Abstract
Nephronophthisis, an autosomal recessive kidney disease, is the most frequent genetic cause of chronic renal failure in the first 3 decades of life. Causative mutations in 8 genes (NPHP1–8) have been identified, and homologous mouse models for NPHP2/INVS and NPHP3 have been described. The jck mouse is another model of recessive cystic kidney disease, and this mouse harbors a missense mutation, G448V, in the highly conserved RCC1 domain of Nek8. We hypothesized that mutations in NEK8 might cause nephronophthisis in humans, so we performed mutational analysis in a worldwide cohort of 588 patients. We identified 3 different amino acid changes that were conserved through evolution (L330F, H425Y, and A497P) and that were absent from at least 80 ethnically matched controls. All 3 mutations were within RCC1 domains, and the mutation H425Y was positioned within the same RCC1 repeat as the mouse jck mutation. To test the functional significance of these mutations, we introduced them into full-length mouse Nek8 GFP-tagged cDNA constructs. We transiently overexpressed the constructs in inner medullary collecting duct cells (IMCD-3 cell line) and compared the subcellular localization of mutant Nek8 to wild-type Nek8. All mutant forms of Nek8 showed defects in ciliary localization to varying degrees; the H431Y mutant (human H425Y) was completely absent from cilia and the amount localized to centrosomes was decreased. Overexpression of these mutants did not affect overall ciliogenesis, mitosis, or centriole number. Our genetic and functional data support the assumption that mutations in NEK8 cause nephronophthisis (NPHP9), adding another link between proteins mutated in cystic kidney disease and their localization to cilia and centrosomes.
Nephronophthisis (NPHP) is an autosomal recessive kidney disease, which leads to kidney cyst formation and progressive renal failure. NPHP is the most frequent genetic cause for end-stage renal failure (ESRF) in the first 3 decades of life. Recently, functions of primary cilia, basal bodies, and centrosomes have been implicated in the pathogenesis of NPHP.1,2 In humans, 8 causative genes, NPHP1–8 (MIM 256100, 602088, 604387, 606966, 609237, 610142, 608539, 610937) have been identified by positional cloning.3–11 In mice, several monogenetic models of recessive cystic kidney disease have been described. Seven proteins were identified as mutated in these murine disease models: Nphp2/inversin in inv, Nphp3 in pcy, cystin in cpk, bicaudal C in bpk and jcpk, polaris/Tg737 in orpk, Nek1 in kat, and Nek8 in jck mice.12 Because of phenotypic disease similarities between human NPHP and murine disease models, we examined by mutational analysis the genes encoding cystin,13 polaris/Tg737 (Otto EA, unpublished observation), and bicaudal C (Otto EA, unpublished observation) as candidate genes by mutational analysis in patients with NPHP but failed to identify any causative mutations. The spontaneously arisen renal cystic mouse model jck is caused by a homozygous G448V substitution in the conserved RCC1 domain of the Nek8 gene.14 Nek1 and Nek8 are members of the NIMA (never in mitosis A)-related kinase (Nek) family. Neks are cell cycle kinases that are thought to coordinate the regulation of cilia and cell cycle progression.15 Like all known nephrocystins (NPHP proteins), Nek8 localizes to primary cilia but seems not to be required for ciliary assembly.16 Knock-down of Nek8 led to the formation of pronephric cysts in zebrafish embryos and in vitro expression of mutated Nek8 resulted in enlarged, multinucleated cells.14 On the basis of the renal cystic disease phenotype of Nek8 missense mutation in mice and knock-down in zebrafish and of the ciliary/centrosomal localization of Nek8 and the known nephrocystins, we evaluated NEK8 as a candidate gene for human NPHP. We identified 3 different functionally significant missense mutations in evolutionary conserved amino acids.
