Abstract
Recent evidence suggests that platelet activation and angiotensin II may each contribute to glomerular inflammation and fibrosis. Clopidogrel inhibits platelet activation and may also reduce inflammation. This study investigated the anti-inflammatory and renoprotective effects of clopidogrel and irbesartan in the five-sixths nephrectomy rat model of chronic kidney disease. After 8 wk of treatment, 24-h proteinuria, serum creatinine, and histologic scores of glomerular sclerosis and tubulointerstitial damage were significantly lower in treated compared with untreated rats. Clopidogrel/irbesartan combination therapy had greater effects than either drug alone. Rats that underwent five-sixths nephrectomy had higher markers of platelet activation (plasma GMP-140 and renal cortical fibrin deposition) than sham-operated rats, and clopidogrel attenuated these effects. Clopidogrel and irbesartan similarly reduced the accumulation of ED-1–expressing macrophages in the cortical glomeruli and the interstitium. Combination therapy almost completely abolished macrophage infiltration and attenuated the expression of monocyte chemoattractant protein-1, intercellular adhesion molecule-1, TGF-β1, and connective tissue growth factor. In conclusion, combination treatment with clopidogrel and irbesartan, more so than either alone, decreases early renal injury induced by five-sixths nephrectomy by inhibiting renal inflammation.
Diffuse glomerular sclerosis and interstitial fibrosis contribute to the progression of renal damage and constitute the final common pathway for almost all forms of kidney diseases.1 In recent years, more attention has been focused on the inflammatory infiltration present in various types of progressive renal diseases in humans and in experimental models. The number of inflammatory cells in the renal tissue closely correlates with the severity of glomerular and tubulointerstitial lesions and loss of renal function. Inflammatory cells and activated intrinsic kidney cells can produce various cytokines, which can promote the progress of glomerular sclerosis and interstitial fibrosis, so it may be a novel therapy strategy for chronic renal disease to reduce the infiltration of inflammatory cells.2
Mounting evidence has shown that platelet activation and angiotensin II (AngII) can promote glomerular inflammation and fibrosis and play a pivotal role in the progression of chronic kidney diseases (CKD).3–5 Although angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) can prevent the renal lesions through interrupting the renin-angiotensin system (RAS), ACEI or ARB alone cannot completely hamper the progression of CKD. The interruption of two or more pathogenic pathways by a combination of drugs with different mechanisms of action is likely to be more effective than the monotherapies, so it is necessary to introduce other drugs to attenuate the renal lesions.
Clopidogrel (CLO) is structurally a thienopyridine derivative and specifically inhibits ADP-dependent platelet aggregation and adhesion via inhibition of the purinergic P2Y12 receptor.6,7 It was reported recently that clopidogrel has a property of anti-inflammation. CLO may exert its beneficial effects by “cooling off” two important pathogenetic pathways, platelet reactivity and inflammation, both deeply involved in atherothrombotic disease8,9; however, its role in chronic renal injury and the mechanism are unknown. Thus, we hypothesized that combining CLO with irbesartan (IRB), an ARB, may be more effective in attenuating the progression of chronic renal injury through their different actions. To test this hypothesis, we used the rat model of five-sixths subtotal nephrectomy to study the effect of CLO/IRB on renal function, pathologic findings, and the expression of proinflammatory and profibrogenic genes in vivo.
RESULTS
Physiologic and Biochemical Data
Values for body weight, systolic BP (SBP), 24-h urinary protein excretion (24hUP), and serum creatinine (Scr) are shown in Table 1. Before surgery, there were no differences among groups with respect to body weight, SBP, 24hUP, or Scr. Body weight increased in all of the groups throughout the study, but the groups with five-sixths nephrectomy showed less body weight and significantly higher SBP, 24hUP, and Scr versus the sham group after 4 and 8 wk of treatment (P < 0.05).
Table 1.
