Abstract
The afferent and efferent arterioles regulate the inflow and outflow resistance of the glomerulus, acting in concert to control the glomerular capillary pressure and glomerular filtration rate. The myocytes of these two vessels are remarkably different, especially regarding electromechanical coupling. This study investigated the expression and function of inward rectifier K+ channels in these two vessels using perfused hydronephrotic rat kidneys and arterioles and myocytes isolated from normal rat kidneys. In afferent arterioles pre-constricted with angiotensin II, elevating [K+]0 from 5 to 15 mmol/L induced hyperpolarization (−27 ± 2 to −41 ± 3 mV) and vasodilation (6.6 ± 0.9 to 13.1 ± 0.6 μm). This manipulation also attenuated angiotensin II-induced Ca2+ signaling, an effect blocked by 100 μmol/L Ba2+. By contrast, elevating [K+]0 did not alter angiotensin II-induced Ca2+ signaling or vasoconstriction in efferent arterioles, even though a significant hyperpolarization was observed (from −30 ± 1 to −37 ± 3 mV, P = 0.003). Both vessels expressed mRNA for Kir2.1 and exhibited anti-Kir2.1 antibody labeling. Patch-clamp measurements revealed prominent inwardly rectifying and Ba2+-sensitive currents in afferent and efferent arteriolar myocytes. Our findings indicate that both arterioles express an inward rectifier K+ current, but that modulation of this current alters responsiveness of only the afferent arteriole. The expression of Kir in the efferent arteriole, a resistance vessel whose tone is not affected by membrane potential, is intriguing and may suggest a novel function of this channel in the renal microcirculation.
Vascular smooth muscle exhibits considerable regional heterogeneity in vasomotor mechanisms and ion channel expression patterns. Understanding circulatory regulation at the vascular level requires knowledge of this smooth muscle diversity and its impact on regional control of vascular resistance. The renal microcirculation represents an extreme example of such heterogeneity. The myocytes of the preglomerular afferent arteriole and postglomerular efferent arteriole differ remarkably in Ca2+ entry mechanisms, myosin expression, and the mechanisms modulating vascular reactivity.1–6 Further regional heterogeneity has been noted for ion channel expression in the juxtamedullary versus cortical efferent arterioles7 and in the activating mechanisms of the contractile pericytes of the descending vasa recta.8 For example, angiotensin II (AngII) responses of the afferent arteriole and descending vasa recta involve membrane depolarization and voltage-gated Ca2+ entry and are attenuated by L-type Ca2+ channel antagonists. In contrast, AngII responses of efferent arterioles are not related to membrane potential and involve a voltage-independent Ca2+ entry mechanism unaffected by L-type Ca2+ channel antagonists.1,2,4,9
Potassium channels are prominent regulators of smooth muscle function. The inward rectifier K+ channel (Kir) exhibits marked regional differences in its expression and involvement in vascular regulation.10,11 In general, Kir expression and the influence of Kir on membrane potential are reported to be greater in smaller resistance vessels than in larger conduit arteries.12,13 Such a trend may also occur in the kidney. Our laboratory found indirect evidence that Kir plays a prominent role in the afferent arteriole,14 whereas Prior et al.15 found no evidence that Kir influences tone or membrane potential in the upstream arcuate artery, a conduit vessel that does not contribute to renal vascular resistance. Recently, Cao et al.16 demonstrated that the pericytes of the postglomerular descending vasa recta express a Kir current and exhibit K+-induced hyperpolarization. The expression of Kir and its role in the efferent arteriole are unknown.
In this study, we investigated these issues using the in vitro perfused hydronephrotic rat kidney model for in situ contractile and membrane potential measurements, as well as afferent and efferent arterioles isolated from normal rat kidneys for studies of Ca2+ signaling and to examine the expression of Kir2.1 by reverse transcriptase–PCR and in situ antibody labeling. We used freshly dispersed myocytes obtained from individually isolated afferent and efferent arterioles to study barium-sensitive Kir currents. Our findings indicate that Kir2.1 is expressed in and contributes to the regulation of membrane potential in the myocytes of both the afferent and efferent arterioles; however, the impact of Kir modulation on Ca2+ signaling, smooth muscle activation, and contractile tone differs in these two vessels.
RESULTS
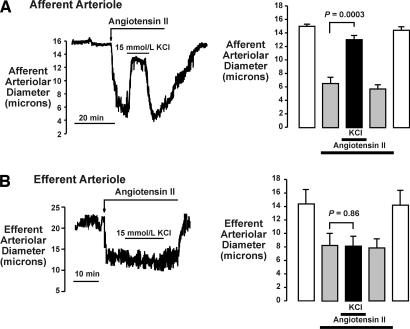
Figure 1 compares the effects of elevating [K+]o from 5 to 15 mmol/L on the responses of cortical afferent and efferent arterioles to AngII. K+-induced vasodilation is a functional marker for the presence of Kir.10 As shown, this manipulation elicited different responses. In the afferent arteriole, 0.1 nmol/L AngII reduced diameters from 15.1 ± 0.3 to 6.6 ± 0.9 μm (n = 7; P = 0.0001) and elevating [K+]o reversed the vasoconstriction, increasing diameters to 13.1 ± 0.6 μm (P = 0.0003 versus AngII alone). Returning [K+]o to 5 mmol/L restored diameters to 5.8 ± 0.6 μm. The efferent arteriole did not exhibit K+-induced vasodilation. Thus, 0.1 nmol/L AngII reduced efferent arteriole diameter from 14.3 ± 2.2 to 8.2 ± 1.8 μm (n = 7; P = 0.003; Figure 1B), and 15 mmol/L KCl had no effect (8.1 ± 1.5 μm; P = 0.86).
