Abstract
The cellular composition of crescents in glomerular disease is controversial. The role of podocytes in crescent formation has been especially difficult to study because podocytes typically lose their characteristic terminally differentiated phenotype under disease conditions, making them difficult to identify. We reasoned that the intermediate filament protein nestin, a marker of progenitor cells that has recently been identified in podocytes, may allow the investigation of podocyte involvement in glomerular crescents. In a series of 35 biopsies with crescentic glomerular disease, all showed nestin-positive cells in the crescents, ranging in number from occasional to approximately 50% of crescent cells. Other podocyte markers, such as podocin and WT1, failed to identify cells in crescents, and no contribution by endothelial or myogenic cells was noted. CD68-positive cells were observed in 80% of cases but were never as numerous as the nestin-positive cells. Nestin and CD68 were not coexpressed by the same cells, providing no evidence of trans-differentiation of podocytes into a macrophage phenotype. Keratin-positive cells were found in crescents in 51% of cases, but only as occasional cells. Up to one third of crescent cells were cycling in 48% of biopsies, and double immunostaining identified these cells as a mixture of nestin-positive cells and “null” cells (negative for nestin, CD68, and keratin). In addition to our observations in human disease, we also identified nestin-positive proliferating podocytes in the crescents of 2 mouse models of crescentic glomerulonephritis. We conclude that podocytes play a role in the formation of glomerular crescents.
Crescent formation occurs in a wide variety of glomerular diseases and is a relatively straightforward pathologic change to recognize on biopsy. More problematic has been determining the cellular composition of crescents. The traditional concepts have come largely from immunohistochemical studies that have concluded crescents are a mixture of parietal epithelial cells, macrophages, and myofibroblasts.1,2 The proportion of these cells is variable; parietal epithelial cells predominate when Bowman's capsule is intact, whereas macrophages and myofibroblasts become more numerous when Bowman's capsule is ruptured,1–3 although such rupture is reported to be a rare event.3 Parietal epithelial cells are usually identified as such by anti-bodies to keratin and macrophages by CD68 immunostaining.1,2,4 However, the picture is an incomplete one because some studies have found that macrophages constitute only a small proportion of the cells in crescents,2,3 a finding supported by studies in animal models.5,6 Moreover, there exists a population of cells in crescents that appear to be epithelial by morphology but are keratin-negative,4,7 sometimes comprising the most abundant cell in the crescent. These cells have been assumed to be parietal epithelial cells, but this assumption is not based on the demonstration of any markers specific for parietal epithelial cells.8
Podocytes were excluded as participants in crescents, largely because cells forming crescents did not express any podocyte markers, including CD10, podocalyxin, synaptopodin, glomerular epithelial protein 1, podocin, CD2AP, nephrin, and Wilms tumor antigen I (WT1).2,3 However, studies in animal models have tended to alter this thinking. In mouse crescentic antiglomerular basement membrane nephritis, podocytes were observed to form bridges between the glomerular tuft and Bowman's capsule, detach from the glomerular basement membrane (GBM), and migrate between parietal epithelial cells.3 This infiltration of podocytes along the parietal basement membrane (PBM) was postulated to initiate proliferation of parietal epithelial cells, resulting in a crescent.3 Concurrently, such podocytes lost their podocyte-specific phenotype. More definitive results have come from studies in which mouse podocytes were genetically tagged, so that cells derived from podocytes could be identified without having to rely on immunophenotyping. Using this approach, 25% to 50% of cells in crescents were found to originate from podocytes,8 in turn leading to the conclusion that podocytes, upon transforming to crescentic cells, lose podocyte-specific antigens so that their origin is no longer identifiable.8
Because genetic tagging is not feasible in humans, the contribution of podocytes in human crescentic disease has been based largely on immunohistochemistry, despite the difficulties in identifying these cells in crescents by this approach. Certainly, occasional cells in crescents have been identified as podocytes by immunostaining for podocyte antigens, such as synaptopodin, podocalyxin, podocin, α-actinin-4, and vimentin.7 Most studies, however, have found that injured podocytes lose their differentiation markers and instead rely on anatomic location (i.e. relative to the GBM) to identify cells as podocytes.7,9,10 This approach has led to some curious results, namely, that cells situated on the GBM sometimes express CD68 or keratin, leading to the conclusion that podocytes not only lose their markers (dedifferentiation) but sometimes acquire new epitopes (transdifferentiation).7,10 Because there were cells in crescents that had similar immunophenotypes (expression of CD68 and/or keratin but not podocyte antigens), it was extrapolated that such cells could also be podocytic in origin. However, the true identity of such cells remains unconfirmed. Such confirmation might be possible if there existed a marker for podocytes that persisted even in disease states. We have found that nestin is expressed in human podocytes in normal kidney and all glomerular diseases studied to date.11 Nestin is an intermediate filament protein that is genetically distinct from other intermediate filaments and was originally identified in neural progenitor cells,12 but only recently, it has been found that nestin is expressed in rat,13 mouse,14 and human11,15 podocytes. We reasoned that nestin might serve as a robust marker for podocytes, and we have carried out a study of nestin expression in crescentic disease, with the goal of assessing the podocyte contribution to crescents.
