Abstract
11β-Hydroxysteroid dehydrogenase (11β-HSD) type 1 and type 2 catalyze the interconversion of inactive and active glucocorticoids. Impaired regulation of these enzymes has been associated with obesity, diabetes, hypertension, and cardiovascular disease. Previous studies in animals and humans suggested that dehydroepiandrosterone (DHEA) has antiglucocorticoid effects, but the underlying mechanisms are unknown. In this study, DHEA treatment markedly increased mRNA expression and activity of 11β-HSD2 in a rat cortical collecting duct cell line and in kidneys of C57BL/6J mice and Sprague-Dawley rats. DHEA-treated rats tended to have reduced urinary corticosterone to 11-dehydrocorticosterone ratios. It was found that CCAAT/enhancer-binding protein-α (C/EBP-α) and C/EBP-β regulated HSD11B2 transcription and that DHEA likely modulated the transcription of 11β-HSD2 in a phosphatidylinositol-3 kinase/Akt-dependent manner by increasing C/EBP-β mRNA and protein expression. Moreover, it is shown that C/EBP-α and C/EBP-β differentially regulate the expression of 11β-HSD1 and 11β-HSD2. In conclusion, DHEA induces a shift from 11β-HSD1 to 11β-HSD2 expression, increasing conversion from active to inactive glucocorticoids. This provides a possible explanation for the antiglucocorticoid effects of DHEA.
Enhanced glucocorticoid effects, mainly as a result of locally disturbed glucocorticoid metabolism, contribute to diseases such as hypertension and the metabolic syndrome.1–3 The adrenal steroid hormone precursor dehydroepiandrosterone (DHEA) is the most abundant circulating steroid in humans, with peak levels between 20 and 30 yr of age, followed by a steady, age-dependent decline. Many metabolic effects, including antiobesity, antidiabetic, and antiaging properties, have been attributed to DHEA.4 In rodents, DHEA attenuated ischemia/reperfusion-induced oxidative stress and renal dysfunction,5 showed beneficial effects in diabetic nephropathy,6,7 and inhibited the age-related development of proteinuria.8 DHEA seems to counteract several adverse effects of excessive glucocorticoid action, including a negative correlation between DHEA concentrations and body mass index, visceral adiposity, and impaired insulin sensi-tivity in elderly individuals9; however, the molecular mechanisms underlying these antigluco-corticoid effects remain unclear.
In peripheral tissues, local glucocorticoid metabolism is mainly controlled by 11β-hydroxysteroid dehydrogenase (11β-HSD1) and 11β-HSD2. 11β-HSD1 catalyzes the reduction of inactive 11-ketoglucocorticoids (cortisone, 11-dehydrocorticosterone) into active 11β-hydroxyglucocorticoids (cortisol, corticosterone).2In vitro, 11β-HSD1 catalyzes both reductase and dehydrogenase reaction, whereas in vivo and in intact cells expressing hexose-6-phosphate dehydrogenase, providing co-substrate NADPH, it predominantly acts as a reductase and is essential for glucocorticoid reactivation in metabolically relevant tissues.10–12 11β-HSD2 acts exclusively as a dehydrogenase using co-substrate NAD+ and has a pivotal role in mineralocorticoid target tissues by protecting mineralocorticoid receptors (MR) from activation by cortisol and in placenta by protecting the fetus from high maternal cortisol.13
Several steroid hormones have been associated with the regulation of 11β-HSD gene expression.14 Homma et al.15 showed that administration of DHEA sulfate decreased BP in spontaneously hypertensive rats. Treated rats had reduced ratios of serum corticosterone to 11-dehydrocorticosterone, and the oxidation of corticosterone was increased in kidneys but decreased in the liver, suggesting that altered 11β-HSD activities contributed to the observed effects; however, these authors did not clearly distinguish between the activities of 11β-HSD1 and 11β-HSD2 or take into account that 11β-HSD1 also functions as a dehydrogenase with NAD+.16 We recently reported reduced 11β-HSD1 expression in response to DHEA in 3T3-L1 adipocytes and in liver and adipose tissue of treated mice.17 We showed that the CCAAT/enhancer-binding protein (C/EBP) family of basic leucin zipper transcription factors C/EBP-α, C/EBP-β, and C/EBP-δ is involved in the DHEA-mediated regulation of 11β-HSD1 expression. C/EBP can act as transcriptional activators as well as repressors.18 C/EBP-α and C/EBP-β were shown to bind directly to the HSD11B1 promoter and act in concert to regulate gene expression in liver cells19 and adipocytes.20 Whether C/EBP might also modulate HSD11B2 gene expression has not been investigated.
Here, we examined the impact of DHEA on 11β-HSD2 expression and activity in cultured cells and in kidneys of Sprague-Dawley rats and C57BL/6J mice. We investigated whether C/EBP are involved in the transcriptional regulation of 11β-HSD2 and compared their effects with those on 11β-HSD1. In particular, we tested whether C/EBP transcription factors might be responsible for the differential regulation of 11β-HSD1 and 11β-HSD2.