Figure 1.
Effects of 15 mmol/L KCl on contractile response of afferent (A) and efferent (B) arterioles to AngII (0.1 nmol/L), as observed in the in vitro perfused hydronephrotic rat kidney model. Original tracings (left) and summary data (n = 7; right) illustrate vasodilator response of afferent arteriole to 15 mmol/L KCl and lack of response of efferent arteriole.
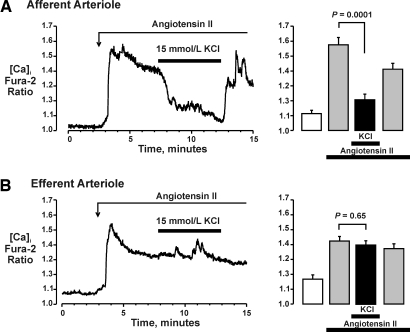
Figure 2 illustrates the effects of 15 mmol/L K+ on AngII-induced Ca2+ signaling in cortical afferent and efferent arterioles isolated from normal rat kidneys. A higher concentration of AngII (10 nmol/L) was used in these in vitro studies to produce a robust Ca signal and facilitate analysis. As shown (Figure 2A), 10 nmol/L AngII increased the fura-2 ratio in afferent arterioles to 142 ± 2% of basal (1.11 ± 0.02 to 1.58 ± 0.05; n = 6; P < 0.0001). The application of 15 mmol/L K+ produced a rapid decrease in [Ca2+], reducing the fura-2 ratio to 108 ± 2% of basal (1.20 ± 0.04; P = 0.0001 versus AngII alone). Returning [K+]o to 5 mmol/L restored the AngII-induced increase in [Ca2+] (1.41 ± 0.04; P = 0.004 versus 15 mmol/L KCl). Figure 2B depicts the effects of KCl on AngII signaling in the efferent arteriole. AngII (10 nmol/L) increased the fura-2 ratio from 1.17 ± 0.03 to 1.42 ± 0.03 (n = 6; P = 0.0002); however, increasing [K+]o from 5 to 15 mmol/L had no effect (1.40 ± 0.03; P = 0.65 versus AngII alone).
Figure 2.
Effects of 15 mmol/L KCl on AngII-induced calcium signaling in afferent (A) and efferent (B) arterioles isolated from normal rat kidneys. As shown in original tracings (left) and mean data (right), 15 mmol/L KCl reduced AngII-evoked increase in calcium in the afferent (A) but not in the efferent (B) arteriole.
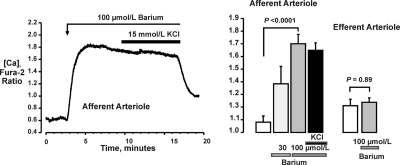
We previously showed that barium (10 to 100 μmol/L) elicited membrane depolarization and vasoconstriction and prevented K+-induced vasodilation in the afferent arteriole of the hydronephrotic kidney.14 As illustrated in Figure 3, barium had comparable effects on [Ca2+] in afferent arterioles isolated from the normal kidney. The fura-2 ratio was 1.08 ± 0.05 (n = 5) in controls and 1.38 ± 0.14 (P = 0.08) after the addition of 30 μmol/L barium and increased to 1.70 ± 0.07 (P < 0.0001) upon the addition of 100 μmol/L barium. Barium also prevented the effects of 15 mmol/L KCl on [Ca2+] (fura-2 ratio 1.65 ± 0.06; P = 0.60 versus barium alone). In contrast to the afferent arteriole, barium had no effect on [Ca2+] in the efferent arteriole (fura-2 ratio 1.21 ± 0.05 versus 1.22 ± 0.05; P = 0.89; n = 5; Figure 3, right). These two vessels differ in the role of membrane depolarization and voltage-gated Ca2+ channels in AngII-induced vasoconstriction and Ca2+ signaling.1,4 Thus, the lack of effect of 15 mmol/L KCl on contractile responses and of barium on Ca2+ signaling in the efferent arteriole might reflect this unique characteristic, rather than a lack of expression of Kir.
Figure 3.
Barium evokes an increase in calcium in the afferent arteriole and blocks the response of this vessel to 15 mmol/L KCl (compare with Figure 2A). Barium has no effect on intracellular calcium in the efferent arteriole (far right).