RESULTS
Immunophenotypes of Cells in Human Crescentic Glomerular Disease
The 35 renal biopsies that were studied included cases of anti-GBM nephritis (n = 4), postinfectious glomerulonephritis (n = 12), IgA nephropathy and Henoch-Schönlein nephritis (n = 8), membranoproliferative glomerulonephritis type I (n = 1), lupus nephritis classes III or IV (n = 4), and pauci-immune glomerulonephritis (n = 6). Most crescents were cellular or mixed cellular and fibrous in morphology. Of the podocyte markers studied, crescents in 35 of 35 (100%) biopsies contained nestin-positive cells (Figure 1), whereas no crescents contained cells positive for WT1 or podocin. In the glomerular tuft, podocytes expressed all 3 antigens, but no expression was seen in other glomerular cells (results not shown). The earliest change noted was bridging of visceral podocytes to the parietal basement membrane followed by the appearance of nestin-positive cells along the parietal basement membrane. Crescents were a mixture of nestin-positive and nestin-negative cells. The number of nestin-positive cells in crescents varied tremendously between glomeruli (both within the same biopsy and in different biopsies) and comprised up to 50% of cells in some crescents as estimated by light microscopy. This did not seem to be related to crescent morphology; nestin-positive cells were present in cellular, mixed fibrous and cellular, and end-stage fibrous crescents.
Figure 1.
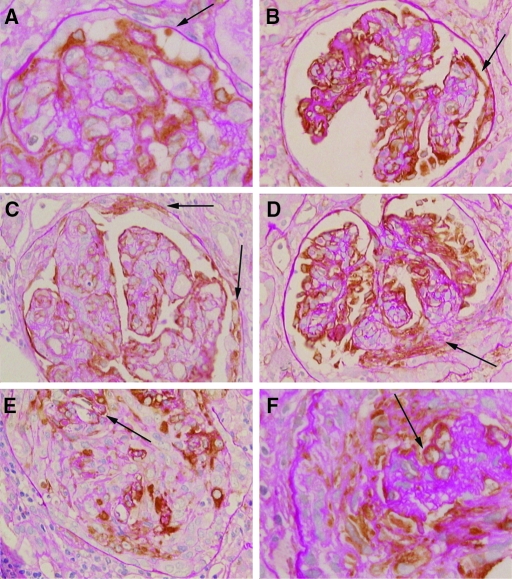
Nestin staining in glomerular crescents at different stages. Podocytes along the glomerular tuft are positive for nestin; the glomerular and parietal basement membranes are highlighted by PAS staining. (A) The earliest change noted was bridging of visceral podocytes to the parietal basement membrane (arrow). (B) Bridging could also be seen to nestin-positive cells along the parietal basement membrane (arrow). (C) Mixtures of nestin-positive and nestin-negative cells were found along the parietal basement membrane (arrows). (D) Crescents extending from the glomerular tuft were a mixture of nestin-positive and nestin-negative cells (arrow). (E) Nestin-positive cells comprised a variable proportion of crescentic cells, with up to 50% positive in some crescents. Arrow points to the residual glomerular basement membrane within this large crescent. (F) Nestin-positive cells were still seen in organized fibrous crescents. The collapsed glomerular tuft with nestin-positive podocytes is marked by an arrow. Immunoperoxidase with PAS counterstain, original magnifications: B, C, D, E, ×200; A, F, ×400.
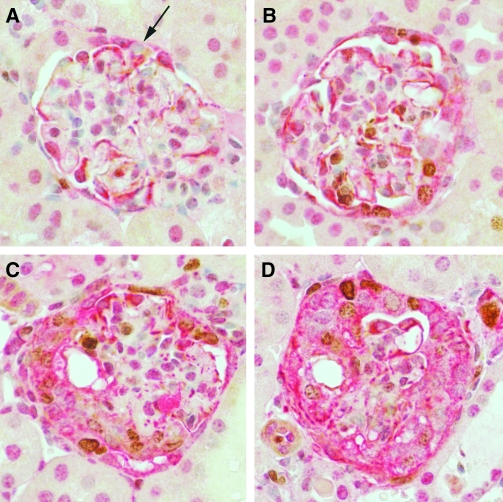
The α3 chain of type IV collagen was detected in crescents in 4 of 7 (57%) biopsies (Figure 2); the remaining 3 cases did not show crescents in the frozen material. The staining had 2 appearances. In some crescents, there were short isolated areas of staining that were distant from the glomerular tuft, in close proximity to Bowman's capsule (Figure 2, A). In other glomeruli, there were bridging from the glomerular tuft to Bowman's capsule and positive areas along Bowman's capsule adjacent to the crescent (Figure 2, B). Bowman's capsule in noncrescentic glomeruli was always negative for the α3 chain. There was also staining that appeared to be fragments of GBM situated near the glomerular tuft, and this was not scored as positive.