RESULTS
DHEA Upregulates 11β-HSD2 Expression and Activity in Renal Cortical Collecting Duct Cells
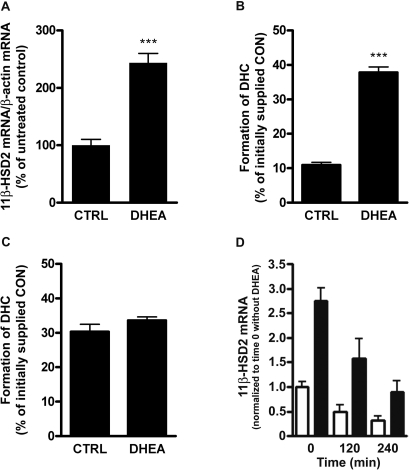
Recently, we reported that DHEA decreases 11β-HSD1 mRNA expression in cultured cells and C57Bl/6J mice.17 Here, we tested the hypothesis that DHEA has antiglucocorticoid effects by inducing a switch from 11β-HSD1–dependent activation to 11β-HSD2–dependent inactivation of glucocorticoids. We investigated the impact of DHEA on 11β-HSD2 expression and activity in RCCD2 rat cortical collecting duct cells.21 Incubation of RCCD2 cells for 24 h with 25 μM DHEA resulted in 2.6-fold higher 11β-HSD2 gene expression (Figure 1A) and 3.5-fold increased reductase activity (Figure 1B). DHEA dosage-dependently enhanced 11β-HSD2 activity, reaching approximately 80% of maximal activation at 25 μM (EC50 approximately 15 μM; data not shown). DHEA up to 100 μM did not affect corticosterone conversion in human embryonic kidney 293 (HEK-293) cells expressing recombinant 11β-HSD2 under the control of a cytomegalovirus promoter, excluding a direct stimulatory effect of DHEA on enzyme activity (Figure 1C). We next examined whether the DHEA-mediated induction of 11β-HSD2 gene expression involves changes in mRNA stability. DHEA treatment increased 11β-HSD2 transcription in RCCD2 cells approximately three-fold (Figure 1D). Addition of the transcription inhibitor actinomycin D (10 μg/ml) after 24 h of incubation with vehicle or 25 μM DHEA resulted in a similar decay of 11β-HSD2 mRNA over the course of 4 h, with estimated half-lives of 138 and 148 min, respectively (Figure 1D). Together, these observations suggest that DHEA stimulates 11β-HSD2 activity in RCCD2 cells primarily through activation of gene transcription. In contrast to renal cortical collecting duct cells, DHEA did not affect 11β-HSD2 expression in Caco-2 and SW620 colon cells (data not shown), suggesting tissue-specific regulation.
Figure 1.
Effect of DHEA on expression and activity of endogenous and recombinant 11β-HSD2. Renal cortical collecting duct RCCD2 cells expressing endogenous 11β-HSD2 were incubated with vehicle (CTRL) or 25 μM DHEA for 24 h, followed by determination of 11β-HSD2 mRNA levels, which were normalized to β-actin mRNA levels (A), and enzyme activity, which was expressed as percentage of converted [1,2,6,7-3H]-corticosterone of an initially supplied concentration of 100 nM (B). A potential direct effect of DHEA (100 μM) on the conversion of corticosterone (100 nM) by recombinant human 11β-HSD2 under the control of a cytomegalovirus promoter was measured in stably transfected HEK-293 cells (C). For measurement of potential effects of DHEA on 11β-HSD2 mRNA stability, RCCD2 cells were incubated in charcoal-treated DMEM for 24 h in the absence (□) or presence (▪) of 25 μM DHEA, before inhibition of transcription by addition of actinomycin D (10 μg/ml) and further incubation for 2 to 4 h. 11β-HSD2 and β-actin mRNA were measured at each time point by real-time reverse transcriptase–PCR. 11β-HSD2 mRNA was normalized to β-actin mRNA, and data are expressed relative to time zero (immediately before addition of actinomycin D), which was set as 1 (D). DHC, 11-dehydrocorticosterone; CON, corticosterone. Data are means ± SD from at least three independent experiments. ***P < 0.001.
DHEA Upregulates 11β-HSD2 Expression and Activity in the Kidney
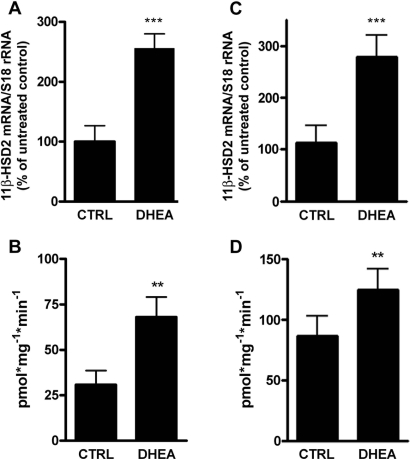
The effect of DHEA on 11β-HSD2 expression and activity was then investigated in vivo by feeding Sprague-Dawley rats standard rodent chow or chow containing 0.2% DHEA. Treatment resulted in 2.8-fold higher 11β-HSD2 mRNA expression (Figure 2A) and 2.2-fold elevated renal 11β-HSD2 enzyme activity (Figure 2B). Comparable results were obtained in DHEA-treated C57Bl/6J mice, indicating species-independent effects. The expression of 11β-HSD2 mRNA was elevated 2.5-fold (Figure 2C) and the activity 1.4-fold (Figure 2D) in kidneys of DHEA-treated mice compared with control mice. Moreover, we measured the ratio of corticosterone (B) to 11-dehydrocorticosterone (A) in 24-h urine samples collected on days 4 and 13 of treatment. DHEA-treated rats showed a tendency for a decreased B/A ratio (1.8-fold on day 4 [P = 0.259] and 1.7-fold on day 13 [P = 0.285]; Figure 3). Given the low number of rats investigated and the variability of urinary steroid metabolite measurements, it was reasonable to calculate the mean value for each rat and compare DHEA-treated with nontreated rats, which yielded a 1.8-fold decrease of the B/A ratio for treated rats (P < 0.05).
Figure 2.
Upregulation of renal 11β-HSD2 mRNA expression and activity by DHEA in Sprague-Dawley rats and C57BL/6J mice. Rats (A and B) and mice (C and D) were treated for 13 and 12 d, respectively, with vehicle (CTRL) or 0.2% DHEA, followed by removal of kidneys and determination of 11β-HSD2 mRNA levels, which were normalized to S18 rRNA. 11β-HSD2–dependent conversion of cortisol to cortisone, expressed as picomoles of converted cortisol per milligram of total protein per minute, was measured in kidney homogenates from rats (B) and mice (D). Data are means ± SD (n = 4 for rats, n = 8 for mice). **P < 0.01; ***P < 0.001.
Figure 3.
Urinary ratio of corticosterone to 11-dehydrocorticosterone measured in 24-h urine samples collected from Sprague-Dawley rats on days 4 and 13 of treatment, respectively, as described in the Concise Methods section. ▪, Control rats; □, DHEA-treated rats. Bars represent means of values obtained from four rats ± SD. *P < 0.05.
