Abstract
Recent evidence suggests that arginine vasopressin (AVP)-dependent aquaporin-2 expression is modulated by the extracellular calcium-sensing receptor (CaSR) in principal cells of the collecting duct, but the signaling pathways mediating this effect are unknown. Using a mouse cortical collecting duct cell line (mpkCCDcl4), we found that increasing the concentration of apical extracellular calcium or treating with the CaSR agonists neomycin or Gd3+ attenuated AVP-dependent accumulation of aquaporin-2 mRNA and protein; CaSR gene-silencing prevented this effect. Calcium reduced the AVP-induced accumulation of cAMP, but this did not occur by increased degradation of cAMP by phosphodiesterases or by direct inhibition of adenylate cyclase. Notably, the effect of extracellular calcium on AVP-dependent aquaporin-2 expression was prevented by inhibition of calmodulin. In summary, our results show that high concentrations of extracellular calcium attenuate AVP-induced aquaporin-2 expression by activating the CaSR and reducing coupling efficiency between V2 receptor and adenylate cyclase via a calmodulin-dependent mechanism in cultured cortical collecting duct cells.
Aquaporins (AQP) facilitate water reabsorption across plasma membranes of renal epithelial cells. The fine-tuning of water reabsorption takes place along the collecting system, where water permeability is controlled by [8-arginine]vasopressin (AVP). An acute increase in AVP concentration enhances water permeability via V2 receptors1 by inducing AQP2 translocation from intracellular vesicles to the apical membrane.2 Water exits the cells via basolateral AQP3 and AQP4.3,4 Conversely, a sustained increase of circulating AVP increases AQP2 and AQP3 abundance and consequently increases collecting duct (CD) water permeability.5
The extracellular calcium-sensing receptor (CaSR), a seven-membrane–spanning G-protein–coupled receptor6 that is expressed in renal epithelial cells,7 is activated by extracellular Ca2+, Mg2+, and other polycations.8 CaSR activation may lead to (1) reduced cAMP levels and protein kinase A (PKA) activity as a result of calcium-sensitive adenylate cyclase (AC) inhibition and phosphodiesterase activation9 and (2) phospholipase C activation and ensuing activation of protein kinase C (PKC) and extracellular signal–regulated kinase (ERK).8 In the CD, CaSR is expressed in the apical membrane, where it senses luminal Ca2+,10 and may antagonize effects of AVP on AQP2 subcellular localization and whole-cell expression. In inner medullary CD (IMCD), elevated luminal Ca2+ blunts AVP-induced acute water permeability.10 In addition, high concentrations of extracellular Ca2+ attenuated forskolin-induced AQP2 plasma membrane targeting in cultured CD cells.11 Moreover, AVP-induced water permeability, AQP2 apical membrane targeting, and protein expression all are decreased in CD of hypercalcemic rats.12,13 We investigated the mechanisms that account for modulated AQP2 expression by extracellular calcium in CD principal cells using the cortical CD (CCD) mpkCCDcl4 cell line exhibiting the major functional properties of CD principal cells.14
Results
High Extracellular Calcium Attenuates AVP-Induced AQP2 Expression
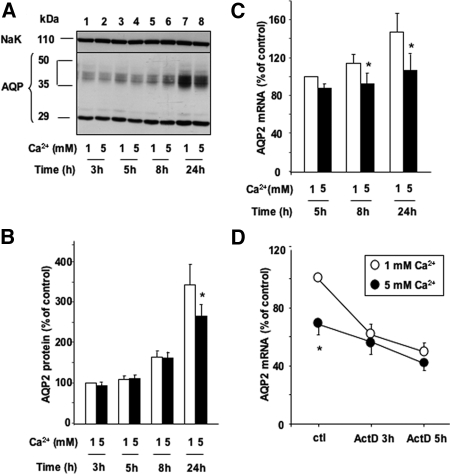
We first examined the effect of extracellular calcium on AVP-dependent AQP2 expression. AVP-treated cells were incubated for 3 to 24 h with either baseline or 5 mM apical calcium. Western blotting analysis showed that AVP-induced AQP2 protein expression was not affected during the first 8 h after calcium supplementation (Figure 1, A, lanes 1 to 6, and B); however, AVP-dependent AQP2 protein accumulation decreased by 31 ± 7.8% 24 h after apical calcium concentration was increased (Figure 1, A, lanes 7 and 8, and B). No significant effect was produced by basolateral calcium supplementation (data not shown). Real-time PCR analysis showed that compared with baseline, 5 mM apical calcium attenuated AVP-induced AQP2 mRNA accumulation in a time-dependent manner (Figure 1C). This effect was significant after 8 h and persisted after 24 h of incubation (as percentage of decreased AQP2 mRNA content from baseline ± SE; 8 h 20.1 ± 7.9; 24 h 25.3 ± 5.6). Elevated calcium did not affect P0 mRNA content (data not shown), which was used as a loading control.
Figure 1.
Effect of high apical calcium on AVP-dependent AQP2 expression in mpkCCDcl4 cells. Confluent mpkCCDcl4 cells grown on filters in culture medium containing baseline (1 mM) calcium were preincubated at 37°C in the presence of 10−9 M AVP for 24 h to induce AQP2 expression. Cells were then incubated in the continuous presence of 10−9 M AVP for different lengths of time (3 to 24 h) with 1 or 5 mM apical calcium. (A and B) Total protein extracts (40 μg) were separated by 10% SDS-PAGE. AQP2 and the Na-K-ATPase α-subunit, used as a loading control, were detected by Western blotting. (A) A representative immunoblot is shown. (B) Densitometric quantification of AQP2 protein expressed as a percentage of control optical density values measured after 3 h of incubation in the presence of 1 mM extracellular calcium (100%). Bars are means ± SEM from 12 independent experiments. (C) RNA was extracted as described in the Concise Methods section. Real-time PCR was performed with primers specific for AQP2. Results are expressed as a percentage of control values determined after 24 h of incubation in the presence of 1 mM calcium (100%). Bars are means ± SEM from seven independent experiments. (D) Cells were preincubated in the continuous presence of AVP and 1 mM (○) or 5 mM (•) apical calcium for 24 h before addition of 5 × 10−6 M actinomycin D (ActD) for 3 or 5 h. RNA was then extracted and real-time PCR was performed. Results are expressed as a percentage of control values determined after 24 h of incubation in the presence of 1 mM calcium and without ActD (control [ctl], 100%). Circles are means ± SEM from four independent experiments. *P < 0.05.