Physiologic and biochemical data
| Parameter | Baseline
|
4 Wk
|
8 Wk
|
|||
|---|---|---|---|---|---|---|
| N | Mean ± SD | n | Mean ± SD | n | Mean ± SD | |
| Body weight (g) | ||||||
| sham | 12 | 336.5 ± 6.5 | 6 | 394.5 ± 10.5 | 6 | 436.9 ± 14.8 |
| nephrectomy | 12 | 336.9 ± 14.4 | 6 | 371.3 ± 16.4a | 5 | 419.1 ± 10.2a |
| CLO | 12 | 327.3 ± 12.1 | 6 | 365.7 ± 9.2a | 5 | 384.0 ± 11.4a |
| IRB | 12 | 337.5 ± 16.2 | 6 | 370.4 ± 15.3a | 5 | 411.0 ± 15.6a |
| CLO/IRB | 12 | 333.5 ± 11.5 | 6 | 373.8 ± 13.3a | 6 | 400.0 ± 10.0a |
| BP (mmHg) | ||||||
| sham | 12 | 110 ± 4 | 6 | 107 ± 3 | 6 | 113 ± 3 |
| nephrectomy | 12 | 111 ± 3 | 6 | 184 ± 6a | 5 | 192 ± 6a |
| CLO | 12 | 114 ± 4 | 6 | 176 ± 9a | 5 | 188 ± 4a |
| IRB | 12 | 118 ± 5 | 6 | 170 ± 7a | 5 | 160 ± 8a,b |
| CLO/IRB | 12 | 112 ± 4 | 6 | 173 ± 8a | 6 | 144 ± 7a,b,c |
| 24hUP (mg/24 h) | ||||||
| sham | 12 | 9.23 ± 2.50 | 6 | 12.16 ± 1.33 | 6 | 12.42 ± 7.26 |
| nephrectomy | 12 | 9.31 ± 1.07 | 6 | 33.21 ± 14.71a | 5 | 90.84 ± 15.92a |
| CLO | 12 | 8.18 ± 1.56 | 6 | 21.52 ± 12.88a | 5 | 51.35 ± 7.06a,b |
| IRB | 12 | 8.79 ± 1.41 | 6 | 20.74 ± 12.33a | 5 | 38.29 ± 4.06a,b,c |
| CLO/IRB | 12 | 8.81 ± 1.96 | 6 | 19.71 ± 12.49a | 6 | 29.74 ± 4.49b,c,d |
| Creatinine (μmol/L) | ||||||
| sham | 12 | 37.60 ± 5.75 | 6 | 38.61 ± 7.75 | 6 | 41.55 ± 3.37 |
| nephrectomy | 12 | 39.60 ± 5.40 | 6 | 56.30 ± 8.46a | 5 | 106.08 ± 6.39a |
| CLO | 12 | 41.80 ± 8.84 | 6 | 55.80 ± 6.05a | 5 | 60.60 ± 3.05a,b |
| IRB | 12 | 40.90 ± 6.51 | 6 | 54.80 ± 6.43a | 5 | 57.35 ± 7.85a,b |
| CLO/IRB | 12 | 41.10 ± 7.84 | 6 | 48.30 ± 6.12a | 6 | 51.60 ± 4.51a,b,c |
P < 0.05 versus sham.
P < 0.05 versus nephrectomy.
P < 0.05 versus CLO.
P < 0.05 versus IRB.
After 8 wk of treatment, CLO/IRB reduced 67% of the elevated urinary protein excretion as shown in NX group. The hypertension that developed in nephrectomy group was unchanged by CLO monotherapy but dropped by approximately 40 mmHg in both IRB and CLO/IRB groups. Scr was lower in the treated groups than in the nephrectomy group (P < 0.05).
Combination of CLO with IRB may have further potential to prevent renal disease progression. Elevated levels of proteinuria and Scr and increased glomerulosclerosis were lessened more in CLO/IRB group than in the IRB group or the CLO group. Furthermore, IRB had a remarkable antihypertensive effect. The therapy combining CLO with IRB dramatically attenuated proteinuria and lowered Scr.
Histopathologic Findings
At the end of 8 wk after surgery, glomerulosclerosis and tubulointerstitial damage were found in periodic acid-Schiff–stained sections (Figure 1, Table 2). In nephrectomized rats, the glomerulosclerosis index (GSI) attained values almost 10-fold as high as in sham rats. Treatment with any of the monotherapies was associated with a less pronounced increment of the GSI. Combined CLO/IRB treatment arrested the progression of glomerular injury. Interstitial expansion was also a prominent component of renal injury after nephrectomy. Unlike CLO and IRB monotherapies, combined CLO/IRB treatment significantly attenuated the progression of interstitial expansion. The glomerular cross-sectional area was significantly lower in the CLO/IRB, CLO, and IRB groups than in the nephrectomy group (P < 0.05; Table 2).
Figure 1.