Figure 2.
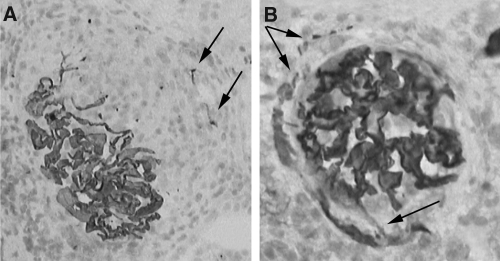
α3 chain of type IV collagen in crescents. The α3 chain of type IV collagen is seen in the residual GBM of glomerular tuft in (A) and (B). In addition, in panel A, staining for the α3 chain is seen at some distance from the tuft close to Bowman's capsule, within a cellular crescent (arrows). In panel B, the α3 chain is seen bridging from the glomerular tuft to Bowman's capsule (lower arrow), and this chain is now abnormally expressed along some aspects of Bowman's capsule where a crescent has formed (upper arrows). The picture is similar to the pattern of nestin staining seen in Figures 1B and 1C. Immunoperoxidase with hematoxylin counterstain, original magnifications ×200.
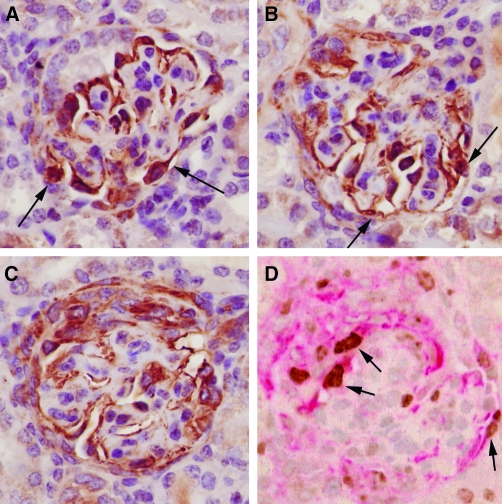
CD68-positive cells were seen in crescents in 28 of 35 (80%) biopsies. C68-negative cases included 2 cases of postinfectious glomerulonephritis, 2 cases of IgA nephropathy, one case of Henoch-Schönlein nephritis, and 2 cases of pauci-immune glomerulonephritis. All cases with keratin-positive cells in crescents also had CD68-positive cells, except one. Using serial sections, it was apparent in some glomeruli that the nestin-positive and the CD68-positive cells in crescents were separate populations, based not only on different distributions of cells within the crescent but also based on different nuclear and cellular morphologies (Figure 3). In 5 biopsies, CD68-positive cells appeared more epithelioid, rather than as typical macrophages. Double immunostaining was performed to investigate whether nestin and CD68 were coexpressed in crescentic cells. In 16 of 16 cases, nestin-positive and CD68-positive cells were intimately mixed in crescents, but no cells coexpressed both antigens (Figure 3). Keratin-positive cells were seen in crescents in 18 of 35 (51%) biopsies, but only as occasional isolated cells and never in the numbers seen for nestin-positive cells (results not shown). No cells in crescents expressed CD31 or smooth muscle actin (results not shown).
Figure 3.
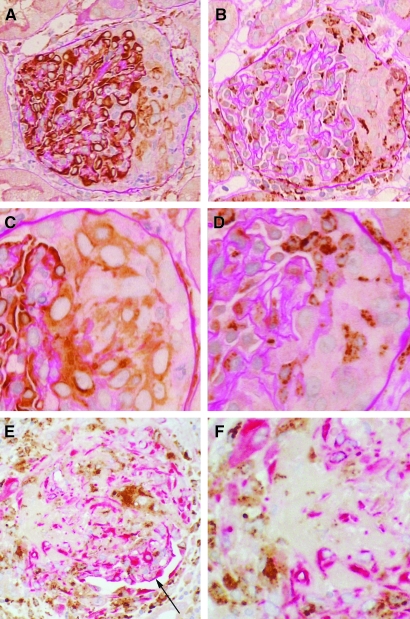
Nestin-positive versus CD68-positive cells in crescents. (A) Glomerulus showing nestin expression in podocytes and in the crescent at the right, where staining is largely confined to the cells immediately adjacent to the glomerular tuft. (B) The same glomerulus as in panel A shows CD68-positive cells in the crescent, which tend to be situated more to the peripheral aspects. (C) A different glomerulus in which the nestin-positive cells in the crescent tend to be epithelioid with large ovoid nuclei. (D) The same glomerulus as in C shows that CD68-positive cells are morphologically distinct as stellate cells with smaller, reniform nuclei. (E) Double immunostaining for nestin (red) and CD68 (brown) showing that in crescents nestin-positive and CD68-positive cells are in separate populations that are intimately mixed. The residual glomerular tuft with nestin-positive podocytes is located around the 4 o'clock position (arrow). (F) Detail of the crescent from same glomerulus as in panel E showing separate populations of nestin-positive and CD68-positive cells. Immunoperoxidase with PAS counterstain, original magnifications: A, B, ×200; C, D, ×400; immunoperoxidase with hematoxylin counterstain, original magnifications: E, F, ×200.