To determine whether extracellular calcium modulates AQP2 mRNA transcription or stability, we measured AQP2 mRNA decay in the presence of actinomycin D, which blocks de novo transcription. AVP-treated cells were incubated for 24 h with either 1 or 5 mM apical calcium and then treated for 3 or 5 h with 5 × 10−6 M actinomycin D. The extent of AQP2 mRNA decay after addition of actinomycin D was similar in cells incubated in the presence of either baseline or high apical calcium (Figure 1D).
The effect of high apical calcium on AQP2 expression did not result from impaired cell growth because transepithelial electrical resistance, an index of cell density and differentiation, was similar in cells incubated 24 h with either 1 or 5 mM apical calcium (as kOhm/cm2± SE; 1 mM Ca2+ 1.52 ± 0.15; 5 mM Ca2+ 1.35 ± 0.29).
CaSR Activation Mediates the Attenuation of AVP-Induced AQP2 Expression by High Extracellular Calcium
To investigate the role of CaSR in modulated AVP-dependent AQP2 expression by extracellular calcium, we assessed the effect of the CaSR agonists neomycin10 and gadolinium.11 AVP-treated cells were incubated for 24 h with or without increasing concentrations of neomycin (100 to 500 μM) or gadolinium (10 to 100 μM) added to the apical medium. Both neomycin (Figure 2, A and B) and gadolinium (Figure 2, C and D) attenuated AVP-induced AQP2 expression in a concentration-dependent manner.
Figure 2.
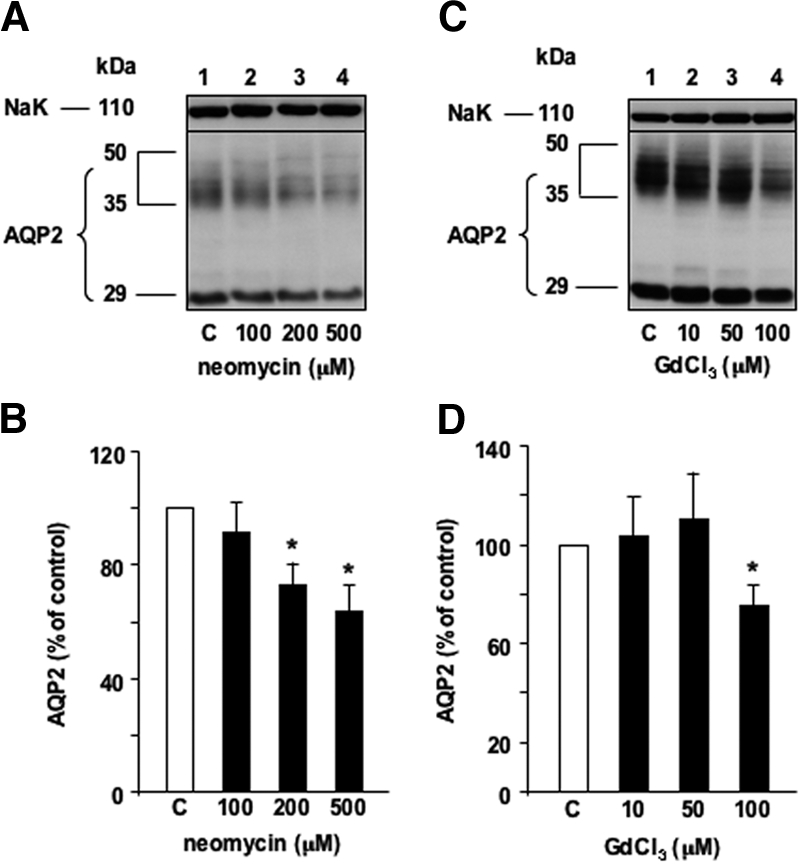
Effect of extracellular CaSR agonists on AQP2 expression. Confluent mpkCCDcl4 cells grown on filters in culture medium containing baseline (1 mM) calcium were preincubated at 37°C with 10−9 M AVP for 24 h. Cells were then incubated in the continuous presence of AVP for another 24 h without (Ctl) or with increasing concentrations of neomycin (A and B) or gadolinium,1 two cationic CaSR agonists. Total protein extracts (40 μg) were separated by 10% SDS-PAGE and AQP2 and the Na-K-ATPase α-subunit, used as a loading control, were detected by Western blotting. (A and C) Representative immunoblot is shown. (B and D) Densitometric quantification of AQP2 protein expressed as a percentage of optical density values measured in the absence of drug (100%). Bars are means ± SEM from four to six independent experiments. *P < 0.05.
The effect of apical calcium on AVP-dependent AQP2 expression is independent of variations of CaSR expression because both CaSR mRNA and protein content remained unchanged after incubation with either 1 or 5 mM apical calcium (Figure 3A). The role of CaSR was further investigated by CaSR knockdown experiments. Both CaSR RNAi exhibited similar efficiencies and dramatically decreased CaSR mRNA and protein expression compared with scramble RNAi (Figure 3B). Increased apical calcium (5 mM) attenuated AVP-induced AQP2 mRNA expression by 24.2 ± 1.7% in cells transfected with scramble RNAi, whereas the inhibitory effect of high apical calcium was abolished in cells transfected with CaSR RNAi (Figure 3C).
Figure 3.