DHEA Modulates Glucocorticoid Metabolism by Inducing a Switch from 11β-HSD1 to 11β-HSD2 Expression
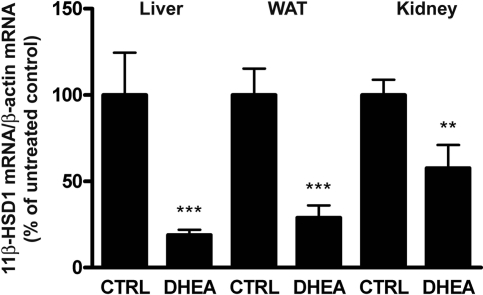
To ensure that the DHEA-mediated suppression of glucocorticoid reactivation reported in a previous study with mice17 is not species specific, we measured 11β-HSD1 mRNA expression in liver, white adipose tissue (WAT), and kidney from Sprague-Dawley rats fed standard rodent chow or chow containing 0.2% DHEA. DHEA significantly lowered 11β-HSD1 mRNA expression in liver (19 ± 3% of control; P < 0.001), WAT (28 ± 5%; P < 0.001), and kidney (57 ± 10%; P < 0.01; Figure 4), in line with previous observations in C57BL/6J mice.17 DHEA treatment for a relatively short time (12 d) did not significantly alter food intake or body weight in C57BL/6J mice and Sprague-Dawley rats (data not shown).
Figure 4.
DHEA downregulates 11β-HSD1 mRNA expression in Sprague-Dawley rats. Male Sprague-Dawley rats were fed standard rodent chow (CTRL) or chow containing 0.2% DHEA for 13 d. The mRNA levels were normalized to β-actin mRNA control and represent percentage of untreated control. Data are means ± SD (n = 4). *P < 0.05; **P < 0.01; ***P < 0.001.
DHEA Modulates C/EBP Expression Levels in Cells and In Vivo
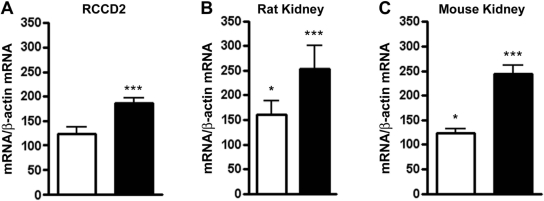
To explore the mechanisms underlying the DHEA-mediated stimulation of 11β-HSD2 expression and to test whether C/EBP transcription factors might differentially regulate 11β-HSD117 and 11β-HSD2, we measured the expression of C/EBP-α and C/EBP-β mRNA in RCCD2 cells treated with 25 μM DHEA for 24 h. C/EBP-α mRNA levels were not significantly altered, whereas C/EBPβ mRNA expression increased almost two-fold upon incubation with DHEA (Figure 5A). In contrast to RCCD2 cells, DHEA treatment did not alter mRNA expression of 11β-HSD2, C/EBP-α, and C/EBP-β in Caco-2 and SW620 colon cancer cells (data not shown). These results suggest a modulatory role for C/EBP-α and C/EBP-β in the DHEA-induced renal HSD11B2 expression.
Figure 5.
Effect of DHEA on the expression of C/EBP-α and C/EBP-β. Expression of C/EBP-α (□) and C/EBP-β mRNA (▪) in DHEA-treated RCCD2 cells (A) and in kidneys of Sprague-Dawley rats (n = 4; B) and C57BL/6J mice (n = 8; C). C/EBP-α and C/EBP-β mRNA expression levels were normalized to β-actin mRNA and represent percentage of untreated control. Data are means ± SD. *P < 0.05; ***P < 0.001.
Next, we analyzed C/EBP-α and C/EBP-β gene expression in various tissues from DHEA-supplemented Sprague-Dawley rats and C57BL/6J mice. In rats, DHEA treatment decreased hepatic C/EBP-α mRNA expression approximately 60%, whereas C/EBP-β mRNA levels remained unchanged (data not shown). In WAT, C/EBP-α gene expression was approximately 50% reduced, whereas C/EBP-β mRNA levels were 1.9-fold higher (data not shown), in line with previous observations in C57BL/6J mice.17 In contrast, both transcripts were increased in kidney, with a clearly more pronounced effect on C/EBP-β both in rats (Figure 5B) and in mice (Figure 5C). Together, these results reveal a DHEA-mediated modulation of C/EBP-α and C/EBP-β expression in vivo, likely responsible for the differential effects on 11β-HSD1 and 11β-HSD2 expression and activity.
Differential Stimulation of HSD11B1 and HSD11B2 Promoters by C/EBP-α and C/EBP-β
To study the impact of C/EBP-α and C/EBP-β on HSD11B1 and HSD11B2 expression, we co-expressed different quantities of each transcription factor with a Renilla luciferase gene driven by the HSD11B1 or HSD11B2 promoter in HEK-293 cells. Both transcription factors greatly enhanced HSD11B1 promoter-dependent luciferase activity, whereby C/EBP-α was a stronger activator, and higher ratios of C/EBP-α to C/EBP-β resulted in correspondingly higher luciferase activities (Figure 6Α). In contrast, the HSD11B2 promoter-dependent transcription was stimulated more potently by C/EBP-β, and higher ratios of C/EBP-α to C/EBP-β resulted in lower luciferase activities (Figure 6B).
Figure 6.
C/EBP-α–and C/EBP-β–dependent activation of luciferase reporter genes under the control of HSD11B1 and HSD11B2 promoters. HEK-293 cells were transfected with plasmids for C/EBP-α and/or C/EBP-β in the relative ratios indicated (0.6 μg of total plasmid DNA) and the Renilla luciferase-HSD11B1 (A) or -HSD11B2 promoter construct (B). Promoter activity is expressed as relative luciferase activity normalized to luciferase activity in the absence of C/EBP. Data are means ± SD from four independent experiments.