Assessment of Cell Cycling by MIB1 (Ki-67) Immunostaining
Very occasionally, mitotic figures were seen in crescents. As a more sensitive indicator of whether cells in crescents were cycling, immunostaining for MIB1 (Ki-67) was used. MIB1-positive cells were seen in crescents in 17 of 35 (48%) biopsies. The proportion of positive cells ranged from zero to about one third of crescentic cells (Figure 4). Multinucleated glomerular cells were never observed to be cycling. In the crescents with the highest MIB1 index, there were frequent nestin-positive cells, suggesting that at least some of the nestin-positive cells were cycling. To be more definitive about which cells were cycling, double immunostaining was performed. Nestin-positive, MIB1-positive cells were seen in 9 of 12 (75%) cases (Figure 4, C and D). Only a few positive cells were seen in any one case. CD68-positive, MIB1-positive cells were seen in only 1 of 12 (8%) cases in cells with a more epithelioid morphology. None of the MIB1-positive cells was keratin-positive. In all biopsies studied, however, the most common MIB1-positive cell was negative for nestin, CD68, and keratin.
Figure 4.
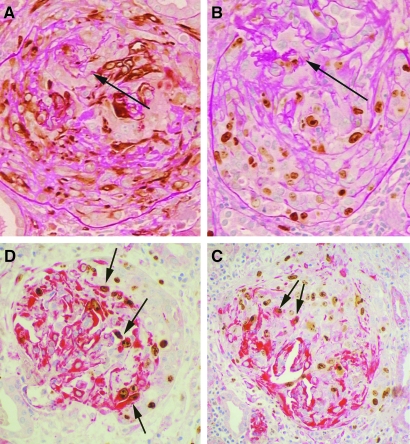
MIB1 expression in crescents. (A) Glomerulus with severe crescentic disease showing numerous nestin-positive cells in the crescent. (B) Same glomerulus stained for MIB1 showing that numerous cells within the crescent are cycling. The remainder of the glomerular tuft is marked by the arrow in panels A and B. (C) Double immunostaining for nestin (red cytoplasm) and MIB1 (brown nucleus) showing that the crescentic cells have a mixture of phenotypes. Several cells in the crescent (the right half of this glomerulus) are MIB1-positive, nestin-positive (arrows). Other cells include MIB1-positive cells that are nestin-negative, nestin-positive cells that are MIB1-negative, and cells that are nestin-negative, MIB1-negative. (D) Same immunostaining as in panel C showing a crescent occupying the entire glomerulus except for some residual tuft in the lower left. There are some MIB1-positive, nestin-positive cells (arrows), but the majority of MIB1-positive cells in crescents are negative for nestin. Original magnifications, A, B, immunoperoxidase with PAS counterstain; C, D, immunoperoxidase with hematoxylin counterstain; all ×200.
Mouse Models of Crescentic Glomerulonephritis
In the anti-GBM model, crescents were observed in approximately 25% of glomeruli at different stages of evolution as previously reported19 (Figure 5). In the von Hippel-Lindau mouse model of crescentic glomerulonephritis, crescents were observed in the majority of glomeruli, ranging from cellular to fibrous as previously reported20 (Figure 6). In both models, nestin-positive cells were present in most crescents, ranging from isolated cells to the majority of the cells in the crescent. Cells in crescents were found to be cycling based on Ki-67 immunostaining. The proportion of cycling cells in both models varied from none to about one third of cells. Double immunostaining for nestin and Ki-67 showed that there were double positive cells in crescents, as well as cells positive for one or the other antigen only, or double-negative. Of the cycling cells, nestin-negative outnumbered the nestin-positive in the von Hippel-Lindau model, whereas most of the cycling cells in the anti-GBM model were nestin-positive. In both models, however, nestin-positive cells were more often Ki-67-negative than positive.
Figure 5.
Crescent formation in mouse anti-GBM glomerulonephritis. Double immunostaining for nestin (red cytoplasm) and Ki-67 (brown nucleus) showing progressive stages in crescent formation. (A) Earliest stage is associated with nestin-positive podocytes bridging (arrow) to cells along the parietal basement membrane. (B through D) Progressively larger crescents in which many cells are nestin-positive and some are cycling. Immunoperoxidase with hematoxylin counterstain, original magnifications ×400.