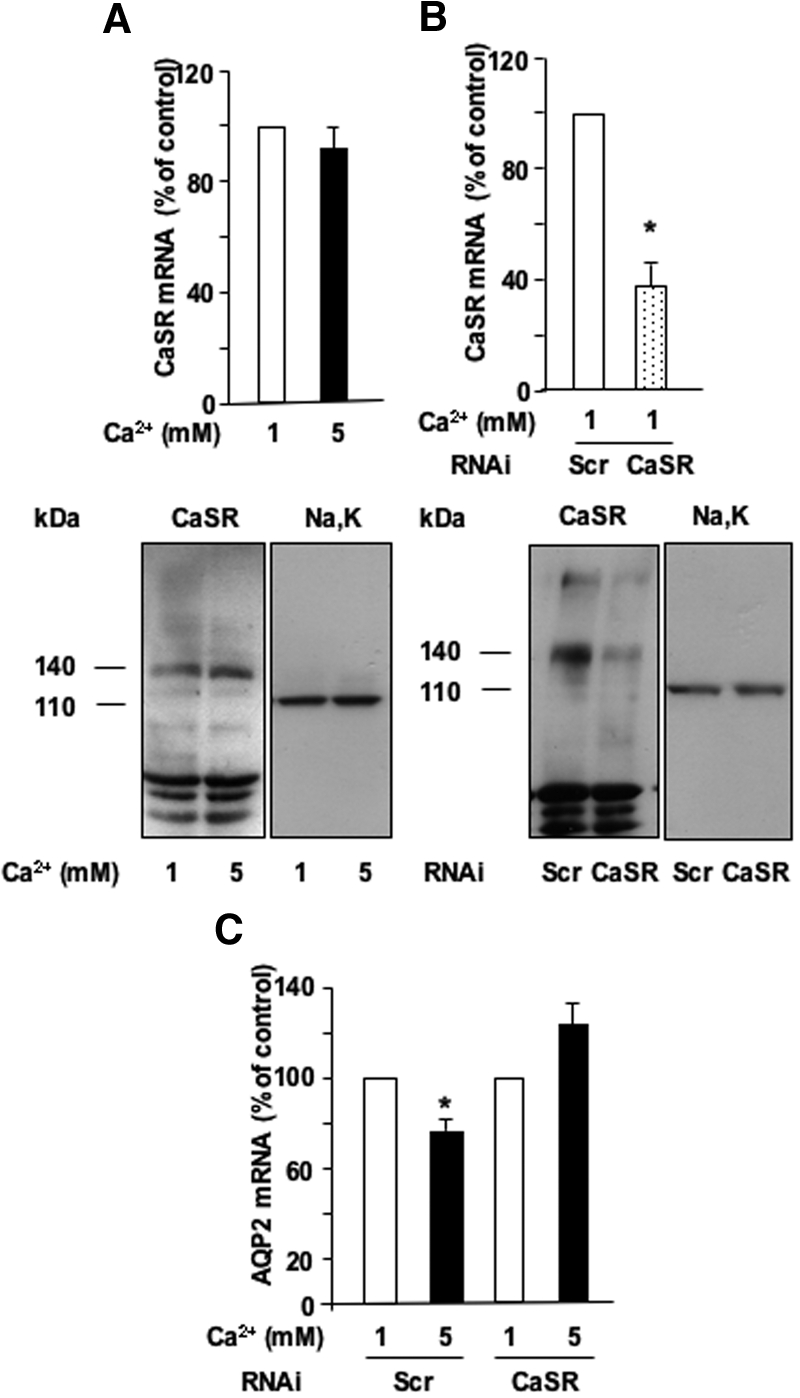
Role of the CaSR in the calcium-induced attenuation of AVP-dependent AQP2 expression. (A) Confluent mpkCCDcl4 cells grown on filters in culture medium containing baseline (1 mM) calcium were preincubated at 37°C with 10−9 M AVP for 24 h and then incubated in the continuous presence of AVP for another 24 h with 1 or 5 mM apical calcium. RNA or protein was then extracted and real-time PCR or Western blotting was performed as described in the Concise Methods section using primers or antibodies specific for CaSR, respectively. (B and C) Two days after transfection with either CaSR scramble RNAi (Scr) or CaSR RNAi, confluent mpkCCDcl4 cells grown on filters in culture medium containing baseline (1 mM) calcium were preincubated at 37°C with 10−9 M AVP for 24 h and then incubated in the continuous presence of AVP for another 14 h with 1 or 5 mM apical calcium. RNA or protein was then extracted and real-time PCR or Western blotting was performed as described in the Concise Methods sections using primers or antibodies specific for CaSR, respectively (B), or primers specific for AQP2 (C). Na-K-ATPase α-subunit was used as a loading control for Western blotting, and representative immunoblots from two independent experiments are shown. CaSR and Na,K-ATPase α-subunit were detected as 140- and 110-kD bands, respectively. PCR results are expressed as a percentage of control values determined after incubation in the presence of 1 mM calcium (100%). Bars are means ± SEM from six independent experiments. *P < 0.05.
CaSR Activation Reduces the Efficiency of Coupling between V2 Receptor and AC
For assessment of the role of ERK and PKC in modulated AQP2 protein expression by extracellular calcium, AVP-treated cells were incubated for 24 h with either 1 or 5 mM apical calcium in the absence or presence of 10−6 M GF109203X or U0126, which specifically inhibit PKC isozymes and the ERK activating kinase MEK-1, respectively. Neither GF109203X nor U0126 altered the attenuation of AVP-induced AQP2 expression by 5 mM apical calcium (Figure 4). It should be mentioned that although U0126 efficiently prevents ERK activation in mpkCCDcl4 cells,15 the efficacy of GF109203X has not been formally demonstrated in this model.
Figure 4.
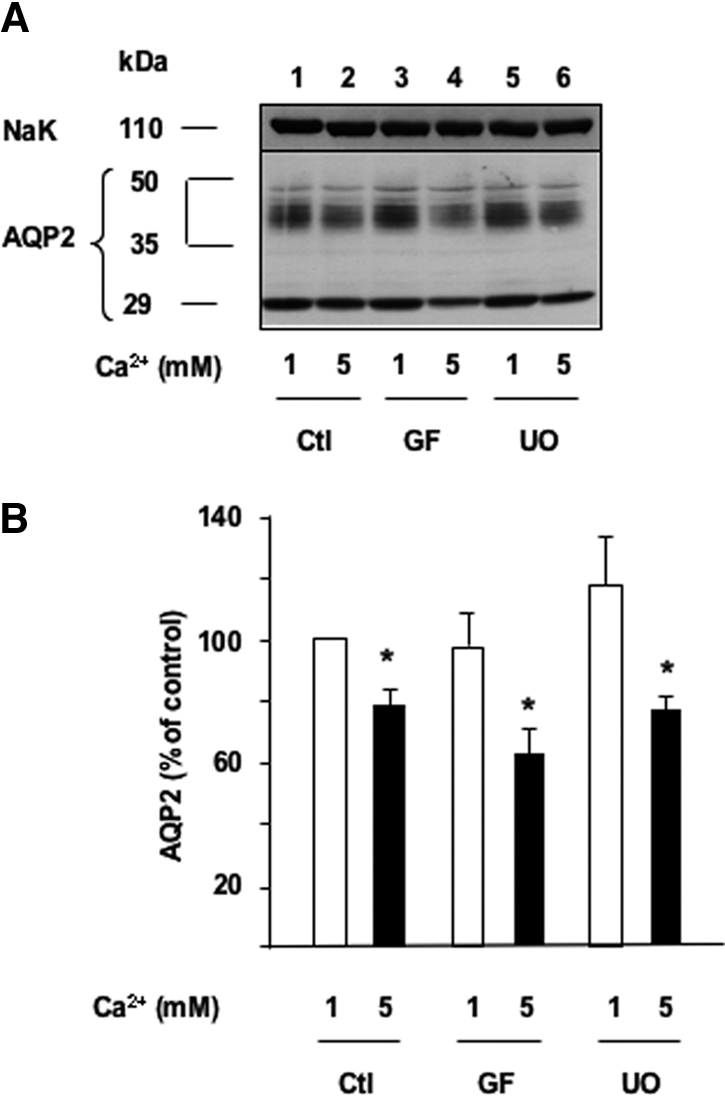
The effect of apical calcium on AVP-dependent AQP2 expression is independent of PKC and ERK activation. Confluent mpkCCDcl4 cells grown on filters in culture medium containing baseline (1 mM) calcium were preincubated for 24 h at 37°C with 10−9 M AVP. Cells were then incubated for another 24 h in the continuous presence of AVP and in the presence of 1 or 5 mM apical calcium and treated or not (Ctl) with either 10−6 M of the GF109203X (GF), a PKC inhibitor, or 10−6 M UO126 (UO), a MEK-1 inhibitor. Total protein extracts (40 μg) were separated by 10% SDS-PAGE and AQP2 and the Na-K-ATPase α-subunit, used as a loading control, were detected by Western blotting. (A) A representative immunoblot is shown. (B) Densitometric quantification of AQP2 protein expressed as a percentage of OD values measured in the presence of 1 mM calcium and without drugs (100%). Bars are means ± SE from four independent experiments. *P < 0.05.
