |
| Clinical chorioamnionitis | 2.1 | 0.8 – 5.6 | NS |
| Histologic chorioamnionitis | 3.0 | 1.6 – 5.6 | <0.001 |
| Funisitis | 1.7 | 0.8 – 3.3 | NS |
| Admission to NICU | 0.7 | 0.3 – 1.6 | NS |
| Composite severe neonatal morbidity | 2.3 | 0.6 – 9.6 | NS |
PROM: prelabor rupture of membranes; MIAC: microbial invasion of the amniotic cavity; NICU: neonatal intensive care unit; OR: odds ratio; NS: not significant.
Composite severe neonatal morbidity was defined as one or more of the following neonatal complications: sepsis or suspected sepsis, use of assisted ventilation due to respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular hemorrhage, or necrotizing enterocolitis.
Explanatory variables: sludge, cervical length <25 mm, gestational age at ultrasound, prior preterm delivery, vaginal bleeding, and cervical cerclage. For the analysis of neonatal outcomes, gestational age at delivery was also included as a covariate.
DISCUSSION
Principal findings of this study
1) The prevalence of AF ‘sludge’ in this population of asymptomatic high-risk patients for preterm delivery between 13 and 29 completed weeks of gestation is 23.5%; 2) AF ‘sludge’ is an independent risk factor for spontaneous preterm delivery at <28 weeks, <32 weeks, and <35 weeks of gestation, preterm PROM, MIAC, and histologic chorioamnionitis; and 3) asymptomatic patients with AF ‘sludge’ had shorter ultrasound-to-delivery and ultrasound-to-preterm PROM intervals than those without AF ‘sludge’.
The prevalence of AF ‘sludge’ in asymptomatic high-risk patients for preterm delivery
Our group had recently reported that the prevalence of AF ‘sludge’ in normal pregnancies at term is 1%, whereas in patients with spontaneous preterm labor and intact membranes is 22.6%.1 Of note, this prevalence is similar to the one presented herein among asymptomatic patients (23.5%). We propose that this reflects the high risk nature of the population included in the present study. Indeed, the prevalence of spontaneous preterm delivery at <32 weeks and <37 weeks of gestation was 21.3% (46/216) and 42.1% (91/216), respectively. The prevalence and clinical significance of AF ‘sludge’ in asymptomatic low-risk patients throughout gestation is unknown. Additional studies should be conducted to address this important clinical question.
AF ‘sludge’ and its association with intra-amniotic infection/inflammation
Patients with AF ‘sludge’ had a significantly higher frequency of MIAC, intra-amniotic inflammation, clinical chorioamnionitis, and histologic chorioamnionitis than patients without ‘sludge’. This is consistent with the previous report that AF ‘sludge’ is associated with positive AF cultures and histologic chorioamnionitis in patients with preterm labor and intact membranes.1 In addition, the frequency of funisitis, which is the histologic counterpart of the fetal inflammatory response syndrome (FIRS),12;21 was significantly higher among patients with AF ‘sludge’.
Since the incidence of vaginal bleeding is higher in symptomatic and asymptomatic patients with AF ‘sludge’ it is possible that, in some patients, the presence of AF ‘sludge’ may represent blood breakdown products; however, the following observations make this possibility less likely: 1) we have obtained samples of AF ‘sludge’ under ultrasound guidance in patients with impending preterm delivery, and the microbiologic examination of these samples indicate that they represent clusters of inflammatory cells and bacteria (Espinoza et al, unpublished observations); 2) a recent study22 reported that MIAC is present in 14% of patients with “idiopathic” vaginal bleeding between 18 to 35 weeks of gestation, and that “idiopathic” vaginal bleeding is associated with preterm delivery <32 weeks and subsequent preterm PROM, suggesting that vaginal bleeding may be the only clinical manifestation of intra-amniotic infection; and 3) vaginal bleeding was included in the logistic regression analysis as a covariate to determine its relationship with the presence of AF ‘sludge’ and other potential explanatory variables in the prediction of the study outcomes. The results of this analysis showed that AF ‘sludge’ was an independent risk factor for the occurrence of the study outcomes.
It has been recently reported that the rate of MIAC in asymptomatic patients with a cervical length of <25 mm in the mid-trimester is 9%.7 Of note, the prevalence of MIAC among patients with AF ‘sludge’ who underwent amniocentesis within 2 weeks of the ultrasound was 21.7%; in contrast, no patients without AF ‘sludge’ had positive AF cultures. Collectively, these results support the notion that AF ‘sludge’ may represent clusters of bacteria and inflammatory cells.
AF ‘sludge’ as a risk factor for adverse pregnancy outcomes
The observation that AF ‘sludge’ is associated with spontaneous preterm delivery at <28 weeks, <32 weeks, and <35 weeks of gestation, preterm PROM, MIAC, and histologic chorioamnionitis in asymptomatic patients at high-risk for spontaneous preterm delivery is novel. In addition, those with AF ‘sludge’ have a higher frequency of short cervix (<25 mm), clinical chorioamnionitis, funisitis, admission to NICU, composite severe neonatal morbidity and neonatal death, earlier gestational age at the time of preterm PROM, as well as shorter ultrasound-to-delivery and ultrasound-to-preterm PROM intervals than those without ‘sludge’. Thus, the sonographic finding of AF ‘sludge’ in asymptomatic patients at high-risk for spontaneous preterm delivery should be considered as a risk factor for adverse pregnancy outcome. To the extent that AF ‘sludge’ may represent intra-amniotic infection/inflammation, these observations are consistent with a solid body of evidence indicating the association between intra-amniotic infection and the aforementioned adverse pregnancy outcomes.3-5;7;23-28
Short cervix and AF ‘sludge’ in the prediction of spontaneous preterm delivery among asymptomatic high-risk patients
Our results that a cervical length of <25 mm between 14-24 weeks is associated with an increased risk for spontaneous preterm delivery at <28, <32, and <35 weeks are consistent with a solid body of evidence that a sonographic short cervix in the mid-trimester is a useful tool for the assessment of risk for spontaneous preterm delivery in low-14;15;18;29 and high-risk populations.16;17;19;20;30-32 However, this study further demonstrates that the presence of AF ‘sludge’ is an independent explanatory variable for the occurrence of spontaneous preterm delivery, and that this sonographic finding confers an odds ratio of 9.1 and 6.2 for spontaneous preterm delivery at <28 and <32 weeks, respectively. In addition, the combination of a cervical length of <25 mm and AF ‘sludge’ confers an odds ratio of 14.8 and 9.9 for spontaneous preterm delivery at <28 and <32 weeks, respectively. However, AF ‘sludge’ did not increase the odds ratios for spontaneous preterm delivery at < 35 weeks (Table VII).
Limitations of the study
The limitations of this study include its retrospective nature, and that the study population may represent a group of patients at very high-risk for preterm delivery, because 42% of them delivered at <37 weeks of gestation. It could be argued that the identification of AF ‘sludge’ is subjective; however, two experienced sonographers, blinded to the clinical information, ascertained the presence of AF ‘sludge’ using a strict definition previously described.1
Conclusions
Our results indicate that AF ‘sludge’ is an independent risk factor for spontaneous preterm delivery, preterm PROM, microbial invasion of the amniotic cavity, and histologic chorioamnionitis in asymptomatic patients at high risk for spontaneous preterm delivery. Moreover, the combination of AF ‘sludge’ and a short cervix (<25 mm) confers a higher risk for spontaneous preterm delivery at <28 and <32 weeks than that conferred by a short cervix alone.
Acknowledgments
The authors wish to acknowledge the contributions of the Registered Diagnostic Medical Sonographers staff of the Perinatology Research Branch: Ms Susan Stites, Ms Lisa Fisher, Ms Anna Peshkess, and Ms Catherine Ducharme, as well as the Nursing staff of the Perinatology Research Branch and Detroit Medical Center: Ms Nancy Hauff, Ms Sandy Field, Ms Lorraine Nikita, Ms Vicky Ineson, Ms Evie Russell, Ms Mahbubeh Mahmoudieh, Ms Julie McKinley, Ms Sue Rehel, Ms Shannon Donegan, Ms Linda Bouey, Ms Carolyn Sudz, Ms Sylvia Warren, Ms Shelley Mullen, Ms Gail Bartley, Ms Denise Bayoneto, Ms Judy Kerman, Ms Barbara Steffy, Ms Milagros Kitchen, and Ms Leandra Ga-Pinlac.
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Espinoza J, Goncalves LF, Romero R, Nien JK, Stites S, Kim YM, et al. The prevalence and clinical significance of amniotic fluid ‘sludge’ in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 2005;25:346–52. doi: 10.1002/uog.1871. [DOI] [PubMed] [Google Scholar]
- 2.Bujold E, Pasquier JC, Simoneau J, Arpin MH, Duperron L, Morency AM, et al. Intra-amniotic sludge, short cervix, and risk of preterm delivery. J Obstet Gynaecol Can. 2006;28:198–202. doi: 10.1016/S1701-2163(16)32108-9. [DOI] [PubMed] [Google Scholar]
- 3.Cassell GH, Davis RO, Waites KB, Brown MB, Marriott PA, Stagno S, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16-20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis. 1983;10:294–302. [PubMed] [Google Scholar]
- 4.Gray DJ, Robinson HB, Malone J, Thomson RB., Jr Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn. 1992;12:111–17. doi: 10.1002/pd.1970120206. [DOI] [PubMed] [Google Scholar]
- 5.Horowitz S, Mazor M, Romero R, Horowitz J, Glezerman M. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med. 1995;40:375–79. [PubMed] [Google Scholar]
- 6.Guzman ER, Shen-Schwarz S, Benito C, Vintzileos AM, Lake M, Lai YL. The relationship between placental histology and cervical ultrasonography in women at risk for pregnancy loss and spontaneous preterm birth. Am J Obstet Gynecol. 1999;181:793–97. doi: 10.1016/s0002-9378(99)70303-0. [DOI] [PubMed] [Google Scholar]
- 7.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–19. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–74. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 10.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–30. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145:1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 13.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 14.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 15.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–17. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 16.Guzman ER, Mellon C, Vintzileos AM, Ananth CV, Walters C, Gipson K. Longitudinal assessment of endocervical canal length between 15 and 24 weeks’ gestation in women at risk for pregnancy loss or preterm birth. Obstet Gynecol. 1998;92:31–37. doi: 10.1016/s0029-7844(98)00120-3. [DOI] [PubMed] [Google Scholar]
- 17.Berghella V, Daly SF, Tolosa JE, DiVito MM, Chalmers R, Garg N, et al. Prediction of preterm delivery with transvaginal ultrasonography of the cervix in patients with high-risk pregnancies: does cerclage prevent prematurity? Am J Obstet Gynecol. 1999;181:809–15. doi: 10.1016/s0002-9378(99)70306-6. [DOI] [PubMed] [Google Scholar]
- 18.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–67. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 19.Guzman ER, Walters C, Ananth CV, O’Reilly-Green C, Benito CW, Palermo A, et al. A comparison of sonographic cervical parameters in predicting spontaneous preterm birth in high-risk singleton gestations. Ultrasound Obstet Gynecol. 2001;18:204–10. doi: 10.1046/j.0960-7692.2001.00526.x. [DOI] [PubMed] [Google Scholar]
- 20.Airoldi J, Berghella V, Sehdev H, Ludmir J. Transvaginal ultrasonography of the cervix to predict preterm birth in women with uterine anomalies. Obstet Gynecol. 2005;106:553–56. doi: 10.1097/01.AOG.0000173987.59595.e2. [DOI] [PubMed] [Google Scholar]
- 21.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–29. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 22.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18:31–37. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 23.Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991;165:955–61. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- 24.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992;166:1382–88. doi: 10.1016/0002-9378(92)91609-e. [DOI] [PubMed] [Google Scholar]
- 25.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941–48. [PubMed] [Google Scholar]
- 26.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol. 1998;92:77–82. doi: 10.1016/s0029-7844(98)00122-7. [DOI] [PubMed] [Google Scholar]
- 27.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–36. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 28.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 29.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18-22 weeks’ gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92:902–07. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 30.Andersen HF. Transvaginal and transabdominal ultrasonography of the uterine cervix during pregnancy. J Clin Ultrasound. 1991;19:77–83. doi: 10.1002/jcu.1870190204. [DOI] [PubMed] [Google Scholar]
- 31.Cook CM, Ellwood DA. The cervix as a predictor of preterm delivery in ‘at-risk’ women. Ultrasound Obstet Gynecol. 2000;15:109–13. doi: 10.1046/j.1469-0705.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 32.Owen J, Yost N, Berghella V, Thom E, Swain M, Dildy GA, III, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340–48. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]