Figure 6.
Crescent formation in von Hippel-Lindau mouse model of crescentic glomerulonephritis. Staining for nestin showing showing progressive stages in crescent formation. (A) Earliest stage is associated with nestin-positive podocytes bridging (arrow) to cells along the parietal basement membrane. (B and C) Progressively larger crescents in which many cells are nestin-positive. (D) Double immunostaining for nestin (red cytoplasm) and Ki-67 (brown nucleus) showing some nestin-positive cells are cycling. Immunoperoxidase with hematoxylin counterstain, original magnifications ×400.
DISCUSSION
In this study, we used nestin as a podocyte marker to investigate the podocyte contribution to crescents in glomerular disease. Nestin expression has recently been documented in rat,13 mouse,14 and human11,15 kidney, where expression in mature kidney is limited to podocytes. We have found that nestin expression in normal mature human glomeruli is confined to podocytes and that nestin expression persists in podocytes in every glomerular disease studied.11 Moreover, we have found that nestin is expressed in metanephric blastema that gives rise to the glomerular podocyte lineage. We postulated that nestin might serve as a reliable podocyte marker regardless of the stage of differentiation or dedifferentiation. In turn, this meant that nestin could be used as marker to investigate the extent of podocytic involvement in crescents, where podocytes cannot normally be identified due to loss of their characteristic terminally differentiated phenotype.3,8
We found that nestin-positive cells were consistently identified in glomerular crescents in all biopsies that we studied. The majority of crescents in any one biopsy contained positive cells, and these cells comprised up to 50% of the cells in a crescent. Although the nestin-positive crescentic cells did not express other podocyte differentiation antigens that we tested, such results are not unexpected given the findings of others.2,3,8 Previous studies have concluded that the most numerous cell is the parietal epithelial cell,1,2,7 with varying numbers of macrophages depending on the severity of the glomerular disease.3,4 However, in these studies, the epithelial cell that is the most abundant component in crescents has been assumed to be a parietal epithelial cell, largely because no podocyte marker can be identified.8
We think that the nestin-positive cells in crescents are podocytic in origin for several reasons. First, no other glomerular cells in normal human kidney consistently express nestin. Second, the α3 chain of type IV collagen was present in crescents in all of the cases studied. Based on the current understanding of the glomerulus, the α3 chain can only have come from podocytes because parietal epithelial cells do not produce this chain.21 Third, in mouse models of crescentic disease,8,20 from 25% to the majority of crescentic cells have been found to originate from podocytes, depending on the stage of the crescent studied, a result comparable to the number of nestin-positive cells we observed in human samples. We also obtained similar numbers of nestin-positive cells in crescents using 2 different mouse models of crescentic disease.19,20
Nestin staining was also found in cells that formed bridges between the glomerular tuft and parietal epithelial cells and/or PBM. These cells have been identified as podocytes in mouse models and in human crescentic disease.3,7,8 It has been postulated that podocytes then detach from the GBM and can migrate between parietal epithelial cells.3 The staining pattern that we noted for the α3 chain of type IV collagen would support both of these observations. One proposed function of nestin is to enable cells to migrate, particularly during differentiation or in response to injury.22 Nestin expression may play such a role in podocytes during crescent formation.
It is possible that some of the nestin-positive crescent cells are parietal epithelial cells that are more “embryonic” in phenotype because, in developing kidney, nestin is expressed in mesenchymal progenitors that give rise to both podocytes and parietal epithelial cells.11 However, parietal epithelial cells in mature kidney do not express nestin. A related concept is the “parietal podocyte.” Electron microscopy and immunohistochemical studies have identified cells with a podocytic phenotype in normal human normal kidney that cover part of the PBM at the vascular pole in continuity with visceral podocytes.23,24 It has been postulated that such cells proliferate and contribute to crescent formation.7 It is difficult to rule out this possibility, except to say that in normal human kidney, we found only very occasional nestin-positive cells along the PBM adjacent to the vascular pole and only in rare glomeruli11; thus, either parietal podocytes are quite uncommon cells or they do not normally express nestin. The nestin-negative cells in crescents could certainly be parietal epithelial cells because there is no specific marker for parietal epithelial cells that is available and only occasionally are these cells keratin-positive. We also observed limited keratin positivity in crescents in our study, and none of the nestin-positive cells coexpressed keratin. Alternatively, the nestin-negative cells might be podocytic cells that have lost nestin-expression.
The other cell component in crescents is the macrophage, and we therefore investigated whether nestin could be marking macrophages. Using CD68 as a macrophage marker, we found that the majority of cases of crescentic glomerulonephritis have CD68-positive cells in crescents, although such cells are less numerous than nestin-positive cells. While often these 2 cell types were intimately mixed, we showed that these were separate populations of cells based on several lines of evidence: 1) it was possible in some cases on serial sections to show that these were spatially separate populations of cells; 2) when such cells were double labeled for MIB1, virtually none of the positive cells was CD68-positive, compared with frequent cells that were nestin-positive; and 3) double labeling for nestin and CD68 showed no cells co-expressed these two antigens.