To assess the role of the PKA pathway, we analyzed the effect of cell-permeable, myristoylated PKI (Calbiochem, Darmstadt, Germany), a specific PKA inhibitor. AVP-treated cells were incubated for 24 h with 1 or 5 mM apical calcium in the absence or presence of 5 × 10−6 M PKI. PKI alone decreased AVP-dependent AQP2 mRNA accumulation by 31.5 ± 5.3% (Figure 5A); however, AQP2 mRNA abundance was not further decreased by 5 mM apical calcium.
Figure 5.
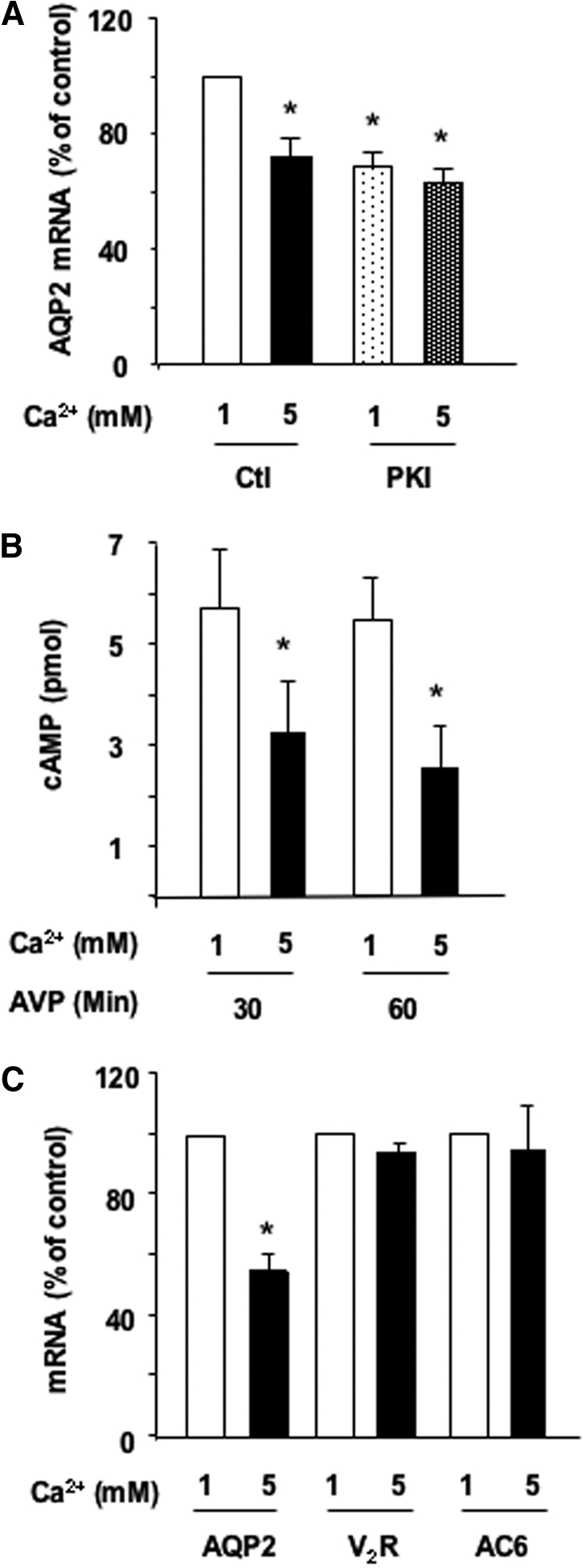
Role of the cAMP-PKA pathway in the calcium-induced attenuation of AVP-dependent AQP2 expression. (A) Confluent mpkCCDcl4 cells grown on filters in culture medium containing baseline (1 mM) calcium were preincubated for 24 h at 37°C with 10−9 M AVP. Cells were then incubated for another 24 h in the continuous presence of AVP and in the presence of 1 or 5 mM apical calcium and treated or not with 5 × 10−6 M myristoylated PKA inhibitor (PKI). After RNA extraction, real-time PCR was performed as described in the Concise Methods section using primers against AQP2. Results are expressed as a percentage of control values determined after 24 h of incubation in the presence of 1 mM calcium (100%). Bars are means ± SEM from four independent experiments. (B) Confluent mpkCCDcl4 cells grown on filters were preincubated for 24 h at 37°C in the presence of 1 or 5 mM apical calcium and treated or not for 30 to 60 min with 10−9 M AVP. Cellular cAMP content was determined as described in the Concise Methods section. Results are expressed as pmol cAMP × 106 cells−1 and are means ± SEM from five independent experiments. (C) Cells were treated as in A before RNA extraction. Real-time PCR was performed as described in the Concise Methods section using primers against AQP2, the vasopressin V2 receptor (V2R), or type 6 AC (AC6). Results are expressed as a percentage of control values determined after 24 h of incubation in the presence of 1 mM calcium (100%). Bars are means ± SEM from four independent experiments. *P < 0.05.