DHEA-Mediated Stimulation of 11β-HSD2 Involves Akt Kinase
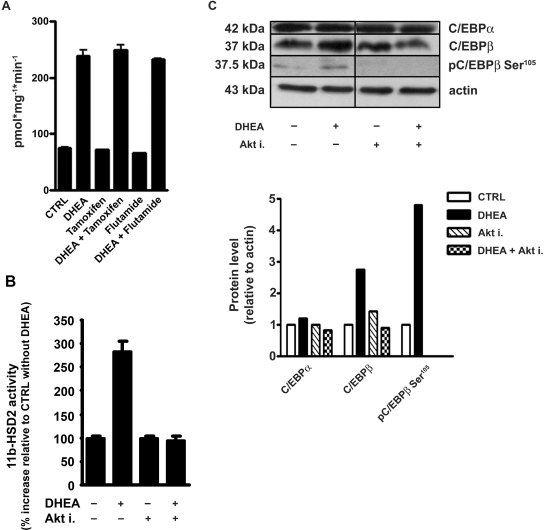
We next investigated whether metabolism of DHEA to testosterone or 17β-estradiol with subsequent activation of androgen and estrogen receptors, respectively, is required for its effect on 11β-HSD2. Neither the estrogen receptor antagonist tamoxifen nor the androgen receptor antagonist flutamide affected the DHEA-mediated upregulation of 11β-HSD2 activity in RCCD2 cells (Figure 7A).
Figure 7.
DHEA-mediated activation of 11β-HSD2 involves Akt kinase but not metabolism to 17β-estradiol and testosterone. RCCD2 cells were incubated with tamoxifen (estrogen receptor antagonist; 100 nM) or flutamide (androgen receptor antagonist; 500 nM) in the absence or presence of DHEA for 24 h, followed by determination of 11β-HSD2 activity (A). 11β-HSD2 activity assay in the presence or absence of DHEA and/or 3μM Akt inhibitor. Activities are expressed as percentage of control in the absence of DHEA, which was set to 100% (B). Immunoblot analysis of C/EBP-β, C/EBP-β phosphorylated on Ser105 (pC/EBP-β), and C/EBP-α. Protein levels are expressed relative to β-actin control protein and normalized to treatment with vehicle, which was set as 1 (C).
Dillon and co-workers22,23 suggested that DHEA exerts its vascular protective effects through a G-protein–coupled membrane receptor and activation of phosphatidylinositol-3 kinase (PI3K)/Akt. Therefore, we examined whether inhibition of Akt kinase might block the DHEA-mediated increase of 11β-HSD2 activity. Incubation of RCCD2 cells with Akt inhibitor blunted the stimulatory effect (Figure 7B), suggesting that activation of Akt is required for the DHEA-mediated increase of 11β-HSD2 activity.
Because phosphorylation of rat C/EBP-β on Ser105 has been shown to be essential for its nuclear translocation and transactivation capacity,24 we tested whether inhibition of Akt kinase can influence C/EBP-β phosphorylation at this residue. DHEA significantly enhanced C/EBP-β protein levels in RCCD2 cells, while having little or no effect on C/EBP-α protein expression (Figure 7C). In addition, incubation of RCCD2 cells with DHEA resulted in elevated phosphorylation of C/EBP-β on Ser105, which might enhance its transactivation potency. Phosphorylation of C/EBP-β on Ser105 was prevented by inhibition of Akt kinase.
DISCUSSION
Impaired intracellular metabolism of glucocorticoids contributes to the development of various pathologic conditions. Here, we investigated the counteracting effects of DHEA on local glucocorticoid metabolism by 11β-HSD enzymes, a mechanism originally proposed by Homma et al.,15 who observed reduced ratios of corticosterone to 11-dehydrocorticosterone and decreased BP in spontaneously hypertensive rats. We previously reported that DHEA downregulated 11β-HSD1–dependent glucocorticoid regeneration in liver, adipose tissue, and kidneys of C57BL/6J mice.17 DHEA had the same effects in Sprague-Dawley rats in this study. Moreover, 11β-HSD2–dependent glucocorticoid inactivation was enhanced in kidneys of DHEA-treated mice and rats. The sum of the locally altered glucocorticoid metabolism (i.e., enhanced renal 11β-HSD2 activity and reduced 11β-HSD1 activity in liver and adipose tissue; Table 1) led to decreased ratios of urinary free corticosterone to 11-dehydrocorticosterone in DHEA-treated Sprague-Dawley rats, in line with Homma et al.15 Because DHEA does not compete with glucocorticoids for binding to glucocorticoid receptors,25 it may protect from excessive glucocorticoid action by means of altered glucocorticoid metabolism.
Table 1.
Overview of the regulation of gene expression in various tissues of Sprague-Dawley rats and C57BI/6J mice by DHEAa
| Tissue | Gene
|
|||
|---|---|---|---|---|
| CEBPA | CEBPB | HSD11B1 | HSD11B2 | |
| WAT | ↓ | ↑ | ↓↓ | ND |
| Liver | ↓ | → | ↓↓ | ND |
| Kidney | ? | ↑↑ | ↓ | ↑↑ |
Arrows indicate the direction of change in gene expression relative to untreated control. Double arrows indicate at least 1.5-fold change in gene expression. Arrow to the right indicates that expression did not change. ? indicates evidence for upregulation was unclear. ND, not determined.
In renal cortical collecting ducts and distal tubules, glucocorticoid inactivation is essential because it renders specificity of MR for aldosterone.26 Impaired 11β-HSD2 activity as a result of genetic mutations or the presence of inhibitors can cause severe hypertension as a consequence of glucocorticoid-induced activation of MR with excessive sodium retention.3,27 Reduced 11β-HSD2 activity has been observed in nephrotic syndrome, upon biliary obstruction, and in liver cirrhosis.28–32 Furthermore, salt sensitivity, at least in part attributable to reduced 11β-HSD2 activity,33 and hypertension are risk factors associated with diabetes.34,35 Lavery et al.36 provided evidence for an association between mutations in HSD11B2 and diabetic nephropathy. Recent studies suggested that DHEA might have beneficial effects in obese Zucker rats with diabetic nephropathy.6,7 Our results might link these findings by demonstrating a role for DHEA in the reduction of local glucocorticoid concentrations (i.e., in the renal proximal tubule37 by downregulation of 11β-HSD1 and in cortical collecting ducts and distal tubules by stimulating 11β-HSD2 expression).