RESULTS
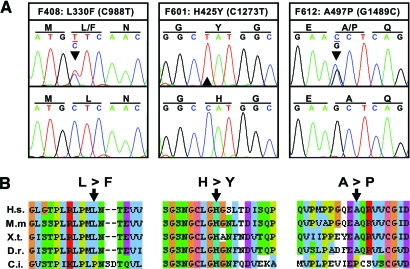
Mutational screening of all exons of NEK8 in 188 patients with NPHP by direct sequencing revealed 3 different nucleotide changes that alter the deduced amino acid sequence of human NEK8 (C988T, L330F, heterozygous; C1273T, H425Y, homozygous; G1489C, A497P, heterozygous) in 3 patients from different families (Table 1; Figure 1, A). Screening of an additional 400 patients with NPHP for homozygosity by typing 3 highly polymorphic microsatellite markers at the NEK 8 locus yielded 2 individuals showing homozygosity. None of these had any further NEK8 mutations. At least 80 DNA samples from healthy control individuals from Central Europe were examined to test the mutations found in the Swiss (F408), Austrian (F612), and the Kurdish (F601) patients. For the Kurdish family (F601) we examined an additional 76 healthy control samples from Turkey. All mutations were absent from the controls investigated. Mutation analysis of all known NPHP genes for these 3 patients revealed an additional homozygous mutation in the NPHP5 gene (424–425delTT, F142fsX146) in the patient F408 who carries the L330F change in NEK8 heterozygously.8 All 3 mutated amino acids (L330, H425, and A497) show evolutionary conservation and are identical in human, mouse, xenopus, and zebrafish. Additionally, H425 is conserved in the chordate Ciona intestinalis (Figure 1, B). The equivalent numbering of the L330F, H425Y, and A497P human mutants is L336F, H431Y, and A503P in mouse, respectively.
Table 1.
NEK8 mutations and clinical characteristics in three patients with NPHP
| Family No. | Ethnic Origin | Nucleotide Changea | Deduced Protein Changea | Exon (allele) | Parental Consanguinity | Age (yr) at ESRF | Renal Cysts | Eye Involvement | Mutation Absent From Controlsb |
|---|---|---|---|---|---|---|---|---|---|
| F408c | Swiss | C988T | L330F | 7 (heterozygous) | − | 24 | ND | RP, blind at age 24 | 0/80, DHPLC |
| F601 | Kurdish | C1273T | H425Y | 9 (homozygous) | + | 3 | Microcysts (renal biopsy consistent with NPHP) | No | 0/85, NcoI 0/76, direct sequencing |
| F612 | Austrian | G1489C | A497P | 11 (heterozygous) | − | 14 | ND | No | 0/85, BlpI |
DHPLC, denaturing high performance liquid chromatography; RP, retinitis pigmentosa; ND, no data available.
Numbering based on NEK8 cDNA position in human reference sequences NM_178170.2; +1 corresponds to the A of the ATG translation initiation codon.
All mutations were absent from at least 80 healthy control subjects. For the Kurdish family (F601), we examined an additional 76 healthy control samples from Turkey. BlpI or NcoI restriction enzyme digest, DHPLC, or direct sequencing were used for examination of healthy control samples.
The patient of family F408 carries an additional homozygous NPHP5 mutation.8
Figure 1.
Human mutations in NEK8 and evolutionary conservation. (A) Chromatograms of 3 different NEK8 mutations detected in 3 individuals with nephronophthisis. Family number, amino acid sequence change, and mutated nucleotide are given above sequence traces. Wild-type sequences are shown below mutated sequences. Reading frame is indicated by underlining codon triplets in the upper panel, and mutated nucleotides are indicated by an arrowhead. The two mutations L330F and A497P occurred in the heterozygous and H425Y in the homozygous state. All mutations were absent from at least 80 healthy control individuals. (B) Alignment of the Nek8 protein sequence of regions mutated in patients with homologues from various species. Amino acid residues that are within the same chemical group are coded in the same color. Mutated amino acids are indicated with arrowheads. The amino acid sequences are aligned with those of Homo sapiens (H.s.), Mus musculus (M.m), Xenopus tropicalis (X.t.), Danio rerio (D.r.), and Ciona intestinalis (C.i.).