We investigated whether high extracellular calcium impairs AVP-induced cAMP accumulation. Cells were first incubated for 24 h in the presence of 1 or 5 mM apical calcium and then challenged with 10−9 M AVP for 30 to 60 min before measuring cellular cAMP in the absence of phosphodiesterase inhibitor. Figure 5B shows that AVP-induced increase of cellular cAMP from background levels (as pmol × 106 cells/min ± SE; 30 min 5.69 ± 1.19; 60 min 5.44 ± 0.82) was attenuated by more than 50% in cells exposed to 5 mM apical calcium (Figure 5B).
The decreased cAMP accumulation in response to high extracellular calcium may result from a responsive deficiency of an initial step of AVP signaling (i.e., the V2 receptor and AC). Real-time PCR analysis revealed similar V2 receptor mRNA content in cells subjected to 1 or 5 mM apical calcium (Figure 5C). Similarly, elevated calcium did not alter the mRNA levels of AC types 3, 5, and 6 (Figure 5C and data not shown) (i.e., the calcium-sensitive AC isoforms expressed in CD).16 It should be mentioned that the expression levels of AC types 3 and 5 isoforms are, respectively, 3000- and 600-fold lower than the expression levels of AC type 6 in mpkCCDcl4 cells.
For assessment of whether extracellular calcium alters coupling between cAMP and PKA, cells were treated or not for 24 h with 10−3 M 8Br-cAMP, which increased AQP2 mRNA expression by 11.66 ± 1.5-fold. In contrast to untreated cells, high extracellular calcium did not alter AQP2 mRNA expression in the presence of 8Br-cAMP (Figure 6A).
Figure 6.
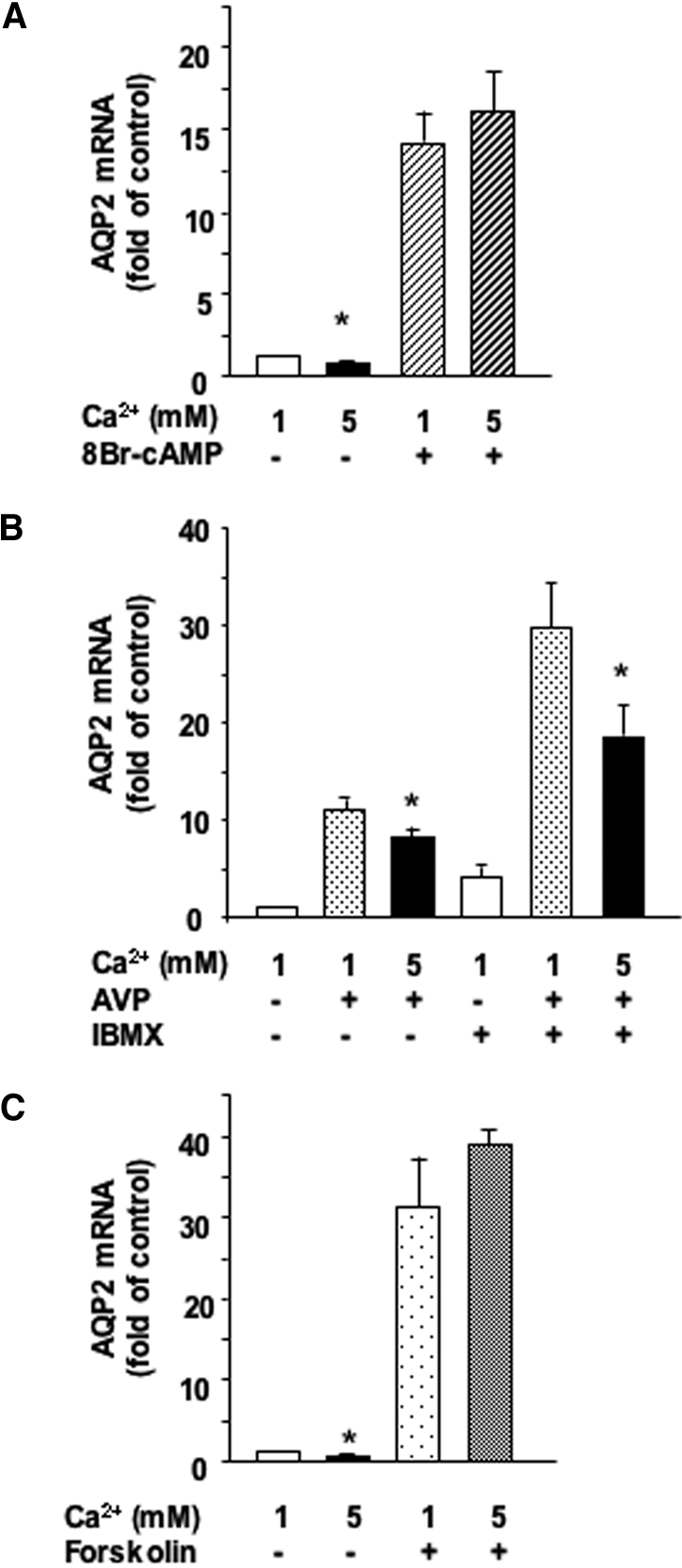
Role of phosphodiesterases and AC in the calcium-induced attenuation of AVP-dependent AQP2 expression. (A) Confluent mpkCCDcl4 cells grown on filters were preincubated for 24 h at 37°C in the presence of 1 or 5 mM apical calcium before incubation for another 24 h with or without 10−3 M 8Br-cAMP, a cell-permeable cAMP analog. (B) Confluent mpkCCDcl4 cells grown on filters were preincubated for 24 h at 37°C with 10−9 M AVP. Cells were then incubated for another 24 h in the continuous presence of AVP and in the presence of 1 or 5 mM apical calcium with or without 10−4 M IBMX, a phosphodiesterase inhibitor. (C) Confluent mpkCCDcl4 cells grown on filters were preincubated for 24 h at 37°C in the presence of 1 or 5 mM apical calcium before incubation for another 24 h with or without 5 × 10−6 M forskolin, a direct activator of AC. After RNA extraction, real-time PCR was performed as described in the Concise Methods section using primers against AQP2. Results are expressed as a percentage of control values determined after 24 h of incubation in the presence of 1 mM calcium and in the absence of drugs (100%). Bars are means ± SEM from four to eight independent experiments. *P < 0.05.