We recently showed that DHEA modulates the expression of C/EBP transcription factors and linked this to reduced 11β-HSD1 expression.17 On the basis of the presence of putative C/EBP binding sites in the HSD11B2 promoter, we investigated whether C/EBP directly regulate this gene and whether DHEA mediates a differential effect on 11β-HSD1 and 11β-HSD2 by changing the relative expression of C/EBP-α and C/EBP-β. C/EBP-α and C/EBP-β directly activated HSD11B1 and HSD11B2 promoters (Figure 6) but with differential effects. Whereas C/EBP-α is a strong activator of HSD11B1 and a weak activator of HSD11B2, C/EBP-β acts in an opposite way and preferentially stimulates HSD11B2 expression. DHEA treatment reduced C/EBP-α mRNA expression levels in liver and WAT and enhanced C/EBP-β expression in WAT and kidneys, respectively, both in C57BL/6J mice and Sprague-Dawley rats. As a net effect, DHEA shifted the equilibrium from C/EBP-α to C/EBP-β expression in these three tissues (Table 1). The approximately three-fold increased expression of C/EBP-β protein, without significant changes in C/EBP-α protein, after treatment of RCCD2 cells with DHEA supports the shift from C/EBP-α to C/EBP-β observed on the RNA level. Regarding 11β-HSD1, our results are in line with observations by Williams et al.19 demonstrating that C/EBP-α is a potent activator of HSD11B1 expression, whereas C/EBP-β was a relatively weak activator, competing with the strong activation mediated by C/EBP-α. Moreover, these authors reported reduced hepatic 11β-HSD1 expression in C/EBP-α- deficient mice and increased levels in C/EBP-β–deficient animals.
Our results from experiments with RCCD2 cells suggest that the effects of DHEA are not due to its metabolism to 17β-estradiol or testosterone with subsequent activation of estrogen receptor and androgen receptor, because neither antagonists of these receptors (Figure 7A) nor the aromatase inhibitor formestane (data not shown) had any influence. Instead, inhibition of Akt kinase prevented the DHEA-induced increase of C/EBP-β protein expression and its phosphorylation on Ser105 as well as stimulation of 11β-HSD2 activity. Recent evidence suggested that DHEA might act through G-protein–coupled membrane receptors and subsequent activation of PI3K/Akt.23 Phosphorylation of Ser105 on rat C/EBP-β has been shown to enhance its transactivation potency.24,38 Moreover, activation of PI3K/Akt has been reported to be important for nuclear translocation and DNA binding of C/EBP-β.39 Together, these observations support a role for PI3K/Akt in the DHEA-mediated regulation of the ratio of C/EBP-α to C/EBP-β protein expression as well as their phosphorylation state. Elucidation of the mechanism underlying DHEA action requires further studies. The phosphorylation state of C/EBP is dynamically regulated by various kinases and phosphatases, and the pathway downstream from the proposed G-protein–coupled membrane receptor is yet to be characterized. Exploration of the mechanisms regulating the transactivation potency of C/EBP-α and C/EBP-β by DHEA is important to understand the fine-tuned control of 11β-HSD1 and 11β-HSD2 activities, which determines glucocorticoid action in a highly tissue-specific manner. By altering expression and transcriptional activity of C/EBP-α and C/EBP-β, DHEA may exert its antiglucocorticoid action in C57BL/6J mice and Sprague-Dawley rats by inducing a switch from 11β-HSD1 reductase activity (liver and fat) to 11β-HSD2 dehydrogenase activity (kidney), thereby reducing the overall intracellular glucocorticoid availability.
A similar but opposite switch (increased 11β-HSD1 reductase and reduced 11β-HSD2 dehydrogenase activity) was previously shown for the cytokine TNF-α.40–42 Importantly, in obese Zucker rats, DHEA treatment reduced serum TNF-α,43 suggesting that the two pathways influence each other. The differential regulation of 11β-HSD1 and 11β-HSD2 is of physiologic relevance, because it modulates the intracellular availability of active glucocorticoids.
Our findings indicate that the tissue-specific expression of 11β-HSD1 and 11β-HSD2 is tightly controlled by C/EBP-α and C/EBP-β. Mechanisms by which DHEA induces a switch from 11β-HSD1–dependent glucocorticoid activation to 11β-HSD2–dependent inactivation, thereby counteracting excessive glucocorticoid action, may involve modulation of PI3K/Akt and a shift in the expression from C/EBP-α to C/EBP-β. Although these effects of DHEA are beneficial in metabolically active tissues to avoid glucocorticoid excess, the uncontrolled consumption of DHEA might cause adverse effects in processes such as cell proliferation and differentiation, in which both C/EBP and 11β-HSD also play crucial roles.
CONCISE METHODS
Materials
Cell culture media, Oligo-dT, and Superscript II reverse transcriptase were from Invitrogen (Carlsbad, CA); steroids were from Steraloids (Wilton, NH); [1,2,6,7-3H]-cortisol and [1,2,6,7-3H]-corticosterone were from Amersham Health AG (Wädenswil, Switzerland); [1,2,6,7-3H]-cortisone was from American Radiolabeled Chemicals (St. Louis, MO), InSolution Akt inhibitor VIII (isozyme selective) was from Calbiochem (VWR Int., Dietikon, Switzerland); and reagents for real-time PCR were from Applied Biosystems (Foster City, CA). All other chemicals were from Fluka AG (Buchs, Switzerland).
Cell Culture, Transfection, and Transactivation Studies
HEK-293 cells and colon cancer–derived SW620 and Caco-2 cells were grown in DMEM and supplemented with penicillin, streptomycin, l-glutamine, and 10% FCS. The rat renal cortical collecting duct cell line RCCD2, provided by Dr. N. Farman (College de France, Paris, France), was cultured as described previously.21 HEK-293 cells, grown to 90% confluence, were transfected using the Ca2+-phosphate method with different combinations of plasmids containing full-length rat C/EBP-α or C/EBP-β (in pcDNA3.1; provided by Dr. P. Johnson,44 National Cancer Institute, Frederick, MD); pGL3-derived plasmids containing the Renilla luciferase gene under the control of human HSD11B1 or HSD11B2 promoter, consisting of approximately 1300 bp of sequence upstream of the initiation codon (provided by Dr. Brigitte Frey,42 Department of Nephrology, Berne, Switzerland); and β-galactosidase control plasmid. Six hours after transfection, the medium was replaced by charcoal-treated DMEM, followed by incubation for another 24 h and determination of luciferase activity using the Dual-Light system (Tropix, Bedford, MA) and a Fluoroskan Ascent FL luminometer (Thermo Electron Corp, Vantaa, Finland).