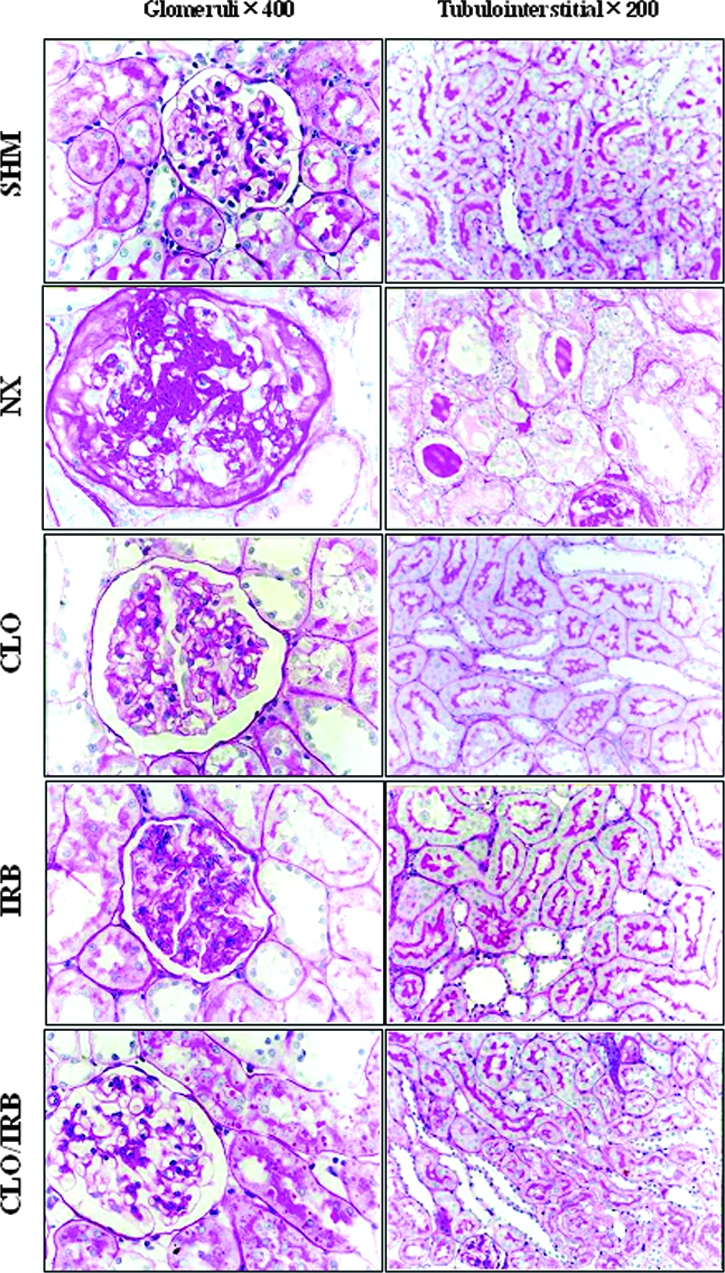
Renal pathologic changes between the groups of rats.
Table 2.
Histologic analysis of remnant kidney tissue after 8 wk of treatmenta
| Group | n | Glomerular Cross-Sectional Area (104 μm2) | Glomerular Damage (Score) | Tubulointerstitial Damage (Score) |
|---|---|---|---|---|
| Sham | 6 | 0.90 ± 0.18 | 0.15 ± 0.11 | 0.10 ± 0.21 |
| Nephrectomy | 5 | 1.96 ± 0.39b | 1.63 ± 0.75b | 1.89 ± 0.65b |
| CLO | 5 | 1.49 ± 0.24b,c | 1.29 ± 0.22c | 0.93 ± 0.43b,c |
| IRB | 5 | 1.36 ± 0.31bc | 0.98 ± 0.31c | 0.89 ± 0.32bc |
| CLO/IRB | 6 | 1.03 ± 0.38c,d | 0.59 ± 0.21c,d,e | 0.41 ± 0.35c,d,e |
Data are means ± SD. CLO, clopidogrel; IRB, irbesartan; CLO/IRB, CLO plus IRB.
P < 0.05 versus sham.
P < 0.05 versus nephrectomy.
P < 0.05 versus CLO.
P < 0.05 versus IRB.
Platelet Activation Data
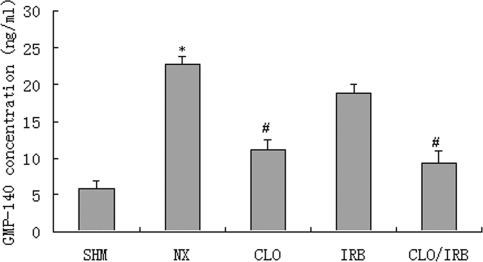
The concentration of GMP-140 (CD62) in plasma is shown in Figure 2. The level of GMP-140 in nephrectomized rats was significantly higher than in the sham group (22.84 ± 1.03 versus 5.98 ± 1.01 ng/ml, respectively), which was reduced by CLO and CLO/IRB (11.16 ± 1.42 versus 9.43 ± 1.50 ng/ml; P < 0.05). The deposit of fibrin in the renal cortex is shown in Figure 3. Compared with the sham group, the deposit of fibrin was increased in the nephrectomy group. CLO alone or combined with IRB could attenuate the fibrin deposit (P < 0.05).
Figure 2.
Comparison of plasma GMP-140 between the groups. *P < 0.05 versus sham (SHM); #P < 0.05 versus nephrectomy (NX).
Figure 3.
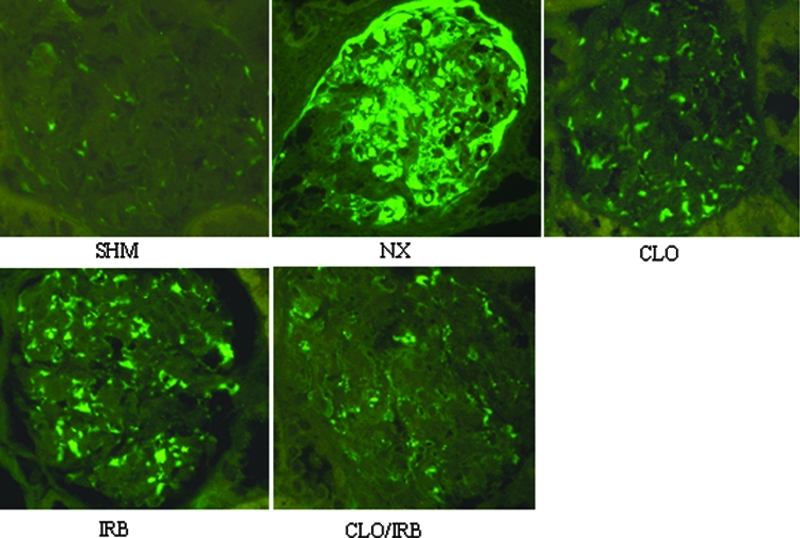
Immunofluorescence of fibrin deposit in the glomeruli. Magnification, ×400 (FITC).
Immunohistochemistry Staining of ED-1, Monocyte Chemoattractant Protein-1, Intercellular Adhesion Molecule-1, TGF-β1, and Connective Tissue Growth Factor
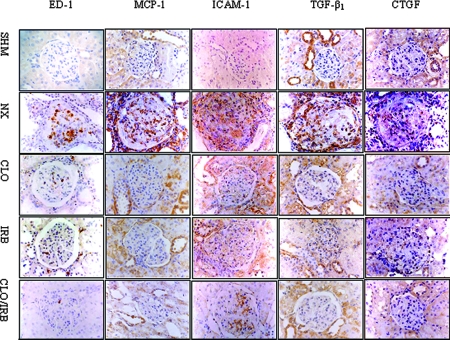
The immunohistochemistry data at 8 wk are shown in Figure 4. ED-1–expressing macrophages accumulated into the cortical glomerulus and interstitium in the nephrectomy group, which was reduced in the CLO, IRB, and CLO/IRB groups.
Figure 4.
Histochemistry analysis of ED-1, MCP-1, ICAM-1, TGF-β1, and CTGF abundance.
Compared with the nephrectomy group, CLO or IRB alone comparably reduced the numbers of ED-1–expressing cells in the rat remnant kidney. The therapy combining CLO with IRB almost completely abolished the infiltration of macrophages/lymphocytes as well as the overexpression of MCP-1 and intercellular adhesion molecule-1 (ICAM-1). In addition, CLO/IRB was found to reduce the expression of TGF-β1 and connective tissue growth factor (CTGF).
mRNA and Protein Expression of MCP-1, ICAM-1, TGF-β1, and CTGF
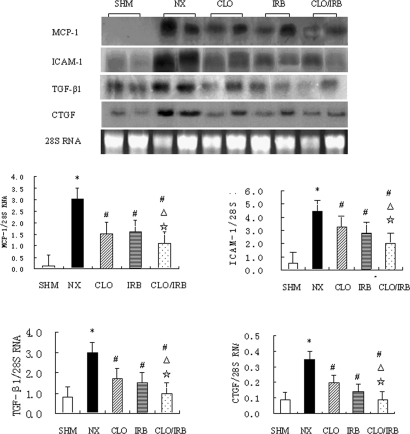
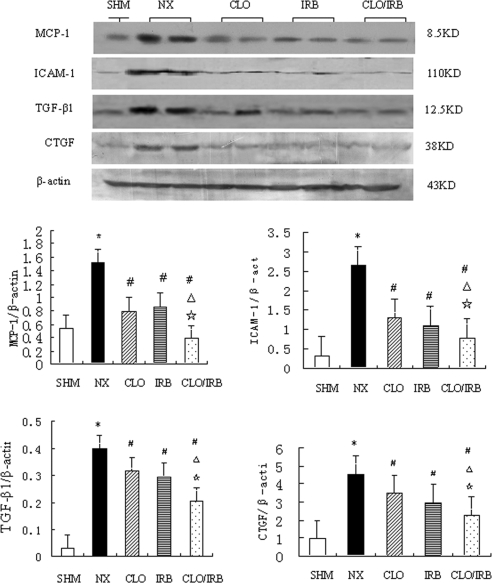
The results showed that CLO/IRB downregulated the mRNA and protein expression of MCP-1, ICAM-1, TGF-β1, and CTGF in the rat remnant kidney tissue. Compared with the sham group, the mRNA and protein expression of MCP-1, ICAM-1, TGF-β1, and CTGF was increased in the renal cortex of the nephrectomy group. In contrast with the nephrectomy group, the three treatment groups decreased the mRNA and protein expression of these molecules, and the combined therapy was more effective than the monotherapy (Figures 5 and 6).
Figure 5.
Expression of MCP-1, ICAM-1, TGF-β1, and CTGF mRNA. *P < 0.05 versus SHM; #P < 0.05 versus NX; ▵P < 0.05 versus CLO; ⋆P < 0.05 versus IRB.
Figure 6.
Expression of MCP-1, ICAM-1, TGF-β1, and CTGF proteins. *P < 0.05 versus SHM; #P < 0.05 versus NX; ▵ 0.05 versus CLO; ⋆P < 0.05 versus IRB.
DISCUSSION
This study demonstrated that CLO prevented early progression of renal injury after five-sixths nephrectomy, and combination therapy with IRB was more effective. As expected, the nephrectomy group promoted growth retardation, systemic arterial hypertension, proteinuria, and impaired renal function. These functional changes were accompanied by severe glomerulosclerosis and interstitial fibrosis, as well as expansion and intense macrophage infiltration of the interstitial area. The detection of inflammatory and profibrotic gene induction and macrophage infiltration in the remnant kidney supports the notion that inflammatory processes may contribute to the progressive renal injury and fibrosis that follows five-sixths nephrectomy. Studies have shown that the protection from progressive renal injury afforded by ARB treatment initiated early after five-sixths nephrectomy is associated with normalization of glomerular capillary hydraulic pressure, suppression of proinflammatory gene induction, and macrophage infiltration.9,10 These data provided support for the hypothesis that after extensive renal mass ablation, expression of ED-1, MCP-1, or ICAM-1 in high numbers of cells in the remnant kidney contributes to progressive renal injury. Although ARB was effective in preventing or attenuating upregulation of several proinflammatory genes in this model, additional interventions that specifically inhibit proinflammatory gene expression may lead to increased therapeutic options for patients with established chronic renal disease progression. In particular, the therapy combining CLO with IRB halted the renal disease progression in this animal model. Such attenuation could be in part due to the effect of CLO/IRB against the inflammatory mononuclear cells. Moreover, we found that CLO/IRB reduced the upregulation of MCP-1 gene in the remnant kidney. Recent studies have shown that the increased MCP-1 in proximal tubular cells and interstitial mononuclear cells of the remnant kidney is downregulated after ACEI or ARB treatment.11 The attenuation of interstitial inflammatory cell infiltration by CLO/IRB may also be due to the downregulation of MCP-1. The inhibitory effect of CLO/IRB on MCP-1 and ICAM-1 may be partially due to the decreased infiltration of monocytes/macrophages; therefore, the first possible mechanism of preventing the renal disease progression may be due to the effect of CLO on attenuating inflammation through reducing the overexpression of MCP-1 and ICAM-1 in the remnant kidney.
Platelet activation is involved in the pathogenesis of chronic renal injury. Intrarenal platelet activation is an important component that contributes to glomerular sclerosis and interstitial fibrosis. There is increasing evidence of platelet activation in the five-sixths nephrectomy rat model.12 Platelet activation also plays an important role in thrombosis and arteriosclerosis.,, Some studies showed that platelet activation is closely related to inflammation, and platelet activation can induce or increase inflammation in vivo.,– Inhibition of the platelet activation contributes to the repression of the inflammation. CLO can inhibit the platelet activation by inhibition of the P2Y12 receptor. A recent study8 showed that the inhibition of P2Y12 receptor can attenuate inflammation. CLO can reduce inflammation that underlies the chronic process of atherosclerosis by reducing platelet-dependent upregulation of inflammatory and proatherothrombotic functions in leukocytes.19 Our study demonstrated that CLO can inhibit the expression of GMP-140 and decrease the deposit of fibrin in the remnant kidney. In the five-sixths nephrectomy rat model, CLO prevented the progression of renal lesions, which may be due to the inhibitory effect of CLO on platelet activation and inflammation. GMP-140 (CD62), a specific marker of platelet activation, is a member of the selectin family, presenting in the α granular membrane of the platelet. Once platelet activation has occurred, GMP-140 translocates to the surface of activated platelets or releases into plasma. GMP-140 participates in adhesion of neutrophils to activated endothelium in a Ca2+-dependent manner and increases the expression of tissue factor by monocyte.20,21 CLO slows the progression of chronic renal injury at least partly through inhibition of platelet activation and inflammation, which is the second possible mechanism of renal protection in our study.
Although there is a wide range in the dosage of CLO used in different rat models (ranging from 1 to 50 mg/kg),22,23 the dosage of 20 mg/kg per d was widely used in rats models with exact effect and without apparent adverse effect.24–26 At the same time, the dosage of CLO in clinical practice is 75 mg/d (1.25 mg/kg per d), so we chose 20 mg/kg per d as the observed dosage, which is 16 times the dosage in human. In addition, although there is a possibility of liver injury during administration of CLO in elderly patients, the serum transaminase level remained normal in our experiments (data not shown), showing that the dosage of 20 mg/kg per d was safe.
A variety of cytokines, including TGF-β1 and CTGF, are important for renal cell proliferation and extracellular matrix production.27–29 In this study, we found that CLO could downregulate the gene expression of these growth factors in the remnant kidney, indicating that CLO may prevent the progressive renal fibrosis by reducing the upregulation of these growth factors and collagen genes in the remnant kidney, which may be the third possible mechanism for its renoprotective effect.
Our results demonstrated that CLO is effective in preventing early progression of renal injury after five-sixths nephrectomy, the mechanism of which is associated with inhibition of inflammation through inhibiting platelet activation, infiltration of monocytes/macrophages, and expression of inflammatory mediators and growth factors, and that combination therapy was more effective in attenuating the early renal injury.
CONCISE METHODS
Animals and Surgery
Sixty male Wistar rats (mean body weight 280 to 300 g) were purchased from the Chinese Academy of Medical Sciences Experimental Animal Center. Animal experiments were performed in accordance with the regulations for the care and use of laboratory animals and were approved by the local authorities. All rats were housed in a constant-temperature room with a consistent light cycle (light from 7:00 a.m. to 7:00 p.m.) and fed a standard rat diet (protein 23.4%). On the day of the operation, all rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally). Renal mass reduction (n = 48) was obtained by ablation of two-thirds mass of the left kidney and subsequent right unilateral nephrectomy 1 wk later. Sham operation (n = 12) was performed by a laparotomy and manipulation of renal pedicle without any removal of renal mass.
Experimental Design
One week after five-sixths nephrectomy, 48 rats survived and were randomly allocated into four groups: Nephrectomy group, given distilled water (vehicle) by gavage for 4 or 8 wk; CLO group, given CLO (Sanofi, France) by gavage in aqueous solution at the daily dosage of 20 mg/kg for 4 or 8 wk; IRB group, given irbesartan (Sanofi, Hang Zhou, China) by gavage in aqueous solution at the daily dosage of 20 mg/kg for 4 or 8 wk; and CLO/IRB group, given simultaneous CLO and IRB treatments, receiving a constant dosage the same as the CLO group or the IRB group for 4 or 8 wk. Body weight and SBP (by tail-cuff method) were measured at regular intervals. Twenty-four-hour urine samples were collected in metabolic cages on day 0 (before administration) and weeks 4 and 8 after administration. Blood samples were collected 8 wk after administration and divided into two parts for assessing creatinine and GMP-140. Rats from each group were killed at weeks 4 and 8. The fragments of remnant kidneys were taken from the center areas and divided into two parts. One part was fixed in 10% neutral buffered formalin for pathologic examination, and the other was dissected to isolate the cortex, which was quickly frozen in liquid nitrogen and stored at −70°C for total RNA and protein extraction.
Assessment of Renal Function
Creatinine levels in serum and urine were measured using the alkaline picrate method. Protein concentration of urine was determined by Coomassie brilliant blue G dye-binding assay, with BSA as the standard.
Pathologic Examinations
The mean glomerular cross-sectional area was determined in 50 glomerular sections for each rat. GSI was assessed in 50 glomeruli per rat.30 The extent of glomerular sclerosis (GS) was graded from 0 to 4 by a semiquantitative score: 0, normal; 1, mesangial expansion/sclerosis involving <25% of the tuft; 2, moderate GS (25 to 50%); 3, severe GS (50 to 75%); and 4, diffuse GS involving >75% of the glomerular tuft. GSI for each rat was calculated as a mean value of all glomerular scores obtained.