For determination of whether high apical calcium attenuates AVP-induced AQP2 expression via increased cAMP degradation by phosphodiesterases, cells were preincubated for 24 h with baseline calcium in the absence or presence of 10−9 M AVP and then incubated for 24 h with 1 or 5 mM apical calcium in the absence or presence of 10−4 M 3-isobutyl-1-methylxanthine (IBMX), an inhibitor of phosphodiesterases. IBMX increased AQP2 mRNA levels in both the absence and the presence of AVP but did not prevent the attenuation of AVP-induced AQP2 mRNA accumulation by high apical calcium (Figure 6B).
For assessment of whether high apical calcium directly inhibits AC, cells were first preincubated for 24 h with 1 or 5 mM apical calcium and treated or not for another 24 h with 5 × 10−6 M forskolin, a direct activator of AC. Forskolin increased AQP2 mRNA expression 31.34 ± 5.84-fold; however, 5 mM apical calcium did not alter AQP2 mRNA expression in the presence of forskolin (Figure 6C).
Recent experimental evidence indicates that calmodulin is required for AVP-induced cAMP accumulation in IMCD cells.17 For assessment of whether calmodulin plays a role in the attenuation of AVP-induced AQP2 expression by high apical calcium, AVP-treated cells were incubated for 24 h with 1 or 5 mM apical calcium and in the absence or presence of either 10−5 M W7 or 10−5 M trifluoperazine, two structurally unrelated inhibitors of calmodulin. Inhibition of calmodulin did not alter AQP2 mRNA expression in the presence of baseline calcium (Figure 7); however, both inhibitors prevented the attenuation of AVP-induced AQP2 mRNA accumulation induced by 5 mM apical calcium.
Figure 7.
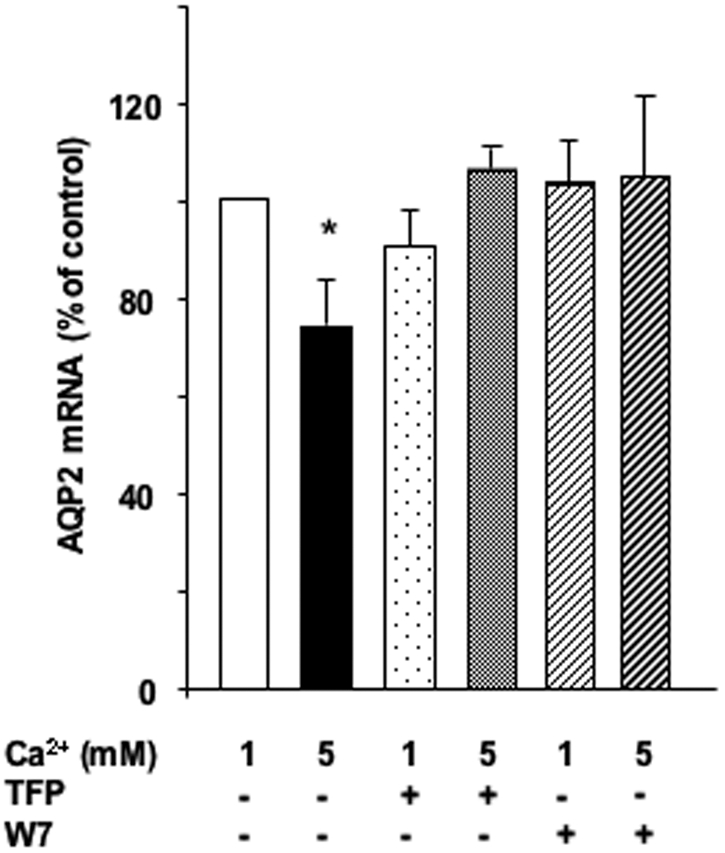
Role of calmodulin in the calcium-induced attenuation of AVP-dependent AQP2 expression. (A) Confluent mpkCCDcl4 cells grown on filters were preincubated for 24 h at 37°C with 10−9 M AVP. Cells were then incubated for another 24 h in the continuous presence of AVP and in the presence of 1 or 5 mM apical calcium with or without 10−5 M W7 or trifluoperazine, two unrelated calmodulin inhibitors. After RNA extraction, real-time PCR was performed as described in the Concise Methods section using primers against AQP2. Results are expressed as a percentage of control values determined after 24 h of incubation in the presence of 1 mM calcium and in the absence of drugs (100%). Bars are means ± SEM from five to eight independent experiments. *P < 0.05.
Discussion
These results show that high concentrations of apical calcium attenuate AVP-induced AQP2 expression via activation of the CaSR and reduced coupling efficiency between V2 receptors and AC activation in mpkCCDcl4 cells, a physiologically relevant and well-characterized CD principal cell model.14,18 Our observation that high apical calcium attenuates AVP-induced AQP2 expression suggests that decreased AQP2 protein abundance, reported in hypercalciuric rats,12,13 relies on a direct effect of luminal calcium acting on CD principal cells. The effect of extracellular calcium on AVP-dependent AQP2 protein expression is observed after a time lag of several hours and is preceded by an attenuation of AVP-induced AQP2 mRNA accumulation without decreased AQP2 mRNA stability. The effect of high apical calcium most likely results from diminished AVP-stimulated AQP2 gene transcription18; however, actinomycin D is a general transcription inhibitor, and we cannot exclude that expression of a modulator of mRNA degradation might also be inhibited. Decreased AQP2 mRNA abundance was not observed in the inner medulla of hypercalcemic rats.12,13 This discrepancy may rely, at least in part, on the confounding effect of increased plasma AVP levels in response to hypercalcemia-induced polyuria.12 Indeed, one can speculate that the same level of polyuria in normocalcemic rats would increase circulating AVP levels and thereby enhance renal AQP2 mRNA expression. In addition, the modest calcium-induced reduction in AVP-dependent AQP2 mRNA accumulation may have been overlooked by the Northern blot technique used in previous studies.12,13 Finally, the mechanisms of controlled AQP2 expression may differ between CCD and IMCD cells. Long-term modulation of AVP-dependent AQP2 expression by extracellular calcium occurs together with an extracellular calcium-induced reduction of cAMP-induced translocation of AQP2 to the plasma membrane.11 Altogether, these observations indicate that extracellular calcium modulates both subcellular repartition and whole-cell expression levels of AQP2.
In mpkCCDcl4 cells, both neomycin and Gd3+, two CaSR agonists,8 reproduced the antagonistic effect of extracellular calcium concentration on AVP-induced AQP2 expression. These CaSR agonists also antagonized the cAMP-induced acute increase of water permeability after translocation of AQP2 to the plasma membrane.10,11 Our results indicate that similar to native CD cells, mpkCCDcl4 cells express functional apical CaSR.10 CaSR expression levels were independent of extracellular calcium, and knockdown of CaSR prevented the attenuation of AVP-induced AQP2 expression by high extracellular calcium, indicating that extracellular calcium modulates AVP-dependent AQP2 expression via changes in CaSR activity but not abundance.
Multiple signaling pathways are affected by CaSR.8 Our findings suggest that the CaSR modulates AQP2 expression independent of PKC and ERK; however, we showed that modulated AQP2 expression by CaSR depends on calmodulin and thereby intracellular calcium in CCD cells. In addition, the effects of high extracellular calcium and a specific inhibitor of PKA were not additive. Taken together with impaired cAMP accumulation by high extracellular calcium, our observations suggest that CaSR controls AQP2 transcription by inhibiting the PKA pathway. This result is in agreement with that of a previous study that showed decreased agonist-induced cAMP accumulation in response to high extracellular calcium in CD8 cells11; however, the effect of extracellular calcium on AVP-induced cAMP accumulation cannot be assessed in CD8 cells that do not express V2 receptor.
Impaired AVP-induced cAMP accumulation in response to high extracellular calcium may result from decreased cAMP synthesis by AC and/or increased cAMP degradation by phosphodiesterases.8,9,19 Our results indicate that phosphodiesterase activation does not mediate the effects of high apical calcium on AVP-dependent AQP2 expression. Moreover, 8Br-cAMP, a hydrolyzable, cell-permeable cAMP analog, and forskolin, a direct activator of AC, induced AQP2 expression equally well in the presence of baseline or high extracellular calcium concentrations. Our finding that IBMX potentiated the effect of AVP on AQP2 expression may explain the improvement of hypercalcemia-induced polyuria by phosphodiesterase inhibitors.20 Consequently, inhibited cAMP generation by AC most likely accounts for the repressive effect produced by extracellular calcium on AVP-dependent cAMP accumulation. High extracellular calcium alters neither the expression levels of V2 receptor nor any of the calcium-sensitive AC isoforms expressed in the CD.16,17 Taken together with the observation that direct activation of adenylyl cyclases stimulated equally well AQP2 expression in the presence of baseline or high extracellular calcium, our results suggest that extracellular calcium modulates coupling between the V2 receptor and adenylyl cyclase. The finding that forskolin-induced AQP2 mRNA expression is not inhibited by high apical calcium contrasts with that of a previous study11 in which high extracellular calcium was shown to antagonize forskolin-induced AQP2 trafficking. Possibly, different mechanisms may be involved in short- and long-term modulation of cAMP-dependent AQP2 expression by extracellular calcium. In IMCD cells, stimulation of calmodulin-dependent AC type 3 is required for AVP-stimulated cAMP accumulation17; however, AVP-dependent AQP2 expression was not altered by calmodulin inhibitors, and the expression levels of AC 3 are very low in mpkCCDcl4 cells. Therefore, modulated AVP-dependent AQP2 expression by extracellular calcium is most likely independent of inhibition of calmodulin-dependent adenylyl cyclase 3 in cultured CD cells.
In conclusion, we showed that long-term exposure of CD principal cells to high apical calcium attenuated AVP-dependent AQP2 expression via CaSR-induced inhibition of cellular cAMP generation. Modulated AVP-dependent AQP2 expression depended on calmodulin and was most likely mediated by altered coupling between the V2 receptor and AC. On the basis of these results together with those demonstrating decreased AQP2 expression in hypercalcemic rats12,13 and reduced AQP2 apical targeting in cultured CD cells that were subjected to high extracellular calcium,11 it is tempting to speculate that luminal CaSR activation in the CD reduces urinary concentration, which prevents calcium salt precipitation and nephrolithiasis.21
Concise Methods
Cell Culture, Incubation, and Transfection
mpkCCDcl4 cells (passages 20 to 32) were grown to confluence for 7 d as described previously.14 Cells were then maintained in serum-free, hormone-deprived medium containing 1 mM calcium for another 48 h before use. Unless otherwise mentioned, cells were preincubated for 24 h with baseline calcium (1 mM) and 10−9 M AVP to induce AQP2 expression. Cells were then incubated in the continuous presence of 10−9 M AVP.
For transfection with RNAi, 4 × 106 cells were transferred to electroporation cuvettes (Gene Pulser cuvette 0.4 cm; Bio-Rad, Hercules, CA) along with 400 μl of culture medium supplemented with 10% serum and 1.2 mmol RNAi and electroporated (300 mV, 960 pF) using a Bio-Rad Gene Pulser.22 Sense primers for RNAi (Invitrogen, Carlsbad, CA) targeting the CaSR were 5′-CAACAUGACUCUGGGUAUAGGAUA-3′ and 5′-CAAAGUCUCGGUUAGAUAGGCAAUA-3′, and that for scramble RNAi (corresponding to the first RNAi) was 5′-CAGGUGGUGGAAGUGAUCCAGAACU-3′. Cells were seeded at a density that allows confluence without division and were then allowed to recover for 24 h in culture medium containing 10% serum. Transfected cells were then maintained in serum-free, hormone-deprived medium for another 48 h. Preliminary experiments showed that silencing is optimal and that cells develop domes, a marker of vectorial solute and water transport, 3 d after transfection.
Western Blot Analysis
After incubation, cells were lysed and proteins (20 μg) were separated by 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA) as described previously.18 AQP2 and Na,K-ATPase α-subunit were detected using polyclonal rabbit antibodies at 1:20,000 dilution.18 CaSR was detected using a polyclonal rabbit antibody at 1:100 dilution (H100; Santa Cruz Biotechnology, Santa Cruz, CA). The antigen–antibody complexes were detected by enhanced chemiluminescence (Amersham-Pharmacia Biotech, Little Chalfont, UK). Results were quantified using a video densitometer and ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Real-Time PCR Analysis
RNA extraction, reverse transcription, and real-time PCR analysis were performed as described previously.23 Primers used for detection of mouse P0 were 5′-AATCTCCAGAGGCACCATTG-3′ and 5′-GTTCAGCATGTTCAGCAGTG-3′, those for AQP2 were 5′-CTTCCTTCGAGCTGCCTTC-3′ and 5′-CATTGTTGTGGAGAGCATTGAC-3′, those for CaSR were 5′-CTGTTTTGAGTGTGCGGAGTGT-3′ and 5′-TCATCCGGGCACTTGTCA-3′, those for vasopressin V2 receptor were 5′-CGTGGGATCCAGAAGCTCC-3′ and 5′-GGCTAGCCAGCAGCATGA-3′, those for AC type 6 were 5′-GACCAAGGACTCTAAGGCATTCC-3′ and 5′-CACCCCGGTTGTCTTTGCT-3′, those for AC type 5 were 5′-TGGAGATGGGAATGGACATGA-3′ and 5′-CGCGCATGTTCACGTTCA-3′, and those for AC type 3 were 5′-TGTCACCGTGGCAAACAAGA-3′ and 5′-TCCATGGTGCTCTGGGAAAT-3′. For each pair of primers, a dissociation plot resulted in a single peak, indicating that a single cDNA species was amplified. The signal was linearly related with the initial amount of cDNA, and the slope of the linear relation was comprised between −3 and −3 to 4, indicating that during each PCR cycle the starting material was amplified by a factor of two. Data were analyzed using ABI Prism software (Applied Biosystems, Foster City, CA), and Po was used as an internal standard. Fold difference in cDNA abundance (F) was calculated using the formula F = 2(Ct1 − Ct2), where Ct1 and Ct2 are the number of cycles required to reach the threshold of amplicon abundance for experimental and control conditions, respectively.
Measurement of Intracellular cAMP
Cells were homogenized in a buffer containing 50 mM Tris and 4 mM EDTA (pH 7.4), and cellular cAMP levels were measured in the absence of phosphodiesterase inhibitor using the Cyclic AMP (3H) System (Amersham Pharmacia Biotech). The radioactivity was then measured by liquid scintillation on 200-μl aliquots containing bound cAMP. Results were expressed as pmol cAMP × 106 cells−1.
Statistical Analyses
Results are means ± SEM from n independent experiments. Each experiment was performed in cells from the same passage. Statistical differences were assessed using the Mann-Whitney U test for comparison of two groups or the Kruskal-Wallis test for comparison of more than two groups. P < 0.05 was considered significant.
Disclosures
None.
Acknowledgments
This work was supported in part by Swiss National Foundation grant 31-67878.02, a Carlos and Elsie de Reuter Foundation grant, an Ernest Boninchi Foundation grant to E. Féraille, an Ernst and Lucie Schmidheiny Foundation grant to M.B., and a Novartis Foundation grant to U.H.
Published online ahead of print. Publication date available at www.jasn.org.
M.B. and U.H. contributed equally to this work.
References
- 1.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA: Aquaporins in the kidney: From molecules to medicine. Physiol Rev 82: 205–244, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW: Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A 90: 11663–11667, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terris J, Ecelbarger CA, Marples D, Knepper MA, Nielsen S: Distribution of aquaporin-4 water channel expression within rat kidney. Am J Physiol 269: F775–F785, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper MA: Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol 269: F663–F672, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Terris J, Ecelbarger CA, Nielsen S, Knepper MA: Long-term regulation of four renal aquaporins in rats. Am J Physiol 271: F414–F422, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC: Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC: Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol 274: F611–F622, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Hofer AM, Brown EM: Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol 4: 530–538, 2003 [DOI] [PubMed] [Google Scholar]
- 9.de Jesus Ferreira MC, Helies-Toussaint C, Imbert-Teboul M, Bailly C, Verbavatz JM, Bellanger AC, Chabardes D: Co-expression of a Ca2+-inhibitable adenylyl cyclase and of a Ca2+-sensing receptor in the cortical thick ascending limb cell of the rat kidney: Inhibition of hormone-dependent cAMP accumulation by extracellular Ca2+. J Biol Chem 273: 15192–15202, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Sands JM, Naruse M, Baum M, Jo I, Hebert SC, Brown EM, Harris HW: Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Invest 99: 1399–1405, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Procino G, Carmosino M, Tamma G, Gouraud S, Laera A, Riccardi D, Svelto M, Valenti G: Extracellular calcium antagonizes forskolin-induced aquaporin 2 trafficking in collecting duct cells. Kidney Int 66: 2245–2255, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Earm JH, Christensen BM, Frokiaer J, Marples D, Han JS, Knepper MA, Nielsen S: Decreased aquaporin-2 expression and apical plasma membrane delivery in kidney collecting ducts of polyuric hypercalcemic rats. J Am Soc Nephrol 9: 2181–2193, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Sands JM, Flores FX, Kato A, Baum MA, Brown EM, Ward DT, Hebert SC, Harris HW: Vasopressin-elicited water and urea permeabilities are altered in IMCD in hypercalcemic rats. Am J Physiol 274: F978–F985, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A: Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Bustamante M, Hasler U, Kotova O, Chibalin AV, Mordasini D, Rousselot M, Vandewalle A, Martin PY, Feraille E: Insulin potentiates AVP-induced AQP2 expression in cultured renal collecting duct principal cells. Am J Physiol Renal Physiol 288: F334–F344, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Chabardes D, Firsov D, Aarab L, Clabecq A, Bellanger AC, Siaume-Perez S, Elalouf JM: Localization of mRNAs encoding Ca2+-inhibitable adenylyl cyclases along the renal tubule. Functional consequences for regulation of the cAMP content. J Biol Chem 271: 19264–19271, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Hoffert JD, Chou CL, Fenton RA, Knepper MA: Calmodulin is required for vasopressin-stimulated increase in cyclic AMP production in inner medullary collecting duct. J Biol Chem 280: 13624–13630, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, Vandewalle A, Feraille E, Martin PY: Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem 277: 10379–10386, 2002 [DOI] [PubMed] [Google Scholar]
- 19.de Jesus Ferreira MC, Bailly C: Extracellular Ca2+ decreases chloride reabsorption in rat CTAL by inhibiting cAMP pathway. Am J Physiol 275: F198–F203, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Li C, Kwon TH, Knepper MA, Frokiaer J, Nielsen S: AQP3, p-AQP2, and AQP2 expression is reduced in polyuric rats with hypercalcemia: Prevention by cAMP-PDE inhibitors. Am J Physiol Renal Physiol 283: F1313–F1325, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, Novarini A: Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 346: 77–84, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Hasler U, Jeon US, Kim JA, Mordasini D, Kwon HM, Feraille E, Martin PY: Tonicity-responsive enhancer binding protein is an essential regulator of aquaporin-2 expression in renal collecting duct principal cells. J Am Soc Nephrol 17: 1521–1531, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Hasler U, Vinciguerra M, Vandewalle A, Martin PY, Feraille E: Dual effects of hypertonicity on aquaporin-2 expression in cultured renal collecting duct principal cells. J Am Soc Nephrol 16: 1571–1582, 2005 [DOI] [PubMed] [Google Scholar]