11β-HSD2 mRNA Decay
RCCD2 cells were incubated in the absence or presence of 25 μM DHEA for 24 h, followed by blocking transcription with 10 μg/ml actinomycin D and further incubation for 2 to 4 h. RNA was isolated, and 11β-HSD2 and β-actin mRNA were analyzed by real-time reverse transcriptase–PCR.
Immunoblot Analysis
Cells were washed with PBS and lysed in buffer containing 25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 1 mM sodium vanadate, 1 mM PMSF, and proteinase inhibitor cocktail (Roche Diagnostics, Rotkreuz, Switzerland). Equivalent amounts of protein (20 to 30 μg) were loaded to SDS-PAGE and subsequently transferred to polyvinylidene difluoride membranes (Hybond-P; Amersham-Pharmacia Biotech, Piscataway, NJ), followed by incubation with antibodies against C/EBP-α, C/EBP-β (Santa Cruz Biotechnology, Santa Cruz, CA), C/EBP-β phosphorylated on Ser105 (Cell Signaling Technology, Beverly, MA), and β-actin (Santa Cruz Biotechnology) as a loading control. Total protein was determined using the BCA assay kit (Pierce, Rockford, IL). Proteins were detected using horseradish peroxidase–conjugated secondary antibodies (Santa Cruz Biotechnology) and ECL enhanced chemiluminescence reagent (Amersham-Pharmacia Biotech).
Animal Experiments
All animal experiments were approved by the Ethical Committee for Animal Research of the University of Berne, equivalent to the NIH guidelines. Male C57BL/6J mice (20 to 22 g body weight; Charles River, Paris, France) were kept in individual cages. For feeding, they were transferred to other cages for 2 h and allowed access to 5 g of standard rodent chow and tap water ad libitum. After 5 d of adaptation, eight animals per condition were fed for 12 d with control chow or with chow containing DHEA (0.4% DHEA in g/g dry food, corresponding to 0.2% DHEA in fresh food). Body weight and food and water intake were recorded daily. No significant changes in body weight were observed during the treatment. On day 12, mice were killed and tissues were harvested. Serum DHEA sulfate was measured using a chemiluminescence immunoassay kit and the Immulite One apparatus following the manufacturer's instruction (Diagnostics Products, Los Angeles, CA).
Sprague-Dawley rats (200 to 220 g body weight; Charles River) were housed in groups of four in a 12:12-h light-dark cycle with standard laboratory chow and tap water ad libitum. Adaptation to individual cages was for 2 d. Four rats received control chow and four others received chow supplemented with DHEA (0.2% DHEA in fresh food) for 13 d before they were killed. Plasma samples and major organs were stored at −70°C.
Analysis of Steroids by Gas Chromatography–Mass Spectrometry
Every morning at 9 a.m., 24-h urine samples were collected from rats. The analysis of urinary steroids was described previously,45 with the exception that medroxyprogesterone was used as an internal standard.
RNA Isolation and Analysis
Total RNA was extracted from adherent cultured cells or from animal tissues, and mRNA levels were analyzed as described previously.17 Data from the analysis of the relative expression of each gene versus S18 rRNA or β-actin mRNA was determined using the 2−ΔΔCT method.46 Cycle threshold (CT) values were determined from at least four independent cell experiments or from tissue samples of eight mice or four Sprague-Dawley rats, each measured in triplicate.
Determination of 11β-HSD2 Enzyme Activity
11β-HSD2–dependent conversion of cortisol to cortisone or corticosterone to 11β-dehydrocorticosterone was measured in intact cells as described previously.47,48 For determination of 11β-HSD2 activity in tissue homogenates, kidneys were pulverized in liquid nitrogen and homogenates were incubated for 10 min in buffer TS2 (100 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM MgCl2, 250 mM sucrose, and 20 mM Tris-HCl [pH 7.4]) containing 250 μM NAD+ and cortisol or corticosterone at a final concentration of 100 nM. Steroids were separated by thin layer chromatography and analyzed by scintillation counting as described previously.49
Statistical Analysis
Statistical comparisons between groups were performed by ANOVA or unpaired t test where appropriate. P < 0.05 was considered significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by grants from the Cloëtta Research Foundation (A.O.), the Swiss National Science Foundation (310000-112279 to A.O., 3100A0-102153 to F.J.F., and 3100A0-105889 to F.R.J.), and the Swiss Cancer League (OCS-01402-08-2003 to A.O.). A.O. is a Novartis Research Foundation Professor. F.R.J. was supported by the European Community (EC) FP6 funding (LSHM-CT-2003-503041).
We thank Heidi Jamin, Marcella Klein, and Balázs Legeza for excellent technical assistance, Dr. Nicolette Farman (Paris, France) for RCCD2 cells, Dr. Peter Johnson (Frederick, MD) for C/EBP expression constructs, and Dr. Brigitte Frey (Berne, Switzerland) for HSD11B1/2 reporter plasmids.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Seckl JR, Walker BR: 11Beta-hydroxysteroid dehydrogenase type 1 as a modulator of glucocorticoid action: From metabolism to memory. Trends Endocrinol Metab 15: 418–424, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Atanasov AG, Odermatt A: Readjusting the glucocorticoid balance: An opportunity for modulators of 11b-hydroxysteroid dehydrogenase type 1 activity? Endocr Metab Immune Disord Drug Targets 7: 125–140, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Frey FJ, Odermatt A, Frey BM: Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr Opin Nephrol Hypertens 13: 451–458, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Tchernof A, Labrie F: Dehydroepiandrosterone, obesity and cardiovascular disease risk: A review of human studies. Eur J Endocrinol 151: 1–14, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Aragno M, Cutrin JC, Mastrocola R, Perrelli MG, Restivo F, Poli G, Danni O, Boccuzzi G: Oxidative stress and kidney dysfunction due to ischemia/reperfusion in rat: Attenuation by dehydroepiandrosterone. Kidney Int 64: 836–843, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Aragno M, Parola S, Brignardello E, Manti R, Betteto S, Tamagno E, Danni O, Boccuzzi G: Oxidative stress and eicosanoids in the kidneys of hyperglycemic rats treated with dehydroepiandrosterone. Free Radic Biol Med 31: 935–942, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Richards RJ, Porter JR, Inserra F, Ferder LF, Stella I, Reisin E, Svec F: Effects of dehydroepiandrosterone and quinapril on nephropathy in obese Zucker rats. Kidney Int 59: 37–43, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Pashko LL, Fairman DK, Schwartz AG: Inhibition of proteinuria development in aging Sprague-Dawley rats and C57BL/6 mice by long-term treatment with dehydroepiandrosterone. J Gerontol 41: 433–438, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Villareal DT, Holloszy JO: Effect of DHEA on abdominal fat and insulin action in elderly women and men: A randomized controlled trial. JAMA 292: 2243–2248, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Frick C, Atanasov AG, Arnold P, Ozols J, Odermatt A: Appropriate function of 11beta-hydroxysteroid dehydrogenase type 1 in the endoplasmic reticulum lumen is dependent on its N-terminal region sharing similar topological determinants with 50-kDa esterase. J Biol Chem 279: 31131–31138, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Atanasov AG, Nashev LG, Schweizer RA, Frick C, Odermatt A: Hexose-6-phosphate dehydrogenase determines the reaction direction of 11beta-hydroxysteroid dehydrogenase type 1 as an oxoreductase. FEBS Lett 571: 129–133, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Lavery GG, Walker EA, Draper N, Jeyasuria P, Marcos J, Shackleton CH, Parker KL, White PC, Stewart PM: Hexose-6-phosphate dehydrogenase knock-out mice lack 11 beta-hydroxysteroid dehydrogenase type 1-mediated glucocorticoid generation. J Biol Chem 281: 6546–6551, 2006 [DOI] [PubMed] [Google Scholar]
- 13.White PC, Mune T, Agarwal AK: 11 Beta-hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev 18: 135–156, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM: 11Beta-hydroxysteroid dehydrogenase type 1: A tissue-specific regulator of glucocorticoid response. Endocr Rev 25: 831–866, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Homma M, Onodera T, Hirabatake M, Oka K, Kanazawa M, Miwa T, Hayashi T: Activation of 11 beta-hydroxysteroid dehydrogenase by dehydroepiandrosterone sulphate as an anti-hypertensive agent in spontaneously hypertensive rats. J Pharm Pharmacol 50: 1139–1145, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Arnold P, Tam S, Yan L, Baker ME, Frey FJ, Odermatt A: Glutamate-115 renders specificity of human 11beta-hydroxysteroid dehydrogenase type 2 for the cofactor NAD(+). Mol Cell Endocrinol 201: 177–187, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Apostolova G, Schweizer RA, Balazs Z, Kostadinova RM, Odermatt A: Dehydroepiandrosterone inhibits the amplification of glucocorticoid action in adipose tissue. Am J Physiol Endocrinol Metab 288: E957–E964, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Pei DQ, Shih CH: Transcriptional activation and repression by cellular DNA-binding protein C/EBP. J Virol 64: 1517–1522, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams LJ, Lyons V, MacLeod I, Rajan V, Darlington GJ, Poli V, Seckl JR, Chapman KE: C/EBP regulates hepatic transcription of 11beta -hydroxysteroid dehydrogenase type 1: A novel mechanism for cross-talk between the C/EBP and glucocorticoid signaling pathways. J Biol Chem 275: 30232–30239, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Gout J, Tirard J, Thevenon C, Riou JP, Begeot M, Naville D: CCAAT/enhancer-binding proteins (C/EBPs) regulate the basal and cAMP-induced transcription of the human 11beta-hydroxysteroid dehydrogenase encoding gene in adipose cells. Biochimie 88: 1115–1124, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Djelidi S, Beggah A, Courtois-Coutry N, Fay M, Cluzeaud F, Viengchareun S, Bonvalet JP, Farman N, Blot-Chabaud M: Basolateral translocation by vasopressin of the aldosterone-induced pool of latent Na-K-ATPases is accompanied by alpha1 subunit dephosphorylation: Study in a new aldosterone-sensitive rat cortical collecting duct cell line. J Am Soc Nephrol 12: 1805–1818, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Liu D, Dillon JS: Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Galpha(i2,3). J Biol Chem 277: 21379–21388, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Si H, Reynolds KA, Zhen W, Jia Z, Dillon JS: Dehydroepiandrosterone protects vascular endothelial cells against apoptosis through a Galphai protein-dependent activation of phosphatidylinositol 3-kinase/Akt and regulation of antiapoptotic Bcl-2 expression. Endocrinology 148: 3068–3076, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Buck M, Poli V, van der Geer P, Chojkier M, Hunter T: Phosphorylation of rat serine 105 or mouse threonine 217 in C/EBP beta is required for hepatocyte proliferation induced by TGF alpha. Mol Cell 4: 1087–1092, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Muller C, Cluzeaud F, Pinon GM, Rafestin-Oblin ME, Morfin R: Dehydroepiandrosterone and its 7-hydroxylated metabolites do not interfere with the transactivation and cellular trafficking of the glucocorticoid receptor. J Steroid Biochem Mol Biol 92: 469–476, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Funder JW, Pearce PT, Smith R, Smith AI: Mineralocorticoid action: Target tissue specificity is enzyme, not receptor, mediated. Science 242: 583–585, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Atanasov AG, Ignatova ID, Nashev LG, Dick B, Ferrari P, Frey FJ and Odermatt A: Impaired protein stability of 11beta-hydroxysteroid dehydrogenase type 2: A novel mechanism of apparent mineralocorticoid excess. J Am Soc Nephrol 18: 1262–1270, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Ackermann D, Vogt B, Escher G, Dick B, Reichen J, Frey BM, Frey FJ: Inhibition of 11beta-hydroxysteroid dehydrogenase by bile acids in rats with cirrhosis. Hepatology 30: 623–629, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Vogt B, Dick B, N′Gankam V, Frey FJ, Frey BM: Reduced 11beta-hydroxysteroid dehydrogenase activity in patients with the nephrotic syndrome. J Clin Endocrinol Metab 84: 811–814, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Quattropani C, Vogt B, Odermatt A, Dick B, Frey BM, Frey FJ: Reduced activity of 11beta-hydroxysteroid dehydrogenase in patients with cholestasis. J Clin Invest 108: 1299–1305, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stauffer AT, Rochat MK, Dick B, Frey FJ, Odermatt A: Chenodeoxycholic acid and deoxycholic acid inhibit 11 beta-hydroxysteroid dehydrogenase type 2 and cause cortisol-induced transcriptional activation of the mineralocorticoid receptor. J Biol Chem 277: 26286–26292, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Thiesson HC, Jensen BL, Bistrup C, Ottosen PD, McNeilly AD, Andrew R, Seckl J, Skott O: Renal sodium retention in cirrhotic rats depends on glucocorticoid-mediated activation of mineralocorticoid receptor due to decreased renal 11beta-HSD-2 activity. Am J Physiol Regul Integr Comp Physiol 292: R625–R636, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Lovati E, Ferrari P, Dick B, Jostarndt K, Frey BM, Frey FJ, Schorr U, Sharma AM: Molecular basis of human salt sensitivity: The role of the 11beta-hydroxysteroid dehydrogenase type 2. J Clin Endocrinol Metab 84: 3745–3749, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Fagerudd JA, Tarnow L, Jacobsen P, Stenman S, Nielsen FS, Pettersson-Fernholm KJ, Gronhagen-Riska C, Parving HH, Groop PH: Predisposition to essential hypertension and development of diabetic nephropathy in IDDM patients. Diabetes 47: 439–444, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Strojek K, Grzeszczak W, Morawin E, Adamski M, Lacka B, Rudzki H, Schmidt S, Keller C, Ritz E: Nephropathy of type II diabetes: Evidence for hereditary factors? Kidney Int 51: 1602–1607, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Lavery GG, McTernan CL, Bain SC, Chowdhury TA, Hewison M, Stewart PM: Association studies between the HSD11B2 gene (encoding human 11beta-hydroxysteroid dehydrogenase type 2), type 1 diabetes mellitus and diabetic nephropathy. Eur J Endocrinol 146: 553–558, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Brereton PS, van Driel RR, Suhaimi F, Koyama K, Dilley R, Krozowski Z: Light and electron microscopy localization of the 11beta-hydroxysteroid dehydrogenase type I enzyme in the rat. Endocrinology 142: 1644–1651, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Trautwein C, Caelles C, van der Geer P, Hunter T, Karin M, Chojkier M: Transactivation by NF-IL6/LAP is enhanced by phosphorylation of its activation domain. Nature 364: 544–547, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Lee SJ, Kim SG: Role of p90 ribosomal S6-kinase-1 in oltipraz-induced specific phosphorylation of CCAAT/enhancer binding protein-beta for GSTA2 gene transactivation. Mol Pharmacol 69: 385–396, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Escher G, Galli I, Vishwanath BS, Frey BM, Frey FJ: Tumor necrosis factor alpha and interleukin 1beta enhance the cortisone/cortisol shuttle. J Exp Med 186: 189–198, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper MS, Bujalska I, Rabbitt E, Walker EA, Bland R, Sheppard MC, Hewison M, Stewart PM: Modulation of 11beta-hydroxysteroid dehydrogenase isozymes by proinflammatory cytokines in osteoblasts: an autocrine switch from glucocorticoid inactivation to activation. J Bone Miner Res 16: 1037–1044, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Kostadinova RM, Nawrocki AR, Frey FJ, Frey BM: Tumor necrosis factor alpha and phorbol 12-myristate-13-acetate down-regulate human 11beta-hydroxysteroid dehydrogenase type 2 through p50/p50 NF-kappaB homodimers and Egr-1. FASEB J 19: 650–652, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Kimura M, Tanaka S, Yamada Y, Kiuchi Y, Yamakawa T, Sekihara H: Dehydroepiandrosterone decreases serum tumor necrosis factor-alpha and restores insulin sensitivity: Independent effect from secondary weight reduction in genetically obese Zucker fatty rats. Endocrinology 139: 3249–3253, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Miller M, Shuman JD, Sebastian T, Dauter Z, Johnson PF: Structural basis for DNA recognition by the basic region leucine zipper transcription factor CCAAT/enhancer-binding protein alpha. J Biol Chem 278: 15178–15184, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Audige A, Dick B, Frey BM, Frey FJ, Corman B, Vogt B: Gluco-corticoids and 11 beta-hydroxysteroid dehydrogenase type 2 gene expression in the aging kidney. Eur J Clin Invest 32: 411–420, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Schweizer RA, Zurcher M, Balazs Z, Dick B, Odermatt A: Rapid hepatic metabolism of 7-ketocholesterol by 11beta-hydroxysteroid dehydrogenase type 1: Species-specific differences between the rat, human, and hamster enzyme. J Biol Chem 279: 18415–18424, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Atanasov AG, Nashev LG, Tam S, Baker ME, Odermatt A: Organotins disrupt the 11beta-hydroxysteroid dehydrogenase type 2-dependent local inactivation of glucocorticoids. Environ Health Perspect 113: 1600–1606, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweizer RA, Atanasov AG, Frey BM, Odermatt A: A rapid screening assay for inhibitors of 11beta-hydroxysteroid dehydrogenases (11beta-HSD): Flavanone selectively inhibits 11beta-HSD1 reductase activity. Mol Cell Endocrinol 212: 41–49, 2003 [DOI] [PubMed] [Google Scholar]