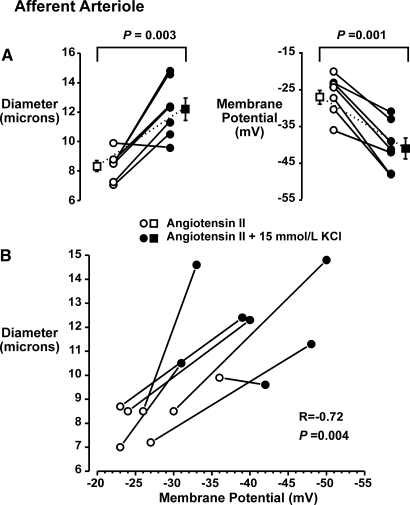
To address this issue, we determined the effects of 15 mmol/L KCl on the membrane potential of in situ afferent and efferent arterioles. In these experiments, kidneys were first treated with 0.1 nmol/L AngII, the arterioles were impaled, and membrane potential and diameter were monitored during the application of 15 mmol/L KCl. AngII reduced afferent arteriolar diameters from 15.4 ± 2.0 to 8.3 ± 0.4 μm (n = 7). After AngII treatment, afferent arteriolar membrane potentials averaged −27 ± 2 mV (n = 7). The application of 15 mmol/L KCl was associated with vasodilation to 12.2 ± 0.7 μm (P = 0.003; Figure 4A, left) and hyperpolarization to −41 ± 3 mV (P = 0.001; Figure 4A, right). These data are presented in Figure 4B as individual diameter and membrane potentials in AngII before and after treatment with 15 mmol/L KCl. Note the relation between diameter and membrane potential (R = −0.72, P = 0.004).
Figure 4.
(A) Simultaneous measurements of diameter (left) and membrane potential (right) in afferent arterioles of the in vitro perfused hydronephrotic rat kidney during 0.1 nmol/L AngII-induced constriction and after 15 mmol/L KCl-induced dilation. (B) Individual diameter and membrane potential responses. Note that the vasodilatory response to KCl was associated with hyperpolarization.
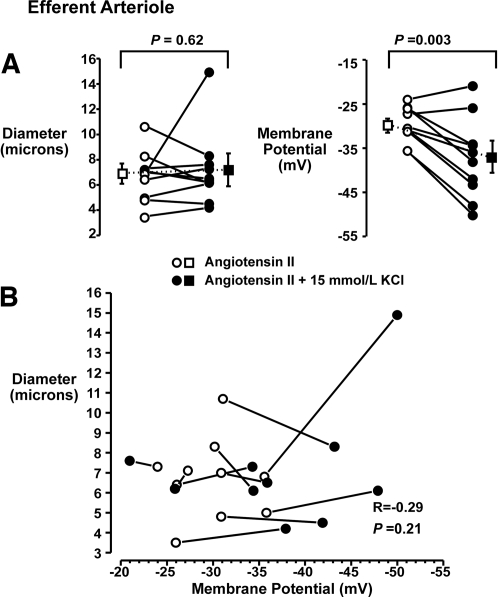
The results of similar experiments on efferent arterioles are depicted in Figure 5. AngII (0.1 nmol/L) reduced diameters from 12.4 ± 1.1 to 6.7 ± 0.6 μm (n = 10). Membrane potentials in the AngII-treated efferent arterioles were −30 ± 1 mV. KCl (15 mmol/L) did not alter diameter (7.2 ± 0.9 μm; P = 0.62; Figure 5A, left) but produced a significant hyperpolarization (−37 ± 3 mV; P = 0.003; Figure 5A, right). Individual efferent arteriolar diameter and membrane potential responses are presented in Figure 5B. Note that the membrane potential responses were dissociated from changes in diameter (R = −0.29, P = 0.2). Thus, K+ elicited hyperpolarization in both vessels but altered vasoconstriction and Ca2+ signaling in only the afferent arteriole.
Figure 5.
(A) Simultaneous measurements of diameter (left) and membrane potential (right) in efferent arterioles of the in vitro perfused hydronephrotic rat kidney during 0.1 nmol/L AngII-induced constriction and after the application of 15 mmol/L KCl. (B) Individual diameter and membrane potential responses. Note that KCl elicited a significant hyperpolarization in the efferent arteriole but that this response was not associated with vasodilation.
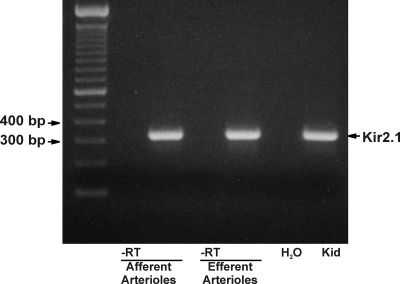
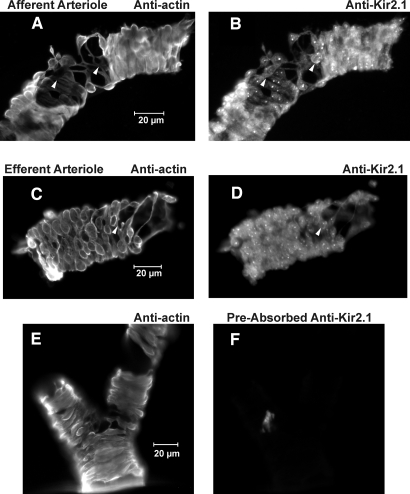
Reverse transcriptase–PCR studies demonstrated message for Kir2.1 in both vessels (Figure 6). Amplification with the primers for Kir2.1 yielded a single 329-bp product using cDNA from both vessels. Sequencing confirmed that this product corresponded to the 862 to 1190 region of the rat Kir2.1 mRNA. No product was seen in the absence of reverse transcriptase. To evaluate the expression of Kir2.1 protein, we used an antibody directed against Kir2.1. Figure 7 depicts afferent and efferent arterioles treated with anti–α-smooth muscle actin (α-SMA; Figure 7, A and C) and anti-Kir2.1 (Figure 7, B and D). Note the anti-Kir2.1 labeling in both α-SMA–positive and -negative (arrows) cells, the latter possibly reflecting Kir2.1 in endothelial cells. Together, these data suggest that both the afferent and efferent arterioles express message for Kir2.1 and Kir2.1 protein.