There is controversy, however, whether expression of CD68 is restricted to macrophages in glomeruli. One study found CD68-positive cells on the GBM and in crescents and concluded that damaged podocytes not only are crescent-forming cells but are phenotypically altered to express macrophage antigens.10 This concept was supported by glomerular culture results in which rat podocytes were reported to change into macrophagic cells.25 From this work and studies on the collapsing glomerulopathy came the concept of the “dysregulated” podocyte. Such podocytes are reported to lose podocyte-specific epitopes (e.g. podocalyxin, synaptopodin, WT1) (“dedifferentiation”) and acquire expression of various cytokeratins and/or macrophagic epitopes such as CD68 (“trans-differentiation”).26,27 Some podocytes could be shown to co-label for podocalyxin and CD68, or cytokeratin and CD68. Other studies have refuted the concept of the trans-differentiated podocyte. A mouse model of crescentic disease in which podocytes were genetically tagged showed that no tagged cells expressed macrophage markers.8 Similarly, a mouse model of focal segmental glomerulosclerosis using tagged podocytes found no evidence for trans-differentiation of podocyte-derived cells. Moreover, when podocytes were injured, proliferating parietal epithelial cells migrated onto the GBM, thereby mimicking podocytes by their location.9 Similar conclusions were reached in other studies on collapsing glomerulopathy.28,29 We also found no evidence to support the concept of trans-differentiation of podocytes toward a macrophage phenotype, as nestin was never coexpressed with CD68 in any human biopsy.
Nestin expression is most prominent in migrating and proliferating embryonal cells in the nervous system as well as some other sites.22,30 Nonetheless, nestin-positive cells can be found in adult tissues and may function as reserve cells that can be reactivated to proliferate and migrate in response to injury.22 The fact that nestin is expressed in the mature podocyte is an intriguing observation given that these cells are usually considered to be nonproliferating. We therefore investigated to what extent cells in crescents are cycling and which cells are cycling, with the goal to determine whether podocytes can become cycling cells in crescentic disease. Other studies have found that MIB1 is frequently expressed in crescents.2,7 In our study, both the human biopsies and the mouse model of crescentic disease, MIB1 (Ki-67) immunostaining readily identified cycling cells in crescents, with some crescents containing up to one third cycling cells. Double labeling confirmed that frequent nestin-positive cells in crescents were cycling, but only very rare CD68-positive cells and no keratin-positive cells. In the mouse models that we used, we were also able to show that there were proliferating cells in crescents that were nestin-positive. However, the largest population of cycling cells was nestin-, CD68-, and keratin-negative. Although these could be podocytes that are too dedifferentiated to express nestin, animal studies suggest some of these cells may be parietal epithelial cells.8,9,20
We conclude that podocytes form an integral cellular component of crescents in glomerular disease. When it becomes a crescentic cell, the podocyte can cycle and proliferate. The mechanism for the transformation from a terminally differentiated nondividing cell to a migratory cycling cell is unclear. Similarly unknown is whether nestin is related to this change or simply a marker for this cell type. Continued studies are needed to help clarify these processes.
CONCISE METHODS
Immunohistochemistry
Sections from human kidneys were obtained from the pathology archives at the Hospital for Sick Children with consent from the Research Ethics Board. A total of 35 renal biopsies were used in this part of the study from patient ages 1 to 17 yr. All specimens had been originally obtained for diagnosis of renal disease, and only material left over after completion of diagnostic workup was used to study nestin expression. Cases were selected solely on the basis of crescent formation and included a variety of glomerular diseases. In our center, renal biopsies are routinely fixed in formalin and cut at 2 μm. Tissue sections were then baked overnight at 60°C before immunostaining, dewaxed in xylene, and hydrated to distilled water through decreasing concentrations of alcohol. Sections underwent heat-induced epitope retrieval with 0.01 M citrate buffer, pH 6.0. All tissue sections were blocked for endogenous peroxidase and biotin. The following primary antibodies were used: human nestin (1:5000 dilution; Chemicon, Temecula, CA), WT1 (1:20 dilution, Novocastra, Newcastle-Upon-Tyne, UK), and podocin (1:1000 dilution; kindly provided by Dr Corinne Antignac16), CD31 (1:20 dilution; Dako, Carpinteria, CA), α-smooth muscle actin (1:40 dilution; Dako), low molecular weight cytokeratin (1:20 dilution; Becton-Dickinson Biosciences, Mountain View, CA), CD68 1:1000 dilution; Dako), and MIB1 (1:20; Dako). All antibodies were monoclonal except nestin and podocin. The staining was performed on the Benchmark auto-immunostainer (Ventana Medical Systems, Tuscon, AZ). Streptavidin-biotin complex immunodetection was performed using the Ventana iView DAB (3–3′-diaminobenzidine) Detection System. Sections were then counterstained lightly with hematoxylin to highlight nuclei or by periodic acid-Schiff to highlight the basement membranes.