Direct yeast-2-hybrid interaction screening of NEK8 (bait) with 5 nephrocystin proteins (NPHP1–5), Bardet Biedl syndrome proteins (BBS1, 2, 4, 5, 7, MKKS, TTC8), cystic mouse model proteins (IFT88/Tg737, KIF3A, KIF3B, NEK1, TGFA, OVOL1), intraflagellar transport proteins (IFT122, −27, −46, −52, −57), and 29 additional NPHP disease candidates (prey) were all negative (Figure S2).
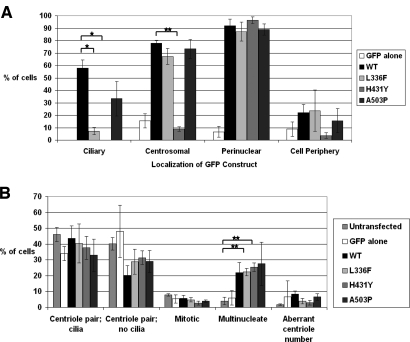
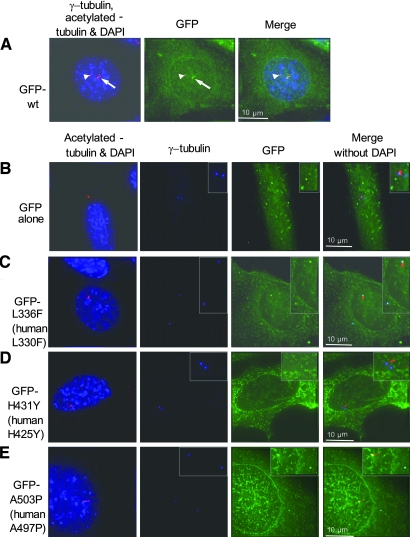
Transient transfection of IMCD-3 cells with wild-type GFP-Nek8 shows cytoplasmic, centrosomal, ciliary, and perinuclear localization, whereas the mutant GFP-Nek8 constructs L336F and H413Y show decreased localization to the cilia (Figures 2, A and 3). The H431Y GFP-Nek8 mutant also shows reduced localization to the centrosome, whereas the other mutations did not (Figure 2, A and 3). Transient transfection of GFP alone, wild-type GFP-Nek8, or GFP-Nek8 mutants in IMCD-3 cells had no effect on whether cells were ciliated (scored as % ciliated cells in the population). We did not detect an effect on mitosis or number of centrioles (Figure 2, B). Transient transfection of IMCD-3 cells with N-terminal myc-tagged wild-type, L336F, H431Y, or A503P Nek8 vectors resulted in similar observations as the GFP-tagged vectors, with all forms of Nek8 causing multinucleated cells and no effect on ciliation, mitosis, or centriole number (data not shown).
Figure 2.
(A) Quantification of differential subcellular localization of GFP-tagged Nek8 constructs. Transiently transfected ciliated, mononucleate IMCD-3 cells with low to medium levels of expression were quantified for ciliary, centrosomal, perinuclear, and cell peripheral localization of GFP-mutant Nek8. The total number of cells counted from 3 independent experiments was n = 45, 50, 55, 55, and 45 for GFP alone, wild-type (WT), L336F, H431Y, and A503P, respectively. Error bars = SEM. Note that ciliary localization was significantly reduced for mutant constructs L336F and H431Y compared with the wild-type. Centrosomal localization was significantly reduced for mutant H431Y. Statistical significance was assessed using T-test assuming 2-sample unequal variances with 2-tailed probability. *P = 0.01, **P < 0.001. (B) Overexpression of Nek8 has no effect on ciliogenesis. Transfected and untransfected cells were categorized as ciliated with a pair of centrioles, lacking cilia with a pair of centrioles, undergoing mitosis, multinucleate, or having an aberrant number of centrioles, including none at all. n = total number of cells counted from three independent experiments. The total number of cells counted from three independent experiments was n = 1095, 150, 300, 250, 300, and 258 for untransfected, GFP alone, WT, L336F, H431Y, and A503P, respectively. Error bars = SEM.
Figure 3.
Mutant forms of Nek8 have defects in subcellular localization. Transient overexpression of N-terminal GFP-tagged mouse Nek8 cDNA in inner medullary collecting duct (IMCD-3) cells. (A) Cells were stained for anti-acetylated tubulin (red) to visualize cilia (arrows) and anti-γ-tubulin (red) to mark centrosomes (arrowheads) and DAPI to indicate the nucleus (blue). Wild-type GFP-Nek8 (green) localizes to the cytoplasm, centrosomes (arrowheads), and cilia (arrows). (B through E) Expression of GFP alone and mutant GFP-Nek8 constructs. Note that amino acid numbering differs in murine mNek8 and human NEK8 (Table 1). Cells were fixed and stained for anti-acetylated tubulin for cilia (red) and for anti-γ-tubulin for centrosomes (blue) and DAPI (blue). (B) GFP alone localizes to the cytoplasm, but not to cilia or centrosomes. (C) The GFP-L336F Nek8 mutant was detected in the cytoplasm and centrosomes, but not in cilia. (D) The GFP-H431Y Nek8 mutant is not associated with either centrosomes or cilia, in this example. (E) GFP-A503P localizes to the cytoplasm and centrosomes, but not to cilia in this example. Insets are ×2 original magnification in panels B and D and ×1.5 magnification in panels C and E.
DISCUSSION
Nephronophthisis is a renal cystic disease with extensive genetic locus heterogeneity. Except for NPHP1, individual genes are mutated only in a few patients in the range of 1% to 3% of all cases. As an example, to date only 8 cases were described with NPHP2/Inversin mutations.6,13 Another extremely rare cause for NPHP are mutations in NPHP7/GLIS2, which were identified in only one family so far.10 After screening 188 patients with NPHP by direct sequencing of NEK8, only 3 mutations were identified. To identify further NEK8 mutations, we followed a homozygosity mapping strategy by typing highly polymorphic microsatellite markers, positioned in close proximity to NEK8 in additional 400 patients. We applied this approach based on the observation that recessive mutations in rare diseases like NPHP1–5 are found to be mostly homozygous, rather than compound heterozygous. No additional homozygous NEK8 mutations were detected. Therefore, we consider NEK8 as another very rare cause for NPHP (NPHP type 9). In total, we identified 3 missense mutations in NEK8 in 3 unrelated patients, one of which was homozygous, and for 2 of which the second NEK8 allele was not detected. In the 2 individuals in whom we only found a heterozygous mutation (F408 and F612), one explanation is that we missed the second NEK8 mutation, which may be not accessible to exon sequencing. This situation is the rule rather than the exception, as in patient cohorts with mutations in NPHP3, NPHP4, and NPHP6 we find a fair number of patients in whom we do not detect the second mutation.7,9,17 Because no parental DNA was available, we could not test for segregation of these mutations. The parents of both individuals did not have an NPHP phenotype. Alternatively, as in the Bardet-Biedl syndrome, oligogenic inheritance may be necessary to manifest the disorder.18 The heterozygous Nek8 mutation might act as a potential genetic modifier of the kidney and eye phenotype. Interestingly, one patient (F408) with a heterozygous NEK8 mutation (L330F) carries an additional homozygous loss of function mutation in NPHP5. Paradoxically, this patient became blind at age 24 yr, which is later compared with all other patients with truncating NPHP5 mutations, who developed blindness within the first 3 yr of life. Whether this may reflect a “gain of function” cannot be decided on the basis of one case only. Interestingly, cilia were significantly lengthened in jck epithelia compared with wild-type cilia accompanied by enhanced expression of polycystins along the cilia.19 We speculate that mutated Nek8 led to analogous changes in connecting cilia of photoreceptors. Crossing jck mice, in which no sign of eye disease has been reported to date, with Nphp5−/− knockout mice may address the question of a potential “gain-of-function modifier.” The mutation H425Y (mNek8_H431Y) found in a NPHP patient (F601) homozygously is localized in close proximity to the murine jck mutation (G448V) in a highly conserved RCC1 (regulator of chromosome condensation) repeat. The role of the RCC1 domain in Nek8 is still unknown. Interestingly, the retinitis pigmentosa GTPase regulator protein RPGR, which is known to be in a complex with NPHP5, contains an RCC1domain as well. All mutations found in NEK8, including the jck cystic mouse model, are “missense” mutations. Truncating mutations, which are common in NPHP1–8, have not been found in NEK8. There is no Nek8 knockout mouse model to address the question if such a mutation would be compatible with life and whether the phenotype will be comparable with the jck phenotype.
Our experiments showed that overexpression of Nek8 gives rise to multinucleated cells regardless of whether wild-type or mutated Nek8 is transfected into cultured cells, which is in conflict with findings that cells expressing jck mutant Nek8 differ compared with wild-type Nek8 regarding cell enlargement and multinucleation.14 The authors did indeed not find multinucleated tubule cells in jck mice.14 The cellular basis of renal cyst formation is still not fully understood. Recent evidence supports a unifying theory of renal cystic disease on the basis of ciliary/centrosomal expression.1,2,20
In this study, we show that mutations in the kinase Nek8 decrease or abolish its localization to the cilium. We speculate that ciliary localization of Nek8 is crucial for proper cilia signaling, which then might affect downstream events, such as cell cycle progression.21,22 Loss of cilary localization of Nek8 was reported in primary kidney epithelial cells derived from jck mice.19 We have recently found decreased ciliary localization of Nek8 in luminal cilia of jck homozygous mouse kidney tubules, but not in heterozygous (unaffected) mice (Trapp ML, unpublished observation).
CONCISE METHODS
Human Subjects
We obtained blood samples, pedigree, and clinical information after receiving informed consent (www.renalgenes.org) from 588 patients/families with NPHP and their parents. Approval for human subjects research was obtained from the University of Michigan Institutional Review Board. In all patients, the diagnosis of NPHP was based on the following criteria: 1) clinical course with characteristic clinical signs of NPHP, including chronic renal failure, polyuria, polydipsia, anemia, and growth retardation; 2) renal ultrasound or renal biopsy compatible with the diagnosis of NPHP as judged by a (pediatric) nephrologist; and 3) pedigree compatible with autosomal recessive inheritance. Homozygous NPHP1 deletions were excluded in all patients, applying a multiplex polymerase chain reaction (PCR) approach.23
PCR Amplification and Sequencing
We screened for NEK8 mutations by direct sequencing from one strand using exon flanking primers in a touchdown PCR reaction for all 15 exons. Sequences and PCR conditions are available on request. PCR products were purified using spin columns according to the manufacturer's instructions (Marligen, Ijamsville, MD) and directly sequenced using the dideoxy chain-termination method on an automatic capillary genetic analyzer (Applied Biosystems, Foster City, CA). Exon-PCR products of ethnically matched healthy control individuals were investigated by restriction enzyme digests, DHPLC analysis, or direct sequencing.
Cell Culture
IMCD-3 cells were cultured in humidified 37°C incubator with 5% CO2 in 1:1 DMEM/Ham's F12 media with 10% FBS (all from Invitrogen, Carlsbad, CA).
GFP Constructs and Transfection
The L336F, H431Y, and A503P mutagenized mouse Nek8 (mNek8) cDNA constructs (corresponding to human amino acid positions L330F, H425Y, and A497P, respectively) were digested with BlpI and XbaI, and the resulting Nek8 fragment containing the mutation was subcloned into the BlpI/XbaI digested pEGFP-C2 (BD Biosciences Clontech, San Jose, CA) vector containing mNek8 with an N-terminal GFP. The reading frame and mutations were verified by sequencing. Western blot of transfected IMCD-3 cell extracts using anti-GFP antibody shows expected band size of 102 kDa for the GFP-Nek8 fusion proteins and 27 kDa for GFP alone (Figure S1). The plasmids were then purified by an Endotoxin-free Maxiprep kit (Qiagen, Hilden, Germany). Transient transfections were performed using Lipofectamine 2000 (Invitrogen) according to manufacturer's protocol with the following exception: Cells grown on coverslips were incubated with DNA complexes in OptiMEM (Invitrogen) for 4 to 6 h, then washed with PBS and incubated in DMEM/Ham's F12 media for 16 to 24 h. Transfection efficiency ranged from 30% to 62%.
Immunofluorescence
The transfected cells grown on coverslips were fixed with −20°C methanol for 10 min and then rehydrated with PBS. They were subsequently incubated for 1 h with the following primary antibodies: polyclonal rabbit anti-γ tubulin (500-fold dilution, Sigma, St. Louis, MO), monoclonal mouse anti-γ tubulin (1000-fold dilution, Sigma), and monoclonal mouse IgG2b anti-acetylated tubulin (7500-fold dilution, Sigma). After washing in PBS, the cells were incubated for 1 h with one or more of the following secondary antibodies: Alexa 594-conguated goat anti-mouse IgG2b (1000-fold dilution, Molecular Probes, San Diego, CA), Alexa 594-conjugated goat anti mouse all IgG (2000-fold dilution, Molecular Probes), and Cy5-conjugated donkey anti-rabbit (500-fold dilution, Jackson ImmunoResearch Laboratories, West Grove, PA). Cell nuclei were stained with 4 to 6-diamidino-2-phenylindole (1 μg/ml). Coverslips were mounted using an antifade medium containing 0.1 mg/ml Mowiol (Calbiochem, San Diego, CA), 50% glycerol, and 100 mM Tris, pH 8.5.16 Microscopy was performed using the Delta Vision system (Applied Precision, Issaquah, WA) as described previously.24
Cell Quantification
In 3 independent experiments, transfected and untransfected cells were categorized as ciliated with a pair of centrioles, lacking cilia with a pair of centrioles, undergoing mitosis, multinucleate, or having an aberrant number of centrioles, including none at all. The ciliated, mononucleate cells with low to medium levels of expression were then quantified for localization of GFP-mutant Nek8 to cilia, centrosomes, to the perinuclear region, and/or to the cell periphery.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Grants DK1069274, DK1068306, and DK064614 (F.H.) and a grant from the Kidney Foundation of Canada (L.M.Q.). M.L.T. is supported by a graduate fellowship from the Michael Smith Foundation for Health Research. F.H. is a Frederick G. L. Huetwell Professor.
The authors thank the affected individuals and their families for participation, R. H. Lyons for excellent large-scale sequencing, and the following physicians for contribution of materials and clinical data from patients: H. Gadner, Vienna, Austria; J. Ehrich, Hannover, Germany; F. Frey, Bern, Switzerland.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Too Much of a Good Thing: Does NeK8 Link Polycystic Kidney Disease and Nephronophthisis?,” on pages 418–420.
Supplemental information for this article is available at http://www.jasn.org/.
E.A.O. and M.L.P. contributed equally to this work.
REFERENCES
- 1.Watnick T, Germino G: From cilia to cyst. Nat Genet 34: 355–362, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Hildebrandt F, Otto E: Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet 6: 928–940, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Hildebrandt F, Otto E, Rensing C, Nothwang HG, Vollmer M, Adolphs J, Hanusch H, Brandis M: A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet 17: 149–153, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Otto E, Hoefele J, Ruf R, Mueller AM, Hiller KS, Wolf MT, Schuermann MJ, Becker A, Birkenhager R, Sudbrak R, Hennies HC, Nurnberg P, Hildebrandt F: A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet 71: 1161–1167, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mollet G, Salomon R, Gribouval O, Silbermann F, Bacq D, Landthaler G, Milford D, Nayir A, Rizzoni G, Antignac C, Saunier S: The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat Genet 32: 300–305, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Otto EA, Schermer B, Obara T, O'Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F: Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 34: 413–420, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A, Wolf MT, Sasmaz G, Trauer U, Reinhardt R, Sudbrak R, Antignac C, Gretz N, Walz G, Schermer B, Benzing T, Hildebrandt F, Omran H: Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis Nat Genet 34: 455–459, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O'Toole JF, Helou J, Attanasio M, Utsch B, Sayer JA, Lillo C, Jimeno D, Coucke P, De Paepe A, Reinhardt R, Klages S, Tsuda M, Kawakami I, Kusakabe T, Omran H, Imm A, Tippens M, Raymond PA, Hill J, Beales P, He S, Kispert A, Margolis B, Williams DS, Swaroop A, Hildebrandt F: Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Løken syndrome and interacts with RPGR and calmodulin. Nat Genet 37: 282–288, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Sayer JA, Otto EA, O'Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson RG, Allen SJ, Wilkinson CJ, Nigg EA, Shou C, Lillo C, Williams DS, Hoppe B, Kemper MJ, Neuhaus T, Parisi MA, Glass IA, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux MR, Hildebrandt F: The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet 38: 674–681, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Attanasio M, Uhlenhaut NH, Sousa VH, O'Toole JF, Otto E, Anlag K, Klugmann C, Treier AC, Helou J, Sayer JA, Seelow D, Nurnberg G, Becker C, Chudley AE, Nurnberg P, Hildebrandt F, Treier M: Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat Genet 39: 1018–1024, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, Moutkine I, Hellman NE, Anselme I, Silbermann F, Vesque C, Gerhardt C, Rattenberry E, Wolf MT, Gubler MC, Martinovic J, Encha-Razavi F, Boddaert N, Gonzales M, Macher MA, Nivet H, Champion G, Bertheleme JP, Niaudet P, McDonald F, Hildebrandt F, Johnson CA, Vekemans M, Antignac C, Ruther U, Schneider-Maunoury S, Attie-Bitach T, Saunier S: The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet 39: 875–881, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Guay-Woodford LM: Murine models of polycystic kidney disease: molecular and therapeutic insights. Am J Physiol Renal Physiol 285: F1034–F1049, 2003 [DOI] [PubMed] [Google Scholar]
- 13.O'Toole JF, Otto EA, Hoefele J, Helou J, Hildebrandt F: Mutational analysis in 119 families with nephronophthisis. Pediatr Nephrol 22: 366–370, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR: A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development 129: 5839–5846, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Quarmby LM, Mahjoub MR: Caught Nek-ing: cilia and centrioles. J Cell Sci 118: 5161–5169, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Mahjoub MR, Trapp ML, Quarmby LM: NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J Am Soc Nephrol 16: 3485–3489, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Hoefele J, Sudbrak R, Reinhardt R, Lehrack S, Hennig S, Imm A, Muerb U, Utsch B, Attanasio M, O'Toole JF, Otto E, Hildebrandt F: Mutational analysis of the NPHP4 gene in 250 patients with nephronophthisis. Hum Mutat 25: 411, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Katsanis N, Lupski JR, Beales PL: Exploring the molecular basis of Bardet-Biedl syndrome. Hum Mol Genet 10: 2293–2299, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Smith LA, Bukanov NO, Husson H, Russo RJ, Barry TC, Taylor AL, Beier DR, Ibraghimov-Beskrovnaya O: Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol 17: 2821–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Pazour GJ: Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol 15: 2528–2536, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Bowers AJ, Boylan JF: Nek8, a NIMA family kinase member, is overexpressed in primary human breast tumors. Gene 328: 135–142, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Quarmby LM, Parker JD: Cilia and the cell cycle? J Cell Biol 169: 707–710, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildebrandt F, Rensing C, Betz R, Sommer U, Birnbaum S, Imm A, Omran H, Leipoldt M, Otto E; Arbeitsgemeinschaft fur Paediatrische Nephrologie (APN) Study Group: Establishing an algorithm for molecular genetic diagnostics in 127 families with juvenile nephronophthisis. Kidney Int 59: 434–445, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Mahjoub MR, Qasim Rasi M, Quarmby LM: A NIMA-related kinase, Fa2p, localizes to a novel site in the proximal cilia of Chlamydomonas and mouse kidney cells. Mol Biol Cell 15: 5172–5186, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.