Figure 6.
Reverse transcriptase–PCR demonstrates the expression of mRNA encoding Kir2.1 in both afferent and efferent arterioles. Standard ladder is shown on far left. Distilled water control and kidney homogenate (Kid) are shown in the two lanes on the far right. Note that no signal is seen in arteriolar preparations in the absence of reverse transcriptase (-RT).
Figure 7.
(A and B) anti–α-SMA and anti-Kir2.1 antibody labeling of afferent arteriole. (C and D) Labeling of efferent arteriole. Note anti-Kir2.1 antibody labeling of both α-SMA–positive (myocytes) and α-SMA–negative (arrows) cells in each vessel. (E) α-SMA labeling. (F) Lack of nonspecific labeling using anti-Kir2.1 antibody preabsorbed with Kir2.1 peptide antigen (afferent arteriolar segments).
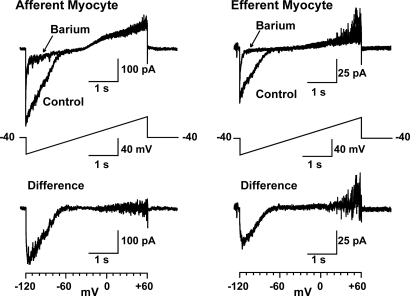
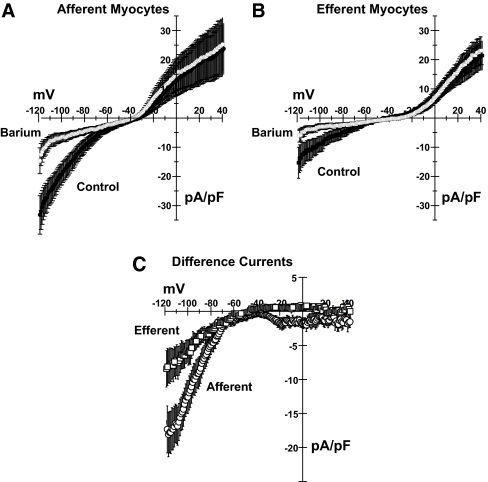
Finally, we measured whole-cell currents in freshly dispersed myocytes from isolated afferent and efferent arterioles. Figure 8 illustrates the protocol and presents examples of current tracings. Figure 9 presents mean data obtained from 11 afferent and 11 efferent arteriolar myocytes. As shown, myocytes from both afferent (left) and efferent (right) arterioles exhibited Ba2+-sensitive inwardly rectifying currents in response to a voltage ramp (−120 to 60 mV; Figure 8, middle). The bottom tracings in Figure 8 and summary of mean data in Figure 9C illustrate the difference currents, obtained by subtracting the current measured in 50 μmol/L Ba2+ from the control currents. The mean currents in Figure 9 are corrected for cell capacitance. As is often the case, the small outward component of the barium-sensitive current is not readily apparent from these data. The small size of the renal arteriolar myocytes and difficulties in obtaining stable patch recordings contribute to this difficulty. Afferent and efferent arteriolar myocytes had capacitances of 6.5 ± 0.5 and 4.9 ± 0.4 pF (P = 0.021), respectively. The barium-sensitive currents clearly display the typical inwardly rectifying characteristics that identify this current as Kir. As shown in Figure 9C, the barium-sensitive current densities were significantly larger in the afferent myocytes (12.6 ± 2.4 versus 6.1 ± 1.5 pA/pF for afferent and efferent myocytes at −100 mV; P = 0.022).
Figure 8.
Examples of original tracings of whole-cell currents obtained in freshly dispersed myocytes isolated from single afferent (left) and efferent (right) arterioles in 20 mmol/L extracellular K+. (Middle) Four-second ramp protocol. Cells were held in voltage-clamp mode at −40 mV and ramped from −120 mV to 60 mV. Note that the inward current is sensitive to 50 μmol/L barium (top). Lower tracings depict examples of difference currents, obtained by subtracting currents obtained in presence of 50 μmol/L barium from the control currents.
Figure 9.
(A and B) Current-voltage plots of mean currents in afferent (A) and efferent (B) myocytes (n = 11). Control currents are shown in solid symbols; currents seen in presence of 50 μmol/L barium are indicated by open symbols. (C) Difference currents (control − barium) for afferent (circles) and efferent (squares) arteriolar myocytes.
