Abstract
Hypokalemic nephropathy is associated with alterations in intrarenal vasoactive substances, leading to vasoconstriction, salt-sensitivity, and progression of interstitial fibrosis. In this study, we investigated whether hypokalemic nephropathy might also involve impaired renal angiogenesis. Sprague-Dawley rats that were fed low-potassium diets developed peritubular capillary loss that began in the inner stripe of the outer medulla (week 2) and progressed to the outer stripe of the outer medulla (week 4) and cortex (week 12). These changes were associated with increased macrophage infiltration, increased expression of both monocyte chemoattractant protein-1 and TNF-α, and a loss of vascular endothelial growth factor and endothelial nitric oxide synthase. Renal thiobarbituric acid–reactive substances, markers of oxidative stress, were increased late in disease. In conclusion, hypokalemic nephropathy is associated with impaired renal angiogenesis, evidenced by progressive capillary loss, reduced endothelial cell proliferation, and loss of VEGF expression.
Hypokalemic nephropathy is a tubulointerstitial disease that clinically presents as prolonged hypokalemia, polyuria, metabolic alkalosis, proteinuria, and progressive loss of renal function.1–3 The renal lesion is characterized by renal hypertrophy and renal tubular cell hyperplasia involving the medullary collecting ducts and, to a lesser extent, the thick ascending limb (mTAL) in association with tubular atrophy, interstitial macrophage infiltration, and interstitial fibrosis.4–6
The pathogenesis of hypokalemic nephropathy is considered multifactorial. Hypokalemia results in renal vasoconstriction, reduced medullary blood flow, and intrarenal ischemia6–8 in association with intrarenal angiotensin II and endothelin-1 generation, and blocking angiotensin II or endothelin I improves the renal lesion.6,9,10 Intrarenal complement activation also occurs and may contribute to the renal disease.1 In addition, growth factors such as IGF-1 and TGF-β are expressed and likely contribute to the renal hypertrophic response.5
Recent studies in other models suggest that loss of glomerular endothelial cells and peritubular capillaries result in chronic hypoxia or ischemia that plays an important role in progression of renal diseases.11–13 Some specific factors such as oxidants and angiotensin II can mediate endothelial cell death.14–16 Kang et al. reported a transient increase of peritubular and glomerular endothelial cell proliferation in the early phase of the remnant kidney model, which was followed by a chronic stage of impaired angiogenesis associated with a reduction in the renal expression of VEGF. In this study the renal macrophage infiltration correlated with the area of decreased VEGF expression and macrophage-associated cytokines (IL-1β, IL-6, TNF-α) were shown to inhibit VEGF mRNA expression and protein secretion in cultured mTAL cells.17
Given the previous demonstration of intrarenal ischemia, renal expression of vasoconstrictor substances, and increased macrophage infiltration in hypokalemic nephropathy, we hypothesized that hypokalemic nephropathy may also be associated with impaired renal angiogenesis.
RESULTS
Effect of Low K+ Diet on Serum K+ and Body Weight
Serum K+ levels of rats on a low-potassium (LK) diet were significantly lower at weeks 2, 4, and 12 (normal-potassium (NK) diet: 4.22 ± 0.06, 4.35 ± 0.16, 4.27 ± 0.1; LK: 3.3 ± 0.21, 3.07 ± 0.34, 2.91 ± 0.25 mEq/L, respectively; P < 0.001 for each time). Urinary K+ excretion was 34-, 28- and seven-fold lower than NK rats at weeks 2, 4, and 12. Hypokalemic rats also developed mild metabolic alkalosis shown at week 12 (serum bicarbonate (mmol/L): NK 20.7 ± 1.8 versus LK 26.2 ± 3.2; P = 0.008). LK rats demonstrated growth retardation limited to the first 4 wk of the study (i.e., during the rapid-growth period). After week 4, the average body weight (BW) of LK rats remained lower than the NK group but the difference was nonsignificant.
Renal Dysfunction and Injury Demonstrated at Weeks 2, 4, and 12
Renal Hypertrophy.
LK rats demonstrated progressive renal hypertrophy defined as an increase of kidney weight (KW) and KW/BW ratio over time (Table 1). When individual rats were examined there was a negative correlation between serum K+ and KW/BW ratios (r = −0.77, P < 0.001).
Table 1.
Body weight, renal weight and renal function at weeks 2, 4, and 12a
| Week 2
|
Week 4
|
Week 12
|
||||
|---|---|---|---|---|---|---|
| NK | LK | NK | LK | NK | LK | |
| KW, g | 0.98 ± 0.1 | 1.11 ± 0.1b | 1.23 ± 0.2 | 1.52 ± 0.2b | 1.69 ± 0.1 | 2.13 ± 0.4b |
| KW/BW, g/100 g BW | 0.31 ± 0.02 | 0.39 ± 0.03c | 0.30 ± 0.03 | 0.41 ± 0.03c | 0.25 ± 0.03 | 0.35 ± 0.08b |
| Serum Cr, mg/dl | 0.30 ± 0.00 | 0.36 ± 0.05b | 0.33 ± 0.05 | 0.40 ± 0.00b | 0.40 ± 0.00 | 0.47 ± 0.05b |
| Cr clearance, ml/min | 1.35 ± 0.1 | 1.23 ± 0.3 | 2.23 ± 0.2 | 1.48 ± 0.4b | 2.70 ± 0.5 | 2.23 ± 0.2b |
Data are expressed as mean±SD. NK, normal-potassium diet group; LK, low-potassium diet group; KW, kidney weight; BW, body weight; Cr, creatinine.
P < 0.05;
P < 0.01 versus NK group at the same time point.
Renal Function.
LK rats had renal dysfunction represented by significant increases in serum creatinine (Cr) at all time points (Table 1). Cr clearance was reduced at weeks 4 and 12, and urine protein/Cr was increased in LK rats at week 12 (NK 3.69 ± 0.33 versus LK 4.40 ± 0.54 mg/mg Cr; P = 0.036).
Renal Histology and Immunohistochemistry.
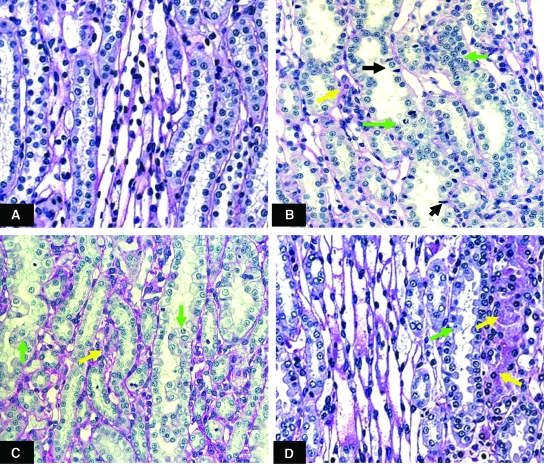
At week 2, LK rats developed characteristic tubulointerstitial injury of hypokalemic nephropathy including hyperplasia and hypertrophy of cortical and medullary collecting and mTAL cells with tubular dilation, cast formation, and interstitial cell infiltration. The most affected area was the inner strip of the outer medulla (ISOM) (Figure 1). The renal injury was more prominent at weeks 4 and 12. Proximal tubular vacuolization and interstitial fibrosis was noted at week 12.
Figure 1.
Periodic acid-Schiff reagent staining within ISOM of normal K+ rats (A) and low K+ rats at weeks 2 (B), 4 (C) and 12 (D). Magnification 400x. Hypokalemic rats developed renal tubular cell hypertrophy and hyperplasia (green arrows) and interstitial cell infiltration (yellow arrows). Pyknotic nuclei representing apoptotic cells were demonstrated in renal tubular cells at week 2 (black arrows).
PCNA expression of renal tubular cells was increased in LK rats especially at week 2, whereas osteopontin expression, which represented renal tubular injury, progressively increased over time (Table 2 and Figure 2). Collagen III deposition within the cortex (percent positive area; NK 3.13 ± 0.7% versus LK 5.08 ± 1.1%; P = 0.006) and outer medulla (NK 4.74 ± 0.9% versus LK 7.43 ± 1.3%; P = 0.003) was observed in LK rats at week 12, which is consistent with interstitial fibrosis.
Table 2.
Tubulointerstitial injury, peritubular capillary loss, and impaired angiogenesis of hypokalemic rats at weeks 2, 4, and 12a
| Week 2
|
Week 4
|
Week 12
|
||||
|---|---|---|---|---|---|---|
| NK | LK | NK | LK | NK | LK | |
| Osteopontin, %b | ||||||
| cortex | 0.96 ± 0.3 | 0.97 ± 0.3 | 1.21 ± 0.1 | 1.37 ± 0.8 | 1.02 ± 0.3 | 2.04 ± 0.5d |
| outer medulla | 3.52 ± 0.5 | 4.45 ± 0.9c | 2.9 ± 1.0 | 5.53 ± 0.5c | 3.36 ± 1.4 | 5.93 ± 0.7d |
| ED-1, cells/mm2 | ||||||
| cortex | 18.8 ± 6.2 | 20.8 ± 7.5 | 13.2 ± 2.1 | 22.8 ± 12.1 | 13.9 ± 3.5 | 35.9 ± 10.6d |
| outer medulla | 24.4 ± 7.5 | 232 ± 180c | 23.7 ± 6.2 | 363 ± 228c | 19.2 ± 4.8 | 470 ± 273d |
| PCNA, cells/mm2 | ||||||
| cortex | 18.7 ± 5.5 | 40.6 ± 8.8d | 17.2 ± 1.6 | 31.9 ± 6.5c | 18.5 ± 7.9 | 35.6 ± 18.5 |
| outer medulla | 28.5 ± 8.3 | 74.6 ± 15.8d | 22.8 ± 1.5 | 44.5 ± 13.1c | 24.9 ± 6.8 | 64.3 ± 30.5c |
| Thrombomodulin-positive area, %b | ||||||
| cortex | 3.61 ± 0.4 | 3.38 ± 0.5 | 3.77 ± 0.9 | 2.96 ± 0.7 | 3.73 ± 0.7 | 2.54 ± 0.6c |
| OSOM | 3.60 ± 0.6 | 3.05 ± 0.3 | 4.05 ± 0.4 | 2.36 ± 0.5c | 3.94 ± 0.8 | 1.82 ± 0.5d |
| ISOM | 4.35 ± 0.6 | 3.12 ± 0.6d | 4.31 ± 0.2 | 2.53 ± 0.4d | 4.65 ± 1.0 | 1.60 ± 0.9d |
| Double staining of thrombomodulin and PCNA, cells/mm2 | ||||||
| outer medulla | 87.3 ± 19 | 72.8 ± 22 | 76.1 ± 19 | 48.5 ± 11c | 63.7 ± 23 | 28.9 ± 17c |
Data are expressed as mean±SD. PCNA, proliferative cell nuclear antigen; OSOM, outer stripe of the outer medulla; ISOM, inner stripe of the outer medulla.
(%) represented the percentage of positive area for the staining.
P < 0.05,
P < 0.01 versus NK group at the same time point.
Figure 2.
Proliferating cell nuclear antigen (PCNA; upper panel) and osteopontin (lower panel) immunostaining within the ISOM area of normal K+ (NK) and low K+ rats (LK) at weeks 2, 4 and 12. Magnification 400x. Hypokalemic rats had more renal tubular cell proliferation as shown with PCNA positive cells especially at week 2. Renal tubular damage represented by osteopontin staining was progressive in hypokalemic rats.
Renal MCP-1 Expression, Macrophage Infiltration, and Renal TNF-α Content.
LK rats demonstrated a progressive increase in renal monocyte chemoattractant protein 1 (MCP-1) protein content, which was significantly higher at week 12 compared with week 2 (P = 0.01). Additionally, renal MCP-1 protein content positively correlated with macrophage infiltration especially within the ISOM (Figure 3). A 5.5-fold increase of MCP-1 mRNA was also observed at week 12 (MCP-1/GAPDH mRNA ratio: NK 0.002 ± 0.003 and LK 0.011 ± 0.009; P < 0.05).
Figure 3.
Renal MCP-1 concentration and ED-1 immunostaining within ISOM area of normal K+ (NK, represented with white bars) and low K+ rats (LK, represented with black bars) at weeks 2, 4 and 12. Magnification 630x. Renal MCP-1 content progressively increased in hypokalemic rats which was significantly higher at week 12 compared with week 2 (P = 0.01) and positively correlated with the density of macrophage infiltration which also increased with time.
Renal TNF-α content was similar between NK and LK rats at week 2 (NK 342 ± 38 versus LK 337 ± 20 pg/mg protein; P = NS); however, it was significantly higher in LK rats at week 4 (NK 352 ± 57 versus LK 443 ± 49 pg/mg protein; P < 0.05) and week 12 (NK 360 ± 54 versus LK 451 ± 74 pg/mg protein; P < 0.05). When individual rats were examined, renal TNF-α content negatively correlated with the percentage positive area of peritubular capillaries (r = −0.53, P = 0.005).
Loss of Peritubular Capillaries in Hypokalemic Nephropathy
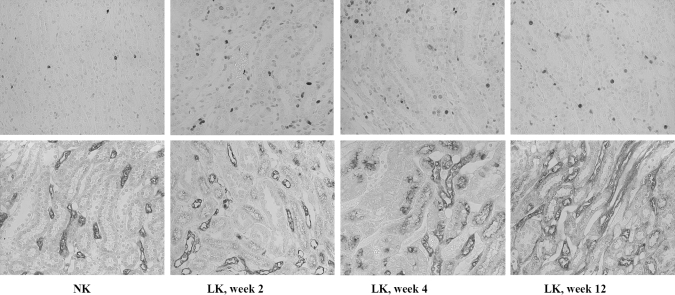
At week 2, LK rats showed a slight but significant reduction of peritubular capillary density demonstrated by both staining of different endothelial markers in the ISOM (percent reduction; 22.3% by JG-12 and 28.3% by thrombomodulin staining) and a tendency of reduction (15.4%) within the outer stripe of the outer medulla (OSOM; P = 0.06). Progressive peritubular capillary loss was observed at week 4 with a significant reduction within the ISOM (40.7% by JG-12 and 41.3% by thrombomodulin staining) and OSOM (41.6%). At week 12, peritubular capillary loss was more severe and extensive (cortex 32%, OSOM 54%, ISOM 66%, and inner medulla 57% reduction) as shown in Table 2 and Figure 4. No significant glomerular capillary loss was present in the hypokalemic rats.
Figure 4.
JG-12 immunostaining within the ISOM area of normal K+ (A) and low K+ rats at weeks 2 (B), 4 (C) and 12 (D). Magnification 400x. The reduction in peritubular capillaries was progressive over time.
Additionally, the severity of peritubular capillary loss within the ISOM negatively correlated with the serum K+ level (r = −0.86, P < 0.001) and positively correlated with the density of macrophage infiltration (r = 0.72, P < 0.001). Areas of peritubular capillary loss also correlated spatially with sites with the greatest macrophage accumulation (Figure 5); peritubular capillary loss also correlated with renal tubular damage represented by osteopontin expression (r = 0.59, P = 0.001) and renal dysfunction demonstrated by a rising serum creatinine (r = 0.58, P = 0.001).
Figure 5.
Correlation between peritubular capillary loss and macrophage infiltration within the ISOM area at week 12. Peritubular capillaries are represented by JG-12 staining (upper panel) and macrophage infiltration by ED-1 staining (lower panel). The severity of both peritubular capillary loss and macrophage infiltration correlated with each other as well as with the level of serum K+. Magnification 400x.
Renal Oxidative Stress in LK Rats
Renal TBARS (TBARS assay kit; Zeptometrix, Buffalo, NY) content was similar between the two groups at weeks 2 and 4. However, TBARS were significantly higher in LK rats at week 12 (NK 2337 ± 327 versus LK 3039 ± 240 nmol/g protein; P < 0.01).
Reduced VEGF Expression and Impaired Angiogenesis in Hypokalemic Nephropathy
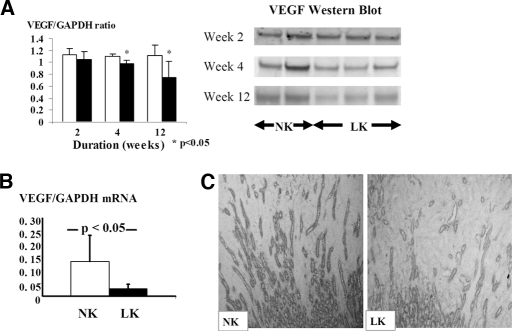
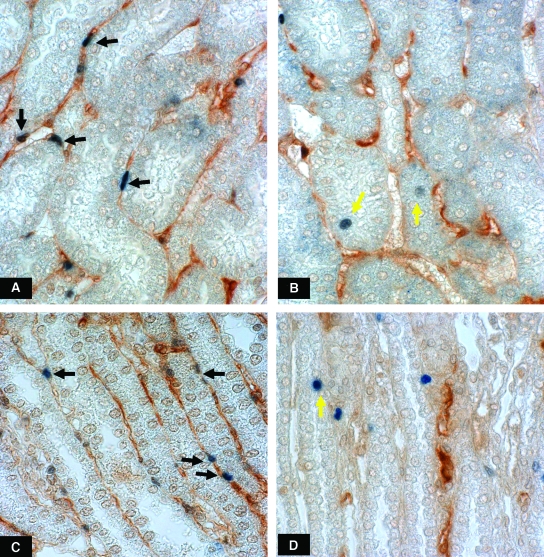
At week 2, endothelial cell proliferation, as noted by positive double staining of endothelial cells for thrombomodulin and PCNA, was preserved (Table 2) along with no difference of renal VEGF protein expression. However, at weeks 4 and 12, LK rats showed significant reduction of both renal VEGF/GAPDH protein (Figure 6) and the number of proliferating endothelial cells (Table 2 and Figure 7). The reduction of tubular VEGF expression at week 12 was demonstrated by immunohistochemical staining and was also associated with a reduction of VEGF mRNA expression (Figure 6).
Figure 6.
Reduction of renal VEGF mRNA and protein expressions in the hypokalemic rats. (A) VEGF protein represented by Western Blot which was reduced at weeks 4 and 12 of hypokalemic nephropathy (normal K+ rats; NK represented with white bars and low K+ rats; LK represented with black bars). (B) VEGF mRNA expression at week 12 of low K+ diet (C) Renal tubular VEGF expression at week 12 of the study demonstrated by immunohistochemistry (200x)
Figure 7.
Endothelial cell proliferation, represented by positive double staining of endothelial cells with thrombomodulin and PCNA (black arrows) within OSOM area of the normal K+ rat (A) and low K+ rat at week 4 (B) and within the ISOM area of normal K+ rat (C) and low K+ rat at week 12 (D). Hypokalemic rats had reduced numbers of proliferating endothelial cells. In contrast, proliferating renal tubular cells were increased in hypokalemic rats (yellow arrows). Magnification 630x.
Renal eNOS Expression in Hypokalemic Rats
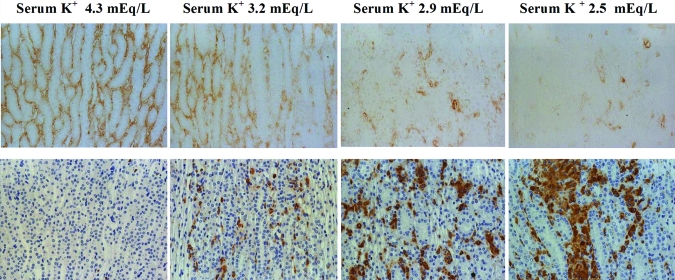
At week 2 there was significant peritubular capillary loss within the ISOM; however, angiogenesis (as noted by endothelial cell proliferation) and renal endothelial nitric oxide synthase (eNOS) protein expression was similar between NK and LK rats. However, as the disease progressed at weeks 4 and 12, peritubular capillary density progressively decreased and was associated with a decline in renal eNOS/GAPDH protein (Figure 8). The reduction of eNOS protein at week 12 was associated with reduced immunohistochemical staining for eNOS protein in peritubular capillaries within the ISOM area, whereas glomerular capillary expression of eNOS was similar between NK and LK rats (Figure 8). eNOS mRNA was also decreased compared with NK rats (Figure 8).
Figure 8.
Rats fed low K diet have decreased renal eNOS expression at weeks 4 and 12. (A) Renal eNOS mRNA expression at week 12 of the study. (B) eNOS protein represented by Western Blot was reduced in hypokalemic rats at weeks 4 and 12 (C) Immunohistochemical staining of eNOS antibody showed reduced eNOS expression demonstrated in peritubular capillaries within ISOM area at week 12 (Magnification 400x).
Correlation between Macrophage Infiltration, Renal TNF-α, VEGF Concentration, and Peritubular Capillary Density in the Kidney
A positive correlation between renal VEGF protein concentration and peritubular capillary density (r = 0.63, P < 0.001) and proliferative endothelial cells (r = 0.46, P = 0.029) was present. Macrophage density and VEGF concentration were negatively correlated (r = −0.60, P = 0.001), as were renal TNF-α and renal VEGF concentrations (r = −0.51, P = 0.006).
DISCUSSION
Chronic hypokalemia is well known to be associated with renal hypertrophy and tubulointerstitial disease in both experimental animals1,2,5,6 and humans.18–20 Previous studies have documented that the site of injury is greatest in the outer medulla and is characterized by tubular hyperplasia, cell proliferation, macrophage infiltration, and interstitial fibrosis.4–6 The mechanisms mediating the renal injury include local ischemia and vasoconstriction,6–8 intrarenal complement activation,1 and local expression of angiotensin II6,7,9 and endothelin.6,10
In this report we have identified two additional mediator systems that are likely involved in the renal injury. First, we demonstrated an increase in MCP-1 protein in renal tissue that was observed as early as 2 w, and was associated with an increase in macrophage infiltration. MCP-1 is a macrophage chemokine that has been shown to have a key role in macrophage recruitment in several models of renal injury.21–23 In addition to MCP-1, the induction of osteopontin expression in tubular cells in response to hypokalemia could play a role in macrophage recruitment.24–26
The second and more important discovery was the observation that there was progressive capillary loss in hypokalemic nephropathy. Injury was first observed in the ISOM at week 2 and expanded to the OSOM at week 4 and to the cortex by week 12. This capillary loss was significantly correlated with local macrophage infiltration. A loss of peritubular capillaries might be expected to result in an angiogenic response to recover the peritubular capillary population. However, endothelial cell proliferation (noted by double labeling PCNA with endothelial cell markers) was depressed, and this was coupled with a fall in VEGF and eNOS expression. VEGF is considered one of the more important angiogenic growth factors for the peritubular capillaries.17,27–30 Thus, a loss of VEGF and reduced endothelial proliferation, coupled with progressive capillary loss, suggests a state of impaired angiogenesis. Furthermore, because the peritubular capillaries are responsible for providing nutrients and oxygen to the tubules, it is likely that a loss of capillaries could contribute to local ischemia that has been previously reported to occur in this model.6–8 In addition, hypoxia is a well-known stimulant for cell proliferation, tubular cell activation, and fibrosis.11,31–33
Although the mechanism for the progressive capillary loss was not specifically elucidated, we found a direct spatial and quantitative association between macrophage infiltration and capillary loss, similar to what we had observed in the remnant kidney model,17 which suggests that these macrophage could play a role. Furthermore, we have previously reported that macrophage cytokines can inhibit VEGF expression in renal tubular epithelial cells by decreasing mRNA stability.17 Some macrophage cytokines such as TNF-α can also induce apoptosis.34–36 Although neither TNF-α nor oxidative stress (as noted by renal TBARS content) were elevated at week 2 in the whole kidney, it is possible that local levels were elevated at the sites of macrophage infiltration but were simply below detection. Indeed, as disease progressed, we were able to detect progressive increases in both TNF-α and oxidative stress in the kidney that correlated with progressive capillary loss and fibrosis.
In conclusion, we provide the first demonstration that there is a significant loss of capillaries in experimental hypokalemic nephropathy and that the capillary loss correlates closely with the development of fibrosis. We suggest that the capillary loss might be caused in part by an MCP-1–and osteopontin-mediated accumulation of macrophages that produce oxidants and cytokines that both injure local cells and reduce local VEGF expression. Thus, our studies suggest that impaired angiogenesis is an additional feature of hypokalemic nephropathy.
CONCISE METHODS
The animal study was approved by the University of Florida Institutional Animal Use and Care Committee (IACUC). Male Sprague-Dawley rats (150 to 200 g) (Charles River, Wilmington, MA) were placed on a normal K+ diet (0.36% K+, 0.1% Na+) (Harlan Teklad, Madison, WI) for a 5-d run-in period and then randomly divided to receive normal K+ diet (n = 16) or low K+ (0.01% K+, 0.1% Na+) diet (n = 18).
BW was recorded weekly. Rats had urine collected (over 18 h) on the day before euthanization at week 2 (NK, n = 6; LK, n = 7), week 4 (NK, n = 4; LK, n = 5), and week 12 (n = 6 for both). After euthanization, kidneys were divided into cross-sectional pieces (2 to 3 mm) and fixed in methyl Carnoys, 10% formalin, or periodate lysine paraformaldehyde fixative for histological studies. The rest of the kidneys were divided into two parts and placed in liquid nitrogen at −80°C. One part of frozen tissue was ground into powder with pestle on dry ice for analysis for TBARS and for VEGF, MCP-1, and eNOS mRNA by reverse-transcriptase PCR. The other part was homogenized in RIPA lysis buffer (Upstate, Lake Placid, NY) and centrifuged at 10,000 rpm for 10 min at 4°C, and protein in the supernatant determined using the BCA protein assay kit (Pierce Biotechnology, Rockford, IL). VEGF and eNOS proteins were measured with Western blot and renal MCP-1 and TNF-α by ELISA analysis.
Biochemical Measurements
Serum and urine K+ concentrations were determined by atomic absorption spectrophotometer (Perkin-Elmer 306, Downers Grove, IL). Routine chemistries were measured using an autoanalyzer (VetAce, Alfa Wassermann, West Caldwell, NJ). Urinary protein was measured with the RC DC protein kit (Bio-Rad, Hercules, CA).
Renal Histological Studies
Methyl Carnoys-fixed tissue was processed and embedded in paraffin, and sections (3 μm thick) stained with periodic acid-Schiff reagent (PAS) to evaluate tubulointerstitial injury.
Immunohistochemistry and Quantification of Staining
An indirect immunoperoxidase method was used on methyl Carnoys-fixed tissue to identify the following antigens: osteopontin, a sensitive marker of tubulointerstitial injury,6 with a goat polyclonal antibody (OP199, gift from C. Giachelli, University of Washington, Seattle, WA); macrophages with ED-1, a mouse monoclonal IgG1 (BD Pharmingen, San Diego, CA) and proliferating cells with mouse monoclonal IgG antibody to proliferative cell nuclear antigen, PCNA (Sigma-Aldrich, St. Louis, MO); collagen III with goat anti-type III collagen (Southern Biotech, Birmingham, AL) and endothelial cells with thrombomodulin (gift from Dr. Y. Yuzawa, Nagoya University Graduate School of Medicine, Nagoya, Japan) and JG-12 (gift of Dr. D. Kerjaschki, Medical University of Vienna, Austria).
Formalin-fixed tissues were deparaffinized, rehydrated, and processed for antigen retrieval using 0.1 M citric acid and microwave oven for VEGF staining and using the Trilogy solution (Cell Marque, Hot Springs, AR) and steamer method for eNOS staining. Goat IgG anti-rat VEGF antibody (R&D Systems, Minneapolis, MN) and mouse IgG1 anti-human NOS type III (BD Transduction, San Jose, CA) were used as primary antibodies to detect VEGF-A 164 and eNOS, respectively.
Double-immunostaining was performed to evaluate endothelial cell proliferation or angiogenesis with anti-endothelial cell antibody (rabbit IgG anti-rat thrombomodulin) and anti-PCNA antibody (mouse monoclonal IgG antibody). Anti-thrombomodulin was incubated overnight at 4°C, followed sequentially by incubation with biotinylated goat anti-rabbit antibody, incubation with the Vectastain ABC-Elite peroxidase detection kit (Vector, Burlingame, CA) and color development with diaminobenzidine and incubation in 3% H2O2 to eliminate any remaining peroxidase activity. Subsequently, sections were incubated with avidin-biotin–blocking reagent and normal horse serum. The anti-PCNA antibody was incubated overnight at 4°C followed by the secondary antibody, the biotinylated horse anti-mouse IgG, and then incubated with Vectastain ABC-alkaline phosphatase reagent and reacted with Vector Blue Alkaline Phosphatase substrate as chromogen.
An Axioplan 2 imaging microscope (Carl Zeiss, Munich, Germany) and Zeiss AutoMeasure software (Axiovision 4.1) were used to determine the percent area occupied by osteopontin-positive injured tubules and area of positive collagen III staining within the cortex and outer medulla, as well as the percent area occupied by peritubular capillaries at the cortex, OSOM, and ISOM and the percent of eNOS-positive area at ISOM. Twenty fields (1100 × 1400 μm) of each area were measured at 200× magnification, and the mean percent area was calculated for each biopsy. The number of macrophages (ED-1–positive cells) and PCNA-positive cells per square millimeter in the entire cortex and outer medulla were also counted at 200× magnification, whereas the number of positive double-staining endothelial cells of thrombomodulin and PCNA per square millimeter in the outer medulla were determined at 630× magnification and the average numbers calculated for each biopsy.
RNA Isolation, Reverse Transcription, and Real-Time PCR
Total RNA was isolated using the TRIzol® reagent (Sigma-Aldrich). Reverse transcription reactions were performed in a one-step protocol using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer's protocol. Primers of MCP-1, VEGF-A, and eNOS were designed by Genetool software (BioTools, Alberta, Canada). Real-time PCR analyses were performed using the Opticon PCR machine (MJ Research, Waltham, MA). The SYBR Green master mix kit (Bio-Rad) was used for all reactions with real-time PCR. GAPDH primers were used as a housekeeping gene to allow quantification. Ratios of MCP-1/GAPDH, VEGF/GAPDH, and e-NOS/GAPDH mRNA were calculated for each sample.
Western Blot Analyses of Homogenized Kidney
Protein samples (30 μg) were mixed with loading buffer (Invitrogen, Carlsbad, CA), boiled, resolved on graded sodium dodecyl sulfate-polyacrylamide gels (NuPage Gel, Invitrogen), and transferred to polyvinylidene fluoride membranes by electroblotting. Membranes were blocked with 5% milk in PBS for 60 min at room temperature. Goat IgG anti-rat VEGF antibody (1/500 dilution) was incubated overnight at 4°C. After incubation of the membrane with secondary antibody, HRP-conjugated rabbit anti-goat antibody (1/1000 dilution), for 1 h, the bound antibody was detected with SuperSignal chemiluminescence substrate (Pierce Biotechnology, Rockford, IL). The membrane was stripped with ImmunoPure IgG Elution Buffer (Pierce Biotechnology) with repeated blocking step with 5% milk and incubated with mouse anti-GAPDH antibody (1/10,000 dilution) and HRP-conjugated goat anti-mouse antibody (1/10,000 dilution), respectively. Positive immunoreactive bands were quantified by densitometry. The VEGF/GAPDH ratio was calculated for each rat. Western blot analysis of eNOS protein was also performed by using mouse IgG1 anti-human NOS type III antibody (1/2500 dilution) and HRP-conjugated goat anti-mouse antibody (1/2000 dilution) as primary and secondary antibody, respectively.
TBARS, the Oxidative Stress Marker
Ground renal tissue was homogenized with PBS 1/10 (wt/vol) and measured for an oxidative stress marker by using the TBARS assay kit and expressed as nmol/g protein.
Renal MCP-1 and TNF-α Proteins by ELISA
Whole-kidney homogenate was diluted 1/15 for MCP-1 and 1/5 for TNF-α and measured for MCP-1 and TNF-α by using ELISA kits (BD Biosciences) according to the manufacturer's protocol. Renal MCP-1 and TNF-α contents were expressed as pg/mg protein.
Statistical Analyses
All data are shown as mean ± SD. The unpaired t test was used to compare the continuous variables of the two groups. The equality of variance was clarified by Levene's test. Pearson correlation was used to address potential associations between groups. Statistical significance was defined as P < 0.05.
Disclosures
Drs. Nakagawa, Reungjui, and Johnson are listed as inventors on several patent applications from the University of Florida or University of Washington related to uric acid and cardiovascular disease. Dr. Johnson is also on the Scientific Board of Nephromics, Inc.
Acknowledgments
Support for this study was provided by NIH grants DK-52121, HL-68607, HL-79352, and support from Gatorade. S.R. is supported by a fellowship from the Anandamahidol Foundation of Thailand. We thank Dr. C. Giachelli (University of Washington, Seattle, WA) for donating an antibody for osteopontin, Dr. Y. Yuzawa (Nagoya University Graduate School of Medicine, Nagoya, Japan) for thrombomodulin antibody, and Dr. D. Kerjaschki (Medical University of Vienna, Austria) for JG-12 antibody.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Tolins JP, Hostetter MK, Hostetter TH: Hypokalemic nephropathy in the rat: Role of ammonia in chronic tubular injury. J Clin Invest 79: 447–458, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amlal H, Krane CM, Chen Q, Soleimani M: Early polyuria and urinary concentrating defect in potassium deprivation. Am J Physiol Renal Physiol 279: F655–F663, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Fourman P, McCance RA, Parker RA: Chronic disease in rats following a temporary deficiency of potassium. Br J Exp Pathol 37: 40–44, 1956 [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura T, Nishino T, Maruyama N, Hamano K, Kubo A, Iwano M, Shiiki H: Expression of Bcl-2 and Bax in hypokalemic nephropathy in rats. Pathobiology 69: 237–248, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Tsao T, Fawcett J, Fervenza FC, Hsu FW, Huie P, Sibley RK, Rabkin R: Expression of insulin-like growth factor-1 and transforming growth factor-beta in hypokalemic nephropathy in the rat. Kidney Int 59: 96–105, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Suga S, Phillips MI, Ray PE, Raleigh JA, Vio CP, Kim YG, Mazzali M, Gordon KL, Hughes J, Johnson RJ: Hypokalemia induces renal injury and alterations in vasoactive mediators that favor salt sensitivity. Am J Physiol Renal Physiol 281: F620–F629, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Linas SL, Dickmann D: Mechanism of the decreased renal blood flow in the potassium-depleted conscious rat. Kidney Int 21: 757–764, 1982 [DOI] [PubMed] [Google Scholar]
- 8.Whinnery MA, Kunau RT Jr: Effect of potassium deficiency on papillary plasma flow in the rat. Am J Physiol 237: F 226–F231, 1979 [DOI] [PubMed] [Google Scholar]
- 9.Suga S, Mazzali M, Ray PE, Kang DH, Johnson RJ: Angiotensin II type 1 receptor blockade ameliorates tubulointerstitial injury induced by chronic potassium deficiency. Kidney Int 61: 951–958, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Suga S, Yasui N, Yoshihara F, Horio T, Kawano Y, Kangawa K, Johnson RJ: Endothelin A receptor blockade and endothelin B receptor blockade improve hypokalemic nephropathy by different mechanisms. J Am Soc Nephrol 14: 397–406, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ: Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa T, Kang DH, Ohashi R, Suga S, Herrera-Acosta J, Rodriguez-Iturbe B, Johnson RJ: Tubulointerstitial disease: Role of ischemia and microvascular disease. Curr Opin Nephrol Hypertens 12: 233–241, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Ho FM, Liu SH, Liau CS, Huang PJ, Shiah SG, Lin-Shiau SY: Nitric oxide prevents apoptosis of human endothelial cells from high glucose exposure during early stage. J Cell Biochem 75: 258–263, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Dimmeler S, Zeiher AM: Reactive oxygen species and vascular cell apoptosis in response to angiotensin II and pro-atherosclerotic factors. Regul Pept 90: 19–25, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Dimmeler S, Rippmann V, Weiland U, Haendeler J, Zeiher AM: Angiotensin II induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ Res 81: 970–976, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ: Impaired angiogenesis in the remnant kidney model. I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol 12: 1434–1447, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Bock KD, Cremer W, Werner U: Chronic hypokalemic nephropathy: A clinical study. Klin Wochenschr 56[suppl 1]: 91–96, 1978 [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Rahman EM, Moorthy AV: End-stage renal disease (ESRD) in patients with eating disorders. Clin Nephrol 47: 106–111, 1997 [PubMed] [Google Scholar]
- 20.Harada K, Akai Y, Iwano M, Nakatani K, Nishino T, Fujimoto T, Shiiki H, Saito Y: Tubulointerstitial macrophage infiltration in a patient with hypokalemic nephropathy and primary Sjogren's syndrome. Clin Nephrol 64: 387–390, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Roncal CA, Mu W, Croker B, Reungjui S, Ouyang X, Tabah-Fisch I, Johnson RJ, Ejaz AA: Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 292: F116–F122, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Mizuno M, Sada T, Kato M, Fukushima Y, Terashima H, Koike H: The effect of angiotensin II receptor blockade on an end-stage renal failure model of type 2 diabetes. J Cardiovasc Pharmacol 48: 135–142, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Chun OY, Ning JP, Zhou QL: Expression and significance of monocyte chemoattractant protein-1 in rat kidney with unilateral ureteral obstruction. Zhong Nan Da Xue Xue Bao Yi Xue Ban 29: 558–561, 2004 [PubMed] [Google Scholar]
- 24.Tian S, Ding G, Jia R, Chu G: Tubulointerstitial macrophage accumulation is regulated by sequentially expressed osteopontin and macrophage colony-stimulating factor: implication for the role of atorvastatin. Mediators Inflamm 2006: 12919, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond JR, Kees-Folts D, Ricardo SD, Pruznak A, Eufemio M: Early and persistent up-regulated expression of renal cortical osteopontin in experimental hydronephrosis. Am J Pathol 146: 1455–1466, 1995 [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly DJ, Wilkinson-Berka JL, Ricardo SD, Cox AJ, Gilbert RE: Progression of tubulointerstitial injury by osteopontin-induced macrophage recruitment in advanced diabetic nephropathy of transgenic (mRen-2) 27 rats. Nephrol Dial Transplant 17: 985–991, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Kang DH, Johnson RJ: Vascular endothelial growth factor: A new player in the pathogenesis of renal fibrosis. Curr Opin Nephrol Hypertens 12: 43–49, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Ohashi R, Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, Yamanaka N: Peritubular capillary regression during the progression of experimental obstructive nephropathy. J Am Soc Nephrol 13: 1795–1805, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Masuda Y, Shimizu A, Mori T, Ishiwata T, Kitamura H, Ohashi R, Ishizaki M, Asano G, Sugisaki Y, Yamanaka N: Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am J Pathol 159: 599–608, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ: Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol 12: 1448–1457, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Eckardt KU, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger: Role of hypoxia in the pathogenesis of renal disease. Kidney Int Supp 99: S46–S51, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Zhang B, Liang X, Shi W, Ye Z, He C, Hu X, Liu S: Role of impaired peritubular capillary and hypoxia in progressive interstitial fibrosis after 5/6 subtotal nephrectomy of rats. Nephrology (Carlton) 10: 351–357, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Norman JT, Fine LG: Intrarenal oxygenation in chronic renal failure. Clin Exp Pharmacol Physiol 33: 989–996, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G: Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21–30, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Xu JW, Ikeda K, Yamori Y: Inhibitory effect of polyphenol cyanidin on TNF-alpha-induced apoptosis through multiple signaling pathways in endothelial cells. Atherosclerosis 193: 299–308, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Xia Z, Liu M, Wu Y, Sharma V, Luo T, Ouyang J, McNeill JH: N-acetylcysteine attenuates TNF-alpha-induced human vascular endothelial cell apoptosis and restores eNOS expression. Eur J Pharmacol 550: 134–142, 2006 [DOI] [PubMed] [Google Scholar]