Tubulointerstitial lesion indexes were determined using a semiquantitative scoring system.30 Ten fields per kidney were examined, and lesions were graded from 0 to 3 (0, no changes; 1, changes affecting <25% of the section; 2, changes affecting 25 to 50% of the section; and 3, changes affecting 50 to 100% of the section), according to the area with tubulointerstitial lesions (tubular atrophy, casts, interstitial inflammation, and fibrosis). The score index in each rat was expressed as a mean value of all scores obtained.
Platelet Activation Study
The expression of GMP-140 in the plasma was determined with ELISA kit (Su Zhou Medical College, Su Zhou, China) according to the instruction. The primary antibody was monoclonal anti–GMP-140. The end compounds reacted with OPD reagent. The deposit of fibrin in the renal cortex was assessed by direct immunofluorescence. Frozen sections (4 μm) were incubated with polyclonal rabbit anti-rat fibrin antibody labeled with dihydroxyfluorane at 4°C overnight, then washed with PBS three times. After mounting with glycerol, the fluorescence density was determined with laser confocal microscopy.
Renal Immunohistochemical Study
Immunohistochemical detection for ED-1, MCP-1, ICAM-1, TGF-β1, and CTGF was performed according to the SP kit method (Maixin Biotechnology Co. Ltd., Fu Zhou, China). The primary antibodies were mouse monoclonal anti-rat ED-1; polyclonal goat anti-rat MCP-1, ICAM-1, and CTGF antibodies (1:100; Santa Cruz Biotechnology, Santa Cruz, CA); and rabbit polyclonal antibody TGF-β1 (1:100; Santa Cruz Biotechnology) antibody. The end compounds reacted with AEC reagent.
Northern Blot Analysis
Total RNA (30 μg) was subjected to electrophoresis and transferred to a nylon membrane. After fixation, membranes were prehybridized for 2 h and then hybridized with 32P-labeled cDNA clones for 16 h.
Western Blot Analysis
Frozen kidney tissue was homogenized. Protein (approximately 60 to 100 μg) was subjected to electrophoresis. Membranes were incubated with goat polyclonal anti–MCP-1, goat polyclonal anti–ICAM-1, goat polyclonal anti-CTGF, and rabbit polyclonal anti–TGF-β1 (all 1:200; Santa Cruz Biotechnology). After washing with TBST, blots were developed with enhanced chemiluminescence reagents (Santa Cruz Biotechnology).
Statistical Analysis
Results are presented as means ± SD and analyzed by ANOVA using SAS/Windows. The difference was considered as statistically significant at P < 0.05.
DISCLOSURES
None.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30121005, 30630033, and 30570856) and National Basic Research Program of China (2007CB507400 and 2006CB503900).
An abstract of this work was presented at the annual meeting of the American Society of Nephrology; November 14 through 29, 2006; San Diego, CA.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Klahr S, Schreiner G, Ichikawa I: The progression of renal disease. N Engl J Med 318: 1657–1666, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Fujihara CK, De Lourdes Noronha I, Malheiros, Antunes GR, de Oliveira IB, Zatz R: Combined mycophenolate mofetil and losartan therapy arrests established injury in the remnant kidney. J Am Soc Nephrol 11: 283–290, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Gawaz M, Langer H, May AE: Platelets in inflammation and atherogenesis. J Clin Invest 115: 3378–3384, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice EK, Tesch GH, Cao Z, Cooper ME, Metz CN, Bucala R, Atkins RC, Nikolic-Paterson DJ: Induction of MIF synthesis and secretion by tubular epithelial cells: a novel action of angiotensin II. Kidney Int 63: 1265–1275, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Ortega M, Lorenzo O, Suzuki Y, Ruperez M, Egido J: Proinflammatory actions of angiotensins. Curr Opin Nephrol Hypertens 10: 321–329, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB: Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409: 202–207, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ Jr, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS: Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest 107: 1591–1598, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Sabate M, Jimenez-Quevedo P, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Costa MA, Bass TA, Macaya C: Clopidogrel withdrawal is associated with proinflammatory and prothrombotic effects in patients with diabetes and coronary artery disease. Diabetes 55: 780–784, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Molero L, Lopez-Farre A, Mateos-Caceres PJ, Fernandez-Sanchez R, Luisa Maestro M, Silva J, Rodriguez E, Macaya C: Effect of clopidogrel on the expression of inflammatory markers in rabbit ischemic coronary artery. Br J Pharmacol 146: 419–424, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu LL, Cox A, Roe CJ, Dziadek M, Cooper ME, Gilbert RE: Transforming growth factor beta 1 and renal injury following subtotal nephrectomy in the rat: role of the renin-angiotensin system. Kidney Int 51: 1553–1567, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Taal MW, Zandi-Nejad K, Weening B, Shahsafaei A, Kato S, Lee KW, Ziai F, Jiang T, Brenner BM, MacKenzie HS: Proinflammatory gene expression and macrophage recruitment in the rat remnant kidney. Kidney Int 58: 1664–1676, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Zoja C, Perico N, Bergamelli A, Pasini M, Morigi M, Dadan J, Belloni A, Bertani T, Remuzzi G: Ticlopidine prevents renal disease progression in rats with reduced renal mass. Kidney Int 37: 934–942, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Vivekananthan DP, Bhatt DL, Chew DP: Effect of clopidogrel pretreatment on periprocedural rise in C-reactive protein after percutaneous coronary intervention. Am J Cardiol 94: 358–360, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Klinkhardt U, Graff J, Harder S: Clopidogrel, but not abciximab, reduces platelet leukocyte conjugates and P-selectin expression in a human ex vivo in vitro model. Clin Pharmacol Ther 71: 176–185, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Siegel-Axel D, Langer H, Lindemann S, Gawaz M: Role of platelets in atherosclerosis and inflammation. Med Klin (Munich) 101: 467–475, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Weyrich AS, Lindemann S, Zimmerman GA: The evolving role of platelets in inflammation. Thromb Haemost 1: 1897–1905, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Gear AR, Camerini D: Platelet chemokines and chemokine receptors: Linking hemostasis, inflammation, and host defense. Microcirculation 10: 335–350, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Ma Y-Q, Plow EF, Geng J-G: P-selectin binding to P-selectin glycoprotein ligand-1 induces an intermediate state of activation and acts cooperatively with extracellular stimuli to support maximal adhesion of human neutrophils. Blood 104: 2549–2556, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Evangelista V, Manarini S, Dell'Elba G, Martelli N, Napoleone E, Di Santo A, Lorenzet PS: Clopidogrel inhibits platelet-leukocyte adhesion and platelet dependent leukocyte activation. Thromb Haemost 94: 568–577, 2005 [PubMed] [Google Scholar]
- 20.Johnston GI, Bliss GA, Newman PJ, McEver RP: Structure of the human gene encoding granule membrane protein-140, a member of the selectin family of adhesion receptors for leukocytes. J Biol Chem 265: 21381–21385, 1990 [PubMed] [Google Scholar]
- 21.Hamburger SA, McEver RP: GMP-140 mediates adhesion of stimulated platelets to neutrophils. Blood 75: 550–554, 1990 [PubMed] [Google Scholar]
- 22.Bernat A, Herbert JM: Effect of various drugs on adriamycin-enhanced venous thrombosis in the rat: Importance of PAF. Thromb Res 75: 91–97, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Borgdorff P, Tangelder GJ, Paulus WJ: Cyclooxygenase-2 inhibitors enhance shear stress-induced platelet aggregation. J Am Coll Cardiol 48: 817–823, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Herbert JM, Dol F, Bernat A, Falotico R, Lalé A, Savi P: The antiaggregating and antithrombotic activity of clopidogrel is potentiated by aspirin in several experimental models in the rabbit. Thromb Haemost 80: 512–518, 1998 [PubMed] [Google Scholar]
- 25.Wang J, Albertson CM, Zheng H, Fink LM, Herbert JM, Hauer-Jensen M: Short-term inhibition of ADP-induced platelet aggregation by clopidogrel ameliorates radiation-induced toxicity in rat small intestine. Thromb Haemost 87: 122–128, 2002 [PubMed] [Google Scholar]
- 26.Daykin HJ, Sturgeon SA, Jones C, Wright CE: Arterial antithrombotic effects of aspirin, heparin, enoxaparin and clopidogrel alone, or in combination, in the rat. Thromb Res 118: 755–762, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kagami S, Border WA, Miller DE, Noble NA: Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest 93: 2431–2437, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, Sugaya T, Kopp JB, Suzuki H: Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol 16: 133–143, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Floege J, Burns MW, Alpers CE, Yoshimura A, Pritzl P, Gordon K, Seifert RA, Bowen-Pope DF, Couser WG, Johnson RJ: Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int 41: 297–309, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Gadola L, Noboa O, Marquez MN, Rodriguez MJ, Nin N, Boggia J, Ferreiro A, Garcia S, Ortega V, Musto ML, Ponte P, Sesser P, Pizarrosa C, Ravaglio S, Vallega A: Calcium citrate ameliorates the progression of chronic renal injury. Kidney Int 65: 1224–1230, 2004 [DOI] [PubMed] [Google Scholar]