DISCUSSION
Using diverse and complimentary approaches, this study demonstrated that afferent and efferent arterioles of cortical nephrons express Kir2.1. A previous study,14 using the hydronephrotic kidney preparation, suggested that Kir is a dominant current in the afferent arteriole. The present study extended these findings using direct approaches in afferent arterioles isolated from normal kidneys. The observation that Kir2.1 is also expressed in the cortical efferent arteriole and that elevated extracellular K+ alters membrane potential but not vascular reactivity in this vessel is a novel finding. It is intriguing that this vessel, which does not exhibit voltage-dependent Ca2+ signaling and does not seem to be regulated by membrane potential, exhibits a Kir current. This finding may suggest a novel role of this channel in the renal microcirculation.
We previously reported that Kir is the dominant current setting membrane potential of the afferent arteriole under basal conditions.14 The present study extended those observations, providing direct evidence that Kir2.1 is expressed in the myocytes of the afferent arteriole and that these cells exhibit a prominent barium-sensitive inwardly rectifying current. This study is the first to demonstrate this current in the contractile vascular myocytes of the afferent arteriole. Consistent with this observation, studies by Kurtz and Penner17 and Leichtle et al.18 reported similar currents in renin-producing juxtaglomerular (JG) cells. Friis et al.,19 however, did not find an inward rectifying current in JG cells. The reasons for these differing observations are unclear; however, JG cells may be recruited from the vascular myocytes,20 which, as this study demonstrated, do express Kir.
The presence of K+-induced hyperpolarization and vasodilation are hallmarks of Kir current in intact vessels.10 Accordingly, the observations of this response in in situ afferent arterioles and the corresponding effects of K+ on Ca2+ signaling in isolated afferent arterioles provide independent confirmation of functioning Kir currents in intact vessels. Previous studies showed that gene deletion of Kir2.1 eliminates K+-induced vasodilation, implicating this specific isoform in this characteristic response.21 Our observation that Kir 2.1 is expressed in renal arterioles is thus consistent with this view. The underlying mechanism is thought to involve an interaction between external K+ ions and polyamine binding sites within the channel pore, such that an elevation in [K+]o at the outer vestibule of the pore shifts the position of the polyamine and reduces inward rectification.22 Reducing [K+]o has the opposite effect of increasing inward rectification, and we showed that this manipulation evokes afferent arteriolar vasoconstriction,14 further implicating Kir current as the dominant basal K+ current in this vessel. Elevation of [K+]o can also elicit hyperpolarization by stimulating the electrogenic Na+/K+ ATPase15,23; however, we found that the K+-induced vasodilation of the afferent arteriole is insensitive to ouabain but blocked by barium,14 suggesting a predominant role of Kir in this response. The ability of barium to block the effects of 15 mmol/L [K+]o on the Ca2+ responses of the afferent arteriole (Figure 3) is consistent with this interpretation.
Efferent arteriolar myocytes also exhibited a prominent barium-sensitive, inwardly rectifying current and expressed message for Kir2.1 and Kir2.1 protein. Moreover, elevated extracellular [K+] elicited a significant hyperpolarization of in situ efferent arterioles. This K-induced hyperpolarization mirrors the response of the afferent arteriole and would be consistent with an augmentation of the outward Kir current by external K+; however, we have not eliminated a possible contribution of the electrogenic Na+/K+-ATPase to membrane potential response of the efferent arteriole to elevated [K+]. In contrast to the afferent arteriole, the K+-induced efferent arteriolar hyperpolarization was not associated with vasodilation or altered Ca2+ signaling. The differing responses of these two arterioles to hyperpolarization may be explained by their differing activation mechanisms. Whereas AngII evokes depolarization4 and activates L-type Ca2+ channels in the afferent arteriole,1 this agonist activates nifedipine-insensitive Ca2+ entry in the efferent arteriole.1 Indeed, efferent arterioles of cortical nephrons do not express L-type Ca2+ channels,7 and the contractile response of these vessels to AngII is dissociated from membrane depolarization.4 Accordingly, we suggest that the divergent responses of these two vessels to K+-induced hyperpolarization simply reflect their differing dependence on voltage-dependent Ca2+ entry. Similarly, the lack of effect of barium on Ca2+ signaling in the efferent arteriole (Figure 3, right) may reflect the lack of depolarization-induced Ca2+ entry. We previously demonstrated that 30 μmol/L barium depolarizes the afferent arteriole.14 Although the membrane potential response of the efferent arteriole to barium was not measured in this study, it is known that depolarization induced by an elevation in extracellular [K+] (30 to 80 mmol/L) does not stimulate Ca2+ entry or vasoconstriction in this vessel.1,5,6
Our finding that Kir is expressed in afferent and efferent arterioles is consistent with the suggestion that this channel is preferentially expressed in resistance vessels. The finding that the postglomerular efferent arterioles express Kir also agrees with a recent report by Cao et al.16 that Kir currents are observed in the contractile pericytes of the postglomerular descending vasa recta. In contrast, Prior et al.15 demonstrated a lack of Kir in the renal arcuate artery, a conduit vessel. Others have shown Kir expression to exhibit regional heterogeneity within vascular beds and suggested that expression is inversely related to vessel diameter or order. Thus, Quayle et al.12 found Kir channel density was greater in myocytes from smaller versus larger coronary arteries, and Edwards et al.13 found that K+-induced hyperpolarization was greater in smaller versus larger segments of rat cerebral vessels. This study, in concert with the observations of Prior et al.15 and Cao et al.,16 suggest a similar distribution within the renal vascular bed.