For type IV collagen, 10 cases were studied for which there was snap-frozen tissue available. Sections were cut at 5-μm sections, fixed in acetone for 10 min, and stained using a monoclonal rat antibody to the α3 chain of human type IV collagen (1:100 dilution).17,18 Sections were pretreated for 10 min with a 100 mM acid-KCL solution (pH 1.5) to expose epitopes, followed by immunostaining on the Benchmark auto-immunostainer using the Ventana Open i-VIEW DAB detection system along with a 1:100 dilution of a biotinylated rabbit anti-rat secondary antibody (Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin.
Double Label Immunohistochemistry
Twelve cases with MIB1-positive crescentic cells were studied by double label immunohistochemistry. Slides were prepared as above, and immunostaining for MIB1 was carried out as described. For the second label, sections were reblocked for endogenous peroxidase and biotin before incubation with one of: nestin, CD68, or low molecular weight keratin (same dilutions as above) for 60 min at room temperature. Immunodetection was performed on the Benchmark auto-immunostainer using the Ventana Alkaline Phosphatase Enhanced Red kit. The chromogen in this kit results in a purple reaction product. Overlap of the brown and purple signals results in black color. Sections were then counterstained with hematoxylin. Sixteen cases with nestin-positive crescentic cells were studied by double label immunohistochemistry for CD68 using a similar approach. Slides were prepared as above, and immunostaining for nestin was carried out first as described. For the second label, sections were reblocked for endogenous peroxidase and biotin before incubation with CD68 for 60 min at room temperature.
Mouse Models of Crescentic Glomerulonephritis
Two models of crescentic glomerulonephritis were studied for nestin expression in crescents. The first was an anti-GBM antibody-mediated model, in which wild-type mice given antibody develop crescents by 7 d.19 Mice were examined at 7 to 28 d after inducing disease. The second model was one in which the von Hippel-Lindau gene was selectively deleted from podocytes.20 In this model, mice spontaneously develop necrotizing crescentic glomerulonephritis at 4 wk of age. Mice were examined at 5.5 wk of age; 5-μm formalin-fixed, paraffin-embedded tissue sections were mounted on positively charged microscope slides and then baked overnight at 60°C before immunostaining. Sections underwent heat-induced epitope retrieval and were blocked for endogenous peroxidase and biotin, as part of the automated staining protocol. The primary monoclonal antibody was mouse nestin (1:50 dilution; Chemicon, Temecula, CA), and Ki-67 (1:100, Labvision, Fremont, CA). The detection system used a rabbit biotinylated secondary antibody (Vector) with the Ventana i-VIEW DAB detection kit. Staining was performed on the Benchmark auto-immunostainer (Ventana). To avoid mouse-on-mouse cross reaction, a modification of the ARK (Dako) detection procedure was adapted for use on the immunostainer. Diaminobenzidine was used as a chromogen and sections were counterstained with hematoxylin.
For double staining for nestin and Ki-67, slides were prepared as above and immunostaining for Ki-67 was carried out as described. For nestin, sections were reblocked for endogenous biotin as part of the automated staining protocol. Immunodetection was performed on the Benchmark auto-immunostainer using the Ventana Alkaline Phosphatase Enhanced Red kit. Sections were then counterstained with hematoxylin.
DISCLOSURES
None.
Acknowledgments
Tissue from the mouse model of anti-GBM nephritis was kindly supplied by Dr Jeffrey Pippin and Dr Stuart Shankland, Division of Nephrology, University of Washington, Seattle, Washington.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Boucher A, Droz D, Adafer E, Noel L-H: Relationship between the integrity of Bowman's capsule and the composition of cellular crescents in human crescentic glomerulonephritis. Lab Invest 56: 526–533, 1987 [PubMed] [Google Scholar]
- 2.Nitta K, Horita S, Honda K, Uchida K, Watanabe T, Nihei H, Nagata M: Glomerular expression of cell cycle regulatory proteins in human crescentic glomerulonephritis. Virchows Arch 435: 422–427, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Le Hir M, Keller C, Eschmann V, Hähnel B, Hosser H, Kriz W: Podocyte bridges between the tuft and Bowman's capsule: an early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol 12: 2060–2071, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Rampino T, Gregorini M, Camussi G, Conaldi P, Soccio G, Maggio M, Bottelli A, Dal Canton A: Hepatocyte growth factor and its receptor Met are induced in crescentic glomerulonephritis. Nephrol Dial Transplant 20: 1066–1074, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Cattell V, Jamieson S: The origin of glomerular crescents in experimental nephrotoxic serum nephritis in the rabbit. Lab Invest 39: 584–590, 1978 [PubMed] [Google Scholar]
- 6.Lan H, Nikolic-Paterson D, Atkins R: Involvement of activated periglomerular leukocytes in the rupture of Bowman's capsule and glomerular crescent progression in experimental glomerulonephritis. Lab Invest 67: 743–751, 1992 [PubMed] [Google Scholar]
- 7.Bariety J, Bruneval P, Meyrier A, Mandet C, Hill G, Jacquot C: Podocyte involvement in human immune crescentic glomerulonephritis. Kidney Int 68: 1109–1119, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Moeller M, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman L: Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol 15: 61–67, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Asano T, Niimura F, Pastan I, Fogo A, Ichikawa I, Matsusaka T: Permanent genetic tagging of podocytes: fate of injured podocytes in a mouse model of glomerular sclerosis. J Am Soc Nephrol 16: 2257–2262, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Orikasa M, Iwanaga T, Kawachi H, Oyanagi A, Li P, Su Q, Kobayashi H, Nakayama H, Kikuchi H, Shimizu F: Monoclonal antibody against rat podocyte-derived macrophagic cells reacts with crescent-forming cells in an experimental model. Nephrology 8: 217–223, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Perry J, Ho M, Viero S, Zheng K, Jacobs R, Thorner P: The intermediate filament nestin is highly expressed in normal human podocytes and podocytes in glomerular disease. Pediatr Develop Pathol 10: 369–382, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Lendahl U, Zimmerman L, McKay R: CNS stem cells express a new class of intermediate filament protein. Cell 60: 585–595, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Zou J, Yaoita E, Watanabe Y, Yoshida Y, Nameta M, Li H, Qu Z, Yamamoto T: Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch 448: 485–492, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Boyle S, Zhao M, Su W, Takahashi K, Davis L, DeCaestecker M, Takahashi T, Breyer M, Hao C-M: Differential expression of the intermediate filament protein nestin during renal development and its localization in adult podocytes. J Am Soc Nephrol 17: 1283–1291, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Bertelli E, Regoli M, Fonzi L, Occhini R, Mannucci S, Ermini L, Toti P: Nestin expression in adult and developing human kidney. J Histochem Cytochem 55: 411–421, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Roselli S, Boute N, Sich M, Benessy F, Attie T, Gubler M, Antignac C: Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol 160: 131–139, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ninomiya Y, Kagawa M, Iyama K, Naito I, Kishiro Y, Seyer J, Sugimoto M, Oohashi T, Sado Y: Differential expression of two basement membrane collagen genes, COL4A6 and COL4A5, demonstrated by immunofluorescence staining using peptide-specific monoclonal antibodies. J Cell Biol 130: 1219–1229, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sado Y, Kagawa M, Kishiro Y, Sugihara K, Naito I, Seyer J, Sugimoto M, Oohashi T, Ninomiya Y: Establishment by the rat lymph node method of epitope-defined monoclonal antibodies recognizing the six different α chains of human type IV collagen. Histochem Cell Biol 104: 267–275, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Ophascharoensuk V, Fero M, Hughes J, Roberts J, Shankland S: The cyclin-dependent kinase inhibitor p27Kip1 safeguards against inflammatory injury. Nat Med 4: 575–580, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Ding M, Cui S, Li C, Jothy S, Haase V, Steer B, Marsden P, Pippin J, Shankland S, Rastaldi M, Cohen P, Kretzler M, Quaggin S: Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med, 12: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hudson B: The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J Am Soc Nephrol 15: 2514–2527, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov K, Tarasova Y, Wersto R, Boheler K, Wobus A: Nestin expression: a property of multi-lineage progenitor cells? Cell Mol Life Sci 61: 2510–2522, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bariety J, Mandet C, Hill G, Bruneval P: Parietal podocytes in normal human glomeruli. J Am Soc Nephrol 17: 2770–2780, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Gibson I, Downie I, Downie T, Han S, More I, Lindop G: The parietal podocyte: a study of the vascular pole of the human glomerulus. Kidney Int 41: 211–214, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Orikasa M, Iwanaga T, Takahashi-Iwanaga H, Kozima K, Shimizu F: Macrophagic cells outgrowth from normal rat glomerular culture: possible metaplastic change from podocytes. Lab Invest 75: 719–733, 1996 [PubMed] [Google Scholar]
- 26.Albaqumi M, Soos T, Barisoni L, Nelson P: Collapsing glomerulopathy. J Am Soc Nephrol 17: 2854–2863, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Bariety J, Bruneval P, Hill G, Irinopoulou T, Mandet C, Meyrier A: Posttransplantation relapse of FSGS is characterized by glomerular epithelial cell transdifