Abstract
Patients with diabetes and chronic kidney disease (CKD) are at particularly high risk for cardiovascular disease (CVD). This post hoc analysis from the PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) investigated the relationship between CKD and incident CVD in a population of patients with diabetes and documented macrovascular disease, as well as the effects of pioglitazone treatment on recurrent CVD. CKD, defined as an estimated GFR <60 ml/min per 1.73m2, was present in 597 (11.6%) of 5154 patients. More patients with CKD reached the primary composite end point (all-cause mortality, myocardial infarction (MI), stroke, acute coronary syndrome, coronary/carotid arterial intervention, leg revascularization, or amputation above the ankle) than patients without CKD (27.5 versus 19.6%; P < 0.0001). Patients with CKD were also more likely to reach a secondary composite end point (all-cause mortality, MI, and stroke). Patients who had CKD and were treated with pioglitazone were less likely to reach the secondary end point (hazard ratio 0.66; 95% confidence interval 0.45 to 0.98), but this association was not observed among those with better renal function. In addition, there was a greater decline in estimated GFR with pioglitazone (between-group difference 0.8 ml/min per 1.73 m2/yr) than with placebo. In conclusion, CKD is an independent risk factor for cardiovascular events and death among patients with diabetes and preexisting macrovascular disease. Patients who had CKD and were treated with pioglitazone were less likely to reach a composite end point of all-cause death, MI, and stroke, independent of the severity of renal impairment.
Chronic kidney disease (CKD), commonly defined as GFR of <60 ml/min per 1.73 m2,1 is associated with substantially increased risk for cardiovascular disease (CVD) morbidity and mortality, independent of traditional cardiovascular risk factors.2,3 Using pooled data from population-based studies, the adjusted relative risk for cardiovascular outcomes and all-cause mortality increased by approximately 30% in patients with CKD compared with individuals with preserved renal function4; however, the impact of CKD on recurrent cardiovascular events among patients with diabetes and established macrovascular disease has not been studied previously, and there is a paucity of data on the impact of medical treatments on major out-comes.5–7 Because CKD, diabetes, and previous cardiovascular events all are independent cardiovascular risk factors, this population may be at especially high risk for subsequent cardiovascular events.
The PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) was designed to compare the effects of the thiazolidinedione pioglitazone with placebo on cardiovascular outcomes in a large cohort of patients with diabetes and a history of macrovascular disease.8 In this high-risk population with diabetes, pioglitazone treatment reduced the combined main secondary end point of all-cause mortality, myocardial infarction (MI; excluding silent MI), and stroke by 16% (P = 0.027) and the primary composite end point (which also included procedural end points, e.g., leg revascularization) by 10% (P = 0.095).9 In this report, we analyzed the influence of CKD on the rates of the primary and the principal secondary end points from PROactive. We also compared the effect of pioglitazone versus placebo on recurrent cardiovascular events in this population of PROactive patients with CKD at baseline.
RESULTS
GFR data were available for 5154 (98.4%) patients. At baseline, evidence of CKD (GFR <60 ml/min per 1.73 m2) was present in 597 (11.6%) of the 5154 patients. Baseline characteristics of the PROactive population stratified by GFR ≥60 versus GFR <60 ml/min per 1.73 m2 are shown in Table 1. Patients with CKD were older, had longer duration of diabetes, and had a higher prevalence of hypertension. There were no differences between treatment groups in baseline characteristics in patients with GFR either ≥60 or <60 ml/min per 1.73 m2 (Table 1).
Table 1.
Baseline characteristicsa
| Characteristic | GFR
|
|||
|---|---|---|---|---|
| <60 ml/min per 1.73 m2 (n = 597)
|
≥60 ml/min per 1.73 m2 (n = 4557)
|
|||
| Pioglitazone (n = 274) | Placebo (n = 323) | Pioglitazone (n = 2292) | Placebo (n = 2265) | |
| Patient | ||||
| male (n [%])b | 163 (59.5) | 181 (56.0) | 1546 (67.5) | 1516 (66.9) |
| white (n [%]) | 272 (99.3) | 314 (97.2) | 2255 (98.4) | 2242 (99.0) |
| age (yr; mean ± SD)b | 65.9 ± 6.0 | 65.2 ± 7.0 | 61.5 ± 7.6 | 61.1 ± 7.7 |
| time since diagnosis of diabetes (yr; mean ± SD)b | 11.2 ± 7.5 | 11.6 ± 8.2 | 9.2 ± 6.8 | 9.3 ± 6.9 |
| BMI (kg/m2; mean ± SD) | 31.0 ± 4.8 | 31.2 ± 4.8 | 30.7 ± 4.7 | 31.0 ± 4.8 |
| BP: mean systolic/diastolic (mmHg) | 144.0/82.2 | 145.8/82.4 | 143.5/82.9 | 143.0/17.4 |
| history of hypertension (n [%])b | 225 (82.1) | 275 (85.1) | 1692 (73.8) | 1695 (74.8) |
| current smoker (n [%])b | 15 (5.8) | 32 (9.9) | 314 (13.7) | 341 (15.1) |
| Blood glucose–lowering treatment (n [%]) | ||||
| metformin only | 24 (8.8) | 23 (7.1) | 226 (9.9) | 234 (10.3) |
| sulfonylureas only | 46 (16.8) | 60 (18.6) | 456 (19.9) | 427 (18.9) |
| metformin + sulfonylurea | 65 (23.7) | 80 (24.8) | 557 (25.2) | 571 (25.2) |
| insulin + metformin | 53 (19.3) | 60 (18.6) | 398 (17.4) | 406 (17.9) |
| insulin + sulfonylurea | 31 (11.3) | 42 (13.0) | 175 (7.6) | 175 (7.7) |
| Laboratory data (mean ± SD) | ||||
| HbA1c (%) | 8.2 ± 1.5 | 8.3 ± 1.5 | 8.0 ± 1.4 | 8.1 ± 1.4 |
| LDL cholesterol (mmol/L) | 3.0 ± 1.0 | 3.0 ± 1.1 | 3.0 ± 0.9 | 3.0 ± 1.0 |
| HDL cholesterol (mmol/L) | 1.1 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 |
| triglycerides (mmol/L) | 2.3 ± 1.6 | 2.5 ± 1.8 | 2.2 ± 1.9 | 2.2 ± 1.8 |
| creatinine (μmol/L)b | 122.6 ± 25.8 | 123.4 ± 38.8 | 77.4 ± 15.0 | 77.2 ± 14.8 |
| GFR (ml/min per 1.73 m2)b | 50.1 ± 7.7 | 50.3 ± 8.8 | 89.3 ± 20.3 | 89.4 ± 19.1 |
| Micral test result | ||||
| negativeb | 120 (43.8) | 157 (48.6) | 1266 (55.2) | 1250 (55.2) |
| 20 mg/L | 48 (17.5) | 58 (18.0) | 487 (21.2) | 484 (21.4) |
| 50 mg/L | 54 (19.7) | 53 (16.4) | 298 (13.0) | 313 (13.8) |
| 100 mg/L | 45 (16.4) | 45 (13.9) | 185 (8.1) | 170 (7.5) |
BMI, body mass index; HbA1c, glycosylated hemoglobin.
P < 0.0001 for GFR <60 versus ≥60 ml/min per 1.73 m2 (using a χ2 test).
The incidence of the primary composite end point was 27.5% in patients with CKD compared with 19.6% in patients with GFR ≥60 ml/min per 1.73 m2 (hazard ratio [HR] 1.51; 95% confidence interval [CI] 1.28 to 1.78; P < 0.0001). The incidence of the principal secondary end point was 18.3% in patients with CKD compared with 11.5% in patients with GFR ≥60 ml/min per 1.73 m2 (HR 1.65; 95% CI 1.35 to 2.03; P < 0.0001). Similarly, a higher proportion of patients with a GFR <60 ml/min per 1.73 m2 died from any cause (n = 65; 10.9%) than those with a GFR ≥60 ml/min per 1.73 m2 (n = 267; 5.9%; HR 1.86; 95% CI 1.42 to 2.43). Multivariate analysis showed that the presence of CKD was an independent risk factor for the primary composite end point (Table 2). Both serum creatinine and GFR were significant in the absence of the other.
Table 2.
Multivariate analysis for the primary composite end point (including GFR; n = 4975)
| Parameter | HR (95% CI) | P |
|---|---|---|
| Duration 5 to 10 yr (versus <5 yr) | 0.92 (0.769 to 1.094) | 0.3382 |
| BMI (kg/m2) | 0.98 (0.968 to 0.996) | 0.0127 |
| Triglycerides 1.7 to 2.2 mmol/l (versus <1.7 mmol/L) | 1.00 (0.844 to 1.183) | 0.9907 |
| Age (yr) | 1.03 (1.017 to 1.036) | <0.0001 |
| Triglycerides >2.2 mmol/l (versus <1.7 mmol/l) | 1.16 (1.004 to 1.332) | 0.0438 |
| Positive Micral test | 1.16 (1.024 to 1.312) | 0.0191 |
| Duration ≥10 yr (versus <5 yr) | 1.20 (1.031 to 1.404) | 0.0188 |
| LDL cholesterol 3 to 4 mmol/L (versus <3 mmol/L) | 1.22 (1.060 to 1.397) | 0.0053 |
| Diuretic use | 1.24 (1.083 to 1.416) | 0.0018 |
| Past smoker (versus never) | 1.25 (1.089 to 1.435) | 0.0015 |
| CKD (GFR<60 ml/min per 1.73 m2) | 1.25 (1.048 to 1.490) | 0.0130 |
| Previous stroke | 1.26 (1.074 to 1.486) | 0.0048 |
| LDL cholesterol >4 mmol/L (versus <3 mmol/L) | 1.27 (1.060 to 1.517) | 0.0096 |
| HbA1c ≥7.5% | 1.32 (1.157 to 1.515) | <0.0001 |
| Previous MI | 1.40 (1.222 to 1.606) | <0.0001 |
| Current smoker (versus never) | 1.49 (1.230 to 1.804) | <0.0001 |
| Peripheral arterial obstructive disease | 1.49 (1.275 to 1.737) | <0.0001 |
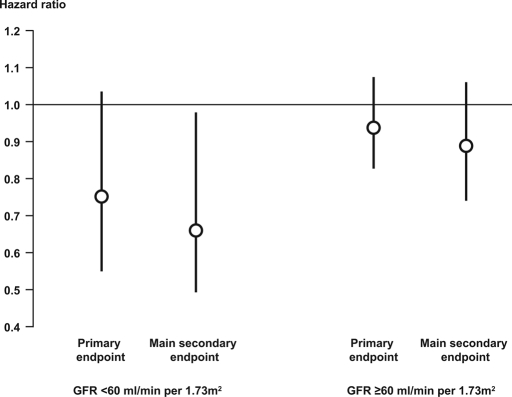
In patients with CKD, the incidence of the primary end point was 23.7% in the pioglitazone-treated group and 30.7% in the placebo group (HR 0.75; 95% CI 0.55 to 1.03; Figure 1). Similarly, the incidence of the principal secondary end point was lower in the pioglitazone-treated group (14.6%) compared with the placebo group (21.4%; HR 0.66; 95% CI 0.45, 0.98; Figure 1). All-cause mortality rates were 7.7% (n = 21) in the pioglitazone group and 13.6% (n = 44) in the placebo group (HR 0.75; 95% CI 0.55 to 1.03).
Figure 1.
HR (95% CI) for the effect of pioglitazone versus placebo treatment on the primary and the principal secondary end points in patients with and without CKD.
In patients with a GFR ≥60 ml/min per 1.73 m2, the incidence of the primary end point was 19.0% with pioglitazone and 20.2% with placebo (HR 0.94; 95% CI 0.83 to 1.07). Similar results were seen for the principal secondary end point (10.9% with pioglitazone and 12.2% with placebo; HR 0.89; 95% CI 0.75 to 1.05). The proportion of patients who had a GFR ≥60 ml/min per 1.73 m2 and subsequently died from any cause was also similar between groups (n = 137 [6.0%] with pioglitazone versus n = 130 [5.7%] with placebo; HR 1.09; 95% CI 0.87 to 1.38). The interaction between baseline renal function and effect of pioglitazone did not reach statistical significance (P = 0.1923 for the primary end point and P = 0.1781 for the principal secondary end point), indicating that the effect of pioglitazone relative to placebo was similar regardless of GFR.
The proportion of patients with a negative Micral test was higher in patients without than with CKD and was slightly lower at final visit than at baseline in both patients with (47.8% at baseline; 42.8% at final visit) and patients without CKD (56.5% at baseline; 54.9% at final visit) at baseline. Multivariate analysis showed that a positive Micral test at baseline was an independent risk factor for the primary composite end point and remained significant when GFR was included in the model (Table 2).
During the mean treatment period of 3 yr, GFR declined by 5.4 and 2.7 ml/min per 1.73 m2 in the pioglitazone and placebo groups, respectively (P < 0.0001). In the patients who had a GFR ≥60 ml/min per 1.73 m2 at baseline, 467 (20.4%) in the pioglitazone group and 370 (16.3%) in the placebo group developed CKD during the study (P = 0.0004). The decreases in BP in the patients with GFR ≥60 ml/min per 1.73 m2 at baseline (−4.3 mmHg in systolic and −3.5 mmHg in diastolic BP with pioglitazone versus −2.5 mmHg and −2.9 mmHg, respectively, in the placebo group) were similar to those observed in the total PROactive population. In the group of patients with CKD at baseline, BP decreased to a similar extent in both treatment groups (−3.0 mmHg in systolic and −3.2 mmHg in diastolic BP with pioglitazone versus −3.4 mmHg and −2.9 mmHg, respectively, in the placebo group). There were no significant differences in the changes of concomitant treatment between the GFR groups (data not shown).
DISCUSSION
Several recent publications have documented that patients with diminished GFR are at high risk for cardiovascular morbidity and mortality.2,3 In this study, we examined the role of baseline renal function in patients with type 2 diabetes and existing macrovascular disease and recurrent cardiovascular events. Our results demonstrate that CKD, as defined by a GFR of <60 ml/min per 1.73 m2, is an independent risk factor for major adverse cardiovascular events and death in this high-risk population.
Several potential mechanisms may explain this increased cardiovascular risk in patients with CKD and diabetes. First, CKD often coexists with other cardiovascular risk factors, including dyslipidemia, hypertension, and smoking.10–13 Second, impaired kidney function is associated with elevated markers of inflammation and other putative risk factors for cardiovascular events,14–18 which contribute to adverse cardiovascular outcomes. Last, patients with renal disease are less likely to receive proven efficacious therapies to prevent CVD.19–22
In the recently published post hoc analysis of Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), a GFR <60 ml/min per 1.73 m2 was associated with an increased incidence for CVD (HR 1.41) in the subgroup of patients with type 2 diabetes.23 Our analysis supports the concept that patients with type 2 diabetes and CKD are at increased risk for recurrent cardiovascular events (HR 1.51 for the primary end point and 1.65 for the principal secondary end point) and highlights the need for intensive therapy in these patients.24
Pioglitazone was more effective than placebo in reducing the rate of both the primary and secondary composite end points in the patients with CKD. There was a nonsignificant 25% risk reduction for pioglitazone relative to placebo for the primary end point (95% CI 0.55 to 1.03) and a significant 34% relative risk reduction for the secondary end point (95% CI 0.45 to 0.98). Likewise, event rates were lowered to a greater degree by pioglitazone relative to placebo among the patients without CKD at baseline, but the difference between treatment groups was not statistically significant. Thus, the effect of pioglitazone relative to placebo seems to be the same regardless of GFR.
The yearly declines in GFR (0.9 ml/min per 1.73 m2 with placebo and 1.8 ml/min per 1.73 m2 with pioglitazone) in our study is considerably lower than the decline of 3 to 4 ml/min per 1.73 m2 observed in patients with diabetes in previous studies25,26 and is more in the range of the GFR decrease found in an aging healthy population (1 ml/min per 1.73 m2/yr).27 This smaller decrease might be explained by the strict treatment algorithm for hyperglycemia and hypertension used in PROactive. The clinical significance of the small difference in GFR decline of 0.8 ml/min per 1.73 m2/yr between placebo and pioglitazone is uncertain considering the suboptimal precision in monitoring the GFR changes using the Modification of Diet in Renal Disease (MDRD) method.28,29 Despite this small change in GFR, our data indicate that treatment with pioglitazone will be of considerable benefit to patients with diabetes and CKD.
A detailed analysis of reported adverse events was not conducted; however, the most critical events, such as cardiovascular events and death, were included in the efficacy parameters. This gives reassurance on the safety profile of pioglitazone in patients with reduced kidney function.
This analysis of PROactive has several limitations. Although the data for this analysis were collected prospectively, the analysis for the presence of CKD was defined and performed retrospectively and treatment randomization was not stratified by CKD. Because this could affect the reliability of the prognostic data, the conclusions about the beneficial effects of pioglitazone in patients with CKD must be viewed with caution until confirmatory data of our findings are provided. It should be emphasized that the participants in PROactive were selected because of their high cardiovascular risk; therefore, caution also should be exercised when extrapolating the estimates of cardiovascular event rates in patients with CKD to those who are at lower cardiovascular risk. Nevertheless, our data suggest strongly that patients with diabetes and high risk identified by CKD can be treated effectively with pioglitazone.
In this retrospective analysis of patients with type 2 diabetes and preexisting macrovascular disease in PROactive, we have shown that CKD identifies a subpopulation of patients who are at even higher risk for CVD and that pioglitazone reduces major cardiovascular end points in these patients.
CONCISE METHODS
Patients
The PROactive protocol has been described in detail elsewhere.8,9 Briefly, 5238 patients who had type 2 diabetes, were aged 35 to 75 yr, and had documented evidence of macrovascular disease and a glycosylated hemoglobin concentration higher than the local laboratory equivalent of 6.5% for a Diabetes Control and Complications Trial (DCCT)-traceable assay, despite existing treatment with diet alone or with oral glucose-lowering agents with or without insulin, were recruited. Patients were excluded when they had type 1 diabetes; were taking only insulin; had planned coronary or peripheral revascularization; had New York Heart Association class II or greater heart failure, ischemic ulcers, gangrene, or leg rest pain; were on hemodialysis on recruitment; or had an alanine aminotransferase >2.5 times the upper limit of normal.
All patients provided written informed consent. The study protocol was approved by local and national ethics committees and regulatory agencies and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Procedures
Patients were randomly assigned to receive pioglitazone (n = 2605) or matching placebo (n = 2633) in addition to their existing guideline-directed glucose-lowering and cardiovascular medications. Pioglitazone was administered as 15 mg/d for the first month, 30 mg/d for the second month, and 45 mg/d thereafter to achieve the maximum tolerated dosage. All patients were followed until the end of the study even when they permanently ceased study medication before the study end. Before the start of therapy, serum creatinine was determined in a central laboratory using the automated Hitachi (Tokyo, Japan) with Roche reagents (Roche Diagnostics, Mannheim, Germany) and standards and controls as recommended by the manufacturer. The central laboratory was certified by a national quality-control program. Urinary albumin concentration was measured locally at the beginning and at the end of the study using the Micral Test strip (Roche Diagnostics, Mannheim, Germany). Blood samples were taken for creatinine determination to examine the natural history of renal disease. GFR was estimated using the simplified MDRD formula: 186.2 × serum creatinine in mg/dl−1.154× age in yr−0.203× 1.212 if black × 0.742 if female.1 Patients with a GFR <60 ml/min per 1.73 m2 were defined as having renal dysfunction (CKD; National Kidney Foundation definition) at baseline. Treatment randomization was not stratified by CKD.
The primary end point in PROactive was time from randomization to the composite end point of all-cause mortality, nonfatal MI (including silent MI), stroke, acute coronary syndrome, coronary/carotid arterial intervention, leg revascularization, or amputation above the ankle. The prespecified principal secondary end point in PROactive was time to the first event of all-cause mortality, MI (excluding silent MI), and stroke, because these represent “hard” end points of greatest concern to patients. Nottingham Clinical Research Group (Nottingham, UK) acted as the coordinating center, providing project management, data management, central randomization services, and statistical analysis. ICON Clinical Research (Southampton, UK) managed and monitored the sites and carried out central laboratory measurements.
Statistical Analyses
Statistical methods used for the sample size calculation and end point analysis for PROactive have been reported previously.9 The data presented here are from the intention-to-treat population. Group comparison was carried out by Mann-Whitney or χ2 testing, for continuous variables and proportion, respectively. Time-to-event analyses were performed by fitting a Cox proportional hazards survival model with either treatment or baseline CKD as a covariate. The interaction between CKD and treatment on event rates was tested in a similar model containing both covariates. Multivariate Cox models were used to identify baseline factors prognostic of outcome; variable selection was carried out using a stepwise selection algorithm at a significance level of 0.05.
DISCLOSURES
C.A.S. has received research grants or honoraria for educational presentations from Bayer, Merck Darmstadt, Roche Diagnostics, and Takeda; E.F. serves on the Advisory Board of Servier, AstraZeneca, Boehringer Ingelheim, GSK, MSD, Novartis, BMS, Roche, and Takeda; R.D. has received research grants from BMS, Amylin, Eli Lilly, Novartis, Pfizer, and Takeda and is on the Speakers’ Bureau for Amylin and Takeda; G.S. is on the board of directors of sanofi aventis, Servier, Astra Zeneca, and Merck and has received honoraria for speaking engagements from sanofi aventis, Takeda, GSK, Novo Nordisk, Eli Lilly, and Bayer; J.Y. was previously employed by Takeda Global Research and Development Center; E.E. is the current chair of the PROactive Executive Committee and has received research grants, as well as honoraria for educational presentations, from Bayer (Germany), Takeda Global Research & Development Center, MSD, and Pfizer.
Acknowledgments
The study was funded by Takeda Pharmaceutical Company and was designed by the International Steering Committee, who approved the protocol and amendments. Data analysis for this report was conducted by Nottingham Clinical Research Limited. Access to data was given freely to the Executive Committee and authors, and the sponsors have not suppressed any data. Data interpretation, writing of this report, and decision to publish were made by all of the authors. All authors have read and approved the final submitted version of the manuscript.
These data were presented as poster presentations at the annual meeting of the American Diabetes Association; June 23, 2007; Chicago, IL.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Cardiovascular Disease, Chronic Kidney Disease, and Type 2 Diabetes Mellitus: Proceeding with Caution at a Dangerous Intersection,” on pages 5–7.
REFERENCES
- 1.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G: National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Wu AY, Kong NC, de Leon FA, Pan CY, Tai TY, Yeung VT, Yoo SJ, Rouillon A, Weir MR: An alarmingly high prevalence of diabetic nephropathy in Asian type 2 diabetic patients: The MicroAlbuminuria Prevalence (MAP) study. Diabetologia 48: 17–26, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Shlipak MG, Smith GL, Rathore SS, Massie BM, Krumholz HM: Renal function, digoxin therapy, and heart failure outcomes: Evidence from the digoxin intervention group trial. J Am Soc Nephrol 15: 2195–2203, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Shlipak MG, Simon JA, Grady D, Lin F, Wenger NK, Furberg CD, the Heart and Estrogen/Progestin Replacement Study (HERS) Investigators: Renal insufficiency and cardiovascular events in postmenopausal women with coronary heart disease. J Am Coll Cardiol 38: 705–711, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Ruilope L, Antonio S, Jamerson K, Hansson L, Warnold I, Wedel H, Zanchetti A: Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) Study. J Am Soc Nephrol 12: 218–225, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Charbonnel B, Dormandy J, Erdmann E, Massi-Benedetti M, Skene A, PROactive Study Group: The Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive): Can pioglitazone reduce cardiovascular events in diabetes? Study design and baseline characteristics of 5238 patients. Diabetes Care 27: 1647–1653, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J, PROactive investigators: Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 366: 1279–1289, 2005. 16214598 [Google Scholar]
- 10.Kasiske BL: The kidney in cardiovascular disease. Ann Intern Med 134: 707–709, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B: Cardiovascular mortality risk in chronic kidney disease: Comparison of traditional and novel risk factors. JAMA 293: 1737–1745, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Hakim RM, Lazarus JM: Progression of chronic renal failure. Am J Kidney Dis 14: 396–401, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, Rogers NL, Teschan PE: Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 51: 1908–1919, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Garg AX, Blake PG, Clark WF, Clase CM, Haynes RB, Moist LM: Association between renal insufficiency and malnutrition in older adults: Results from the NHANES III. Kidney Int 60: 1867–1874, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Stenvinkel P, Wanner C, Metzger T, Heimburger O, Mallamaci F, Tripepi G, Malatino L, Zoccali C: Inflammation and outcome in end-stage renal failure: Does female gender constitute a survival advantage? Kidney Int 62: 1791–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Stuveling EM, Hillege HL, Bakker SJ, Asselbergs FW, de Jong PE, Gans RO, de Zeeuw D, PREVEND study group: C-reactive protein and microalbuminuria differ in their associations with various domains of vascular disease. Atherosclerosis 172: 107–114, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Knight EL, Rimm EB, Pai JK, Rexrode KM, Cannuscio CC, Manson JE, Stampfer MJ, Curhan GC: Kidney dysfunction, inflammation, and coronary events: A prospective study. J Am Soc Nephrol 15: 1897–1903, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Clausen P, Jensen JS, Jensen G, Borch-Johnsen K, Feldt-Rasmussen B: Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation 103: 1869–1874, 2001 [DOI] [PubMed] [Google Scholar]
- 19.McClellan W, Flanders W, Langston R, Jurkovitz C, Presley R: Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: A population-based study. J Am Soc Nephrol 13: 1928–1936, 2002 [DOI] [PubMed] [Google Scholar]
- 20.McCullough PA, Sandberg KR, Borzak S, Hudson MP, Garg M, Manley HJ: Benefits of aspirin and beta-blockade after myocardial infarction in patients with chronic kidney disease. Am Heart J 144: 226–232, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB: Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med 137: 555–562, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Tonelli M, Keech A, Shepherd J, Sacks F, Tonkin A, Packard C, Pfeffer M, Simes J, Isles C, Furberg C, West M, Craven T, Curhan G: Effect of pravastatin in people with diabetes and chronic kidney disease. J Am Soc Nephrol 16: 3748–3754, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Rahman M, Pressel S, Davis BR, Nwachuku C, Wright JT Jr, Whelton PK, Barzilay J, Batuman V, Eckfeldt JH, Farber MA, Franklin S, Henriquez M, Kopyt N, Louis GT, Saklayen M, Stanford C, Walworth C, Ward H, Weigmann T, for the ALLHAT Collaborative Research: Cardiovascular outcomes in high-risk hypertensive patients stratified by baseline glomerular filtration rate. Ann Intern Med 144: 172–180, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Fox CS, Larson MG, Leip EP, Meigs JB, Wilson PW, Levy D: Glycemic status and development of kidney disease: The Framingham Heart Study. Diabetes Care 28: 2436–2440, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH: Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol 16: 1404–1412, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies DF, Shock NW: Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 29: 496–507, 1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beauvieux MC, Le Moigne F, Lasseur C, Raffaitin C, Perlemoine C, Barthe N, Chauveau P, Combe C, Gin H, Rigalleau V: New predictive equations improve monitoring of kidney function in patients with diabetes. Diabetes Care 30: 1988–1994, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Rossing P, Rossing K, Gaede P, Pedersen O, Parving HH: Monitoring kidney function in type 2 diabetic patients with incipient and overt diabetic nephropathy. Diabetes Care 29: 1024–1030, 2006 [DOI] [PubMed] [Google Scholar]