The preferential expression of Kir channels in resistance vessels suggests an importance in the regulation of blood flow and BP; however, the physiologic roles of Kir in the renal microcirculation are not understood. In the cerebral and skeletal muscle vascular beds, elevated interstitial [K+] is an important signal associated with increased activity and is thought to trigger increased blood flow in part via Kir.24 It is not known whether such a mechanism is important in the kidney. It has been suggested that endothelium-derived K+ is a hyperpolarizing factor (EDHF) and that K+ efflux through endothelial charybdotoxin- and apamin-sensitive K+ channels elevates [K+]o to evoke hyperpolarization, in part, by Kir.25 Responses attributed to EDHF are commonly observed in resistance vessels, but not all vessels exhibiting such responses express Kir channels.26 Moreover, we found that the charybdotoxin- and apamin-sensitive EDHF response of the afferent arteriole is insensitive to barium, ruling out a contribution of Kir.27 A recent study28 suggested that Kir may be involved in pressure-induced depolarization and myogenic signaling, in that hypotonic cell swelling reduced Kir currents, implicating regulation by mechanosensitive mechanisms. Our previous attempts to ascertain the involvement of Kir in afferent arteriolar myogenic signaling were compromised by the marked vasoconstrictor response to barium,14 and a possible role of Kir in this response remains an intriguing possibility.
When considering the role of K+ channels in the vasculature, one generally focuses on their potential involvement in the membrane-dependent regulation of tone. This is reasonable when considering the role of Kir currents in the afferent arteriole; however, what could be the function of Kir in the efferent arteriole, a vessel that is not modulated by membrane potential? An interesting possibility relates to recent suggestions that Kir plays an essential role in transmitting electrical signals along the microvasculature, an important characteristic of small vessels expressing Kir channels. The transmission of such signals is blocked by barium, suggesting an essential role of Kir channels.29–32 Whether Kir plays a physiologic role in the efferent arteriole or is simply a vestigial current in this vessel is not known; however, one might speculate that its presence in this vessel could facilitate the transmission of electrical signals to the upstream preglomerular afferent arteriole, where electrical signals might modulate preglomerular tone and GFR.
Although the physiologic role of Kir in the renal microcirculation is not resolved, this study demonstrates that this current is expressed in the myocytes of both afferent and efferent arterioles. Modulation of the outward Kir current alters tone and Ca2+ signaling in the afferent arterioles but not in the efferent arteriole, a vessel that lacks electromechanical coupling. The finding that Kir is expressed in the afferent arteriole and that its modulation alters vessel reactivity is consistent with a traditional view that, by affecting membrane potential, Kir plays an important role in the regulation of vasomotor tone and blood flow in the resistance circulation. Our finding that this current is also prominent in the myocytes of the efferent arteriole, a vessel whose tone is not modulated by membrane potential, may suggest a role in the microcirculation that extends beyond this traditional view.
CONCISE METHODS
All animal protocols were approved by the University of Calgary Animal Care Committee in accordance with the Canadian Council on Animal Care. The in vitro perfused hydronephrotic rat kidney was used to assess contractile and membrane potential responses. Unilateral hydronephrosis was induced in male Sprague-Dawley rats by ligation of the left ureter. After 6 to 8 wk, the rats were anesthetized, the renal artery was cannulated in vivo, and the kidney was excised with continuous perfusion. Kidneys were perfused in vitro with modified DMEM (Life Technologies, Gaithersburg, MD) containing (in mmol/L) 1.6 Ca2+, 26 bicarbonate, 5 glucose, 1 pyruvate, and 5 HEPES. Ibuprofen (10 μmol/L) was added to eliminate the effects of renal prostanoids.33 Perfusion pressure within the renal artery was held at 80 mmHg. Vessel diameters were measured by on-line image processing, and membrane potentials were measured using a dual-pipette system as described previously.4,14,34 Elevated KCl solutions were prepared by isotonic substitution for NaCl.
Afferent and efferent arterioles were isolated from the renal cortex (excluding the juxtamedullary region) of normal rat kidneys using the gel perfusion technique previously described.1 For Ca2+ signaling, fura-2–loaded vessels were superfused (2 ml/min) in a custom chamber held at 37°C and equilibrated with 5% CO2. The 340/380 emission fluorescence ratio was used as a qualitative index of cellular Ca2+ signaling as described previously.1 The expression of Kir2.1 was examined using a nested PCR approach. Total RNA was extracted (RNEasy micro kit; Qiagen, Mississauga, Ontario), and an outer reaction (20 cycles) was performed using primers corresponding to nucleotides 758 to 777 (gttcgatagcggaatcgac) and 1214 to 1234 (cctggttgtggagatctatgc). The nested reaction (35 cycles) was performed using primers corresponding to nucleotides 862 to 883 (gacaatgcagacttgaaatcg) and 1168 to 1190 (ctctctggaactccgttctcac) to yield a 329-bp amplicon. The products were sequenced at the University Sequencing Facility (www.sequencing.ucalgary.ca). For determination of the presence of immunoreactive Kir2.1 protein, vessels were fixed (1% formalin) and treated with 2% Triton X-100. Primary antibodies against α-SMA (mouse monoclonal, A2547; Sigma, St. Louis, MO) and Kir2.1 (rabbit polyclonal, AB5374; Chemicon, Temecula, CA) and secondary antibodies (Cy3–anti-rabbit [Jackson Laboratories, Bar Harbor, ME] and Alexa 488–anti-mouse [Molecular Probes, Eugene, OR]) were used. For ruling out nonspecific interactions, vessels were treated with Kir2.1 antibody pre-equilibrated with the peptide antigen.
Patch-clamp studies were performed on myocytes isolated from single afferent and efferent arterioles, obtained as described previously. Only cells exhibiting the characteristic spindle-shape morphology for the afferent arteriole and elongated smooth muscle morphology with characteristic bifurcated ends for the efferent arteriole (see Loutzenhiser and Loutzenhiser1) were studied. Borosilicate glass pipettes were pulled to a 2- to 3-μm tip diameter, fire polished to a resistance of 4 to 6 MΩ, and coated with Sylgard polymer. Pipettes were filled with an internal solution containing (in mmol/L) 120 K-gluconate, 20 KCl, 1.5 ATP-Mg, 0.1 GTP, 10 HEPES, 5 EGTA, 2 MgCl2, and 0.1 CaCl2 (pH 7.2). The external solution contained 105 mmol/L NaCl, 20 mmol/L KCl, 2 mmol/L MgSO4, 1.5 mmol/L CaCl2, 0.2 μmol/L nifedipine, 5 μmol/L glibenclamide, and 10 mmol/L HEPES (pH 7.4). A 4-s voltage ramp protocol (0.045 mV/ms, −120 to 60 mV) was applied in the whole-cell configuration using pClamp (v8) and an Axoclamp 200B amplifier (Axon Instruments, Union City, CA). Data were acquired at 10 KHz with low-pass filter at 5 KHz. Voltages were not corrected for the liquid junction potential (15 mV, calculated using pClamp).
Data are expressed as means ± SEM. Differences between means for single comparisons were evaluated by paired or unpaired t test. P < 0.05 was considered significant. For multiple measurements, ANOVA followed by Bonferroni t test were applied to assess significance.
Disclosures
None.
Acknowledgments
This study was supported by grants from the Canadian Institutes for Health Research, the Heart and Stroke Foundation of Alberta and Nunavet, the Natural Sciences and Engineering Research Council, and the Alberta Heritage Foundation for Medical Research (AHFMR). R.L. is an AHFMR Scientist, and L.C. was supported by studentships from AHFMR and the Medical Research Council and is an Honorary Killam Memorial Scholar.
This work was previously presented, in part, at the annual meeting of the American Society of Nephrology (November 12 through 17, 2003; San Diego, CA) and published in abstract form.35
We gratefully acknowledge the technical assistance of Bob Winkfein in designing the PCR studies. We also thank Dr. Robert Clark and Dr. Philip Aaronson for advice in the preparation of this article.
Published online ahead of print. Publication date available at www.jasn.org.
L.C. and K.L. contributed equally to this work.
References
- 1.Loutzenhiser K, Loutzenhiser R: Angiotensin II-induced Ca2+ influx in renal afferent and efferent arterioles: Differing roles of voltage-gated and store-operated Ca2+ entry. Circ Res 87: 551–557, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Carmines PK, Navar LG: Disparate effects of Ca channel blockade in afferent and efferent arteriolar responses to ANG II. Am J Physiol 256: F1015–F1020, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Shiraishi M, Wang X, Walsh MP, Kargacin G, Loutzenhiser K, Loutzenhiser R: Myosin heavy chain isoform expression in renal afferent and efferent arterioles: Relation to contractile kinetics and function. FASEB J 17: 2284–2286, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Loutzenhiser R, Chilton L, Trottier G: Membrane potential recordings of afferent and efferent arterioles in the in vitro perfused hydronephrotic rat kidney: Actions of angiotensin II. Am J Physiol 273: F307–F314, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Loutzenhiser R, Hayashi K, Epstein M: Divergent effects of KCl-induced depolarization on afferent and efferent arterioles. Am J Physiol 257: F561–F564, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Carmines PK, Fowler BC, Bell PD: Segmentally distinct effects of depolarization on intracellular [Ca2+] in renal arterioles. Am J Physiol 265: F677–F685, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Hansen PB, Jensen BL, Andreasen D, Skott O: Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ Res 89: 630–638, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Pallone TL, Zhang Z, Rhinehart K: Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol 284: F253–F266, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Pallone TL, Huang JM: Control of descending vasa recta pericyte membrane potential by angiotensin II. Am J Physiol Renal Physiol 282: F1064–F1074, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Nelson MT, Quayle JM: Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 268: C799–C822, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Cole WC, Chomienne-Clément O: Properties, regulation, and role of potassium channels in smooth muscle. In: Advances in Organ Biology, edited by Barr L, Christ GJ, Stamford, CT, JAI Press, 2000, pp 247–317
- 12.Quayle JM, Dart C, Nelson MT: The properties and distribution of inward rectifier potassium channels in pig coronary arterial smooth muscle. J Physiol 494: 715–726, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards FR, Hirst GC, Silverberg GD: Inward rectification in rat cerebral arterioles: Involvement of potassium ions in autoregulation. J Physiol 404: 455–466, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chilton L, Loutzenhiser R: Functional evidence of inward rectifier potassium channel current in rat renal afferent arterioles. Circ Res 88: 152–158, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Prior HM, Webster N, Quinn K, Beech DJ, Yates MS: K+-induced dilation of a small renal artery: No role for the inward rectifier K+ channel. Circ Res 37: 780–790, 1989 [DOI] [PubMed] [Google Scholar]
- 16.Cao C, Goo JH, Lee-Kwon W, Pallone TL: Vasa recta pericytes express a strong inward rectifier K+ conductance. Am J Physiol Regul Integr Comp Physiol 290: R1601–R1607, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kurtz A, Penner R: Angiotensin II induces oscillations of intracellular calcium and blocks anomalous inward rectifying potassium current in mouse renal juxtaglomerular cells. Proc Natl Acad Sci U S A 86: 3423–3427, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leichtle A, Rauch U, Albinus M, Benohr P, Kalbacher H, Mack AF, Veh RW, Quast U, Russ U: Electrophysiological and molecular characterization of the inward rectifier in juxtaglomerular cells from rat kidney. J Physiol 560: 365–376, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friis UG, Jorgensen F, Andreasen D, Jensen BL, Skott O: Molecular and functional identification of cyclic AMP-sensitive BKCa potassium channels (ZERO variant) and L-type voltage-dependent calcium channels in single rat juxtaglomerular cells. Circ Res 93: 213–220, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Gomez RA, Chevalier RL, Carey RM, Peach MJ: Molecular biology of the renal renin-angiotensin system. Kidney Int Suppl 30: S18–S23, 1990 [PubMed] [Google Scholar]
- 21.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL: Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ Res 87: 160–166, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Lu Z: Mechanism of rectification in inward-rectifier K+ channels. Annu Rev Physiol 66: 103–129, 2004 [DOI] [PubMed] [Google Scholar]
- 23.McCarron JG, Halpern W: Potassium dilates rat cerebral arteries by two independent mechanisms. Am J Physiol 259: H902–H908, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Haddy FJ, Vanhoutte PM, Feletou M: Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol 290: R546–R552, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Edwards G, Weston AH: Potassium and potassium clouds in endothelium-dependent hyperpolarizations. Pharmacol Res 49: 535–541, 2004 [DOI] [PubMed] [Google Scholar]
- 26.McGuire JJ, Ding H, Triggle CR: Endothelium-derived relaxing factors: A focus on endothelium-derived hyperpolarizing factor(s). Can J Physiol Pharmacol 79: 443–470, 2001 [PubMed] [Google Scholar]
- 27.Wang X, Loutzenhiser R: Determinants of renal microvascular response to ACh: Afferent and efferent arteriolar actions of EDHF. Am J Physiol Renal Physiol 282: F124–F132, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Wu BN, Luykenaar KD, Brayden JE, Giles WR, Corteling RL, Wiehler WB, Welsh DG: Hyposmotic challenge inhibits inward rectifying K+ channel in cerebral arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 292: H1085–H1094, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Rivers RJ, Hein TW, Zhang C, Kuo L: Activation of barium-sensitive inward rectifier potassium channels mediates remote dilation of coronary arterioles. Circulation 104: 1749–1753, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Horiuchi T, Dietrich HH, Hongo K, Dacey RG Jr: Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke 33: 2692–2699, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Goto K, Rummery NM, Grayson TH, Hill CE: Attenuation of conducted vasodilatation in rat mesenteric arteries during hypertension: Role of inwardly rectifying potassium channels. J Physiol 561: 215–231, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jantzi MC, Brett SE, Jackson WF, Corteling R, Vigmond EJ, Welsh DG: Inward rectifying potassium channels facilitate cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol 291: H1319–H1328, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Tang L, Loutzenhiser K, Loutzenhiser R: Biphasic actions of PGE2 on the renal afferent arteriole: Role of EP3 and EP4 receptors. Circ Res 86: 663–670, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Loutzenhiser R: In situ studies of renal arteriolar function using the in vitro perfused hydronephrotic rat kidney. Int Rev Exp Pathol 36: 145–160, 1996 [PubMed] [Google Scholar]
- 35.Loutzenhiser R, Chilton L, Loutzenhiser K, Morales E, Kargacin GJ: Differing roles of the inward rectifier K current in renal afferent and efferent arterioles [Abstract]. J Am Soc Nephrol 14: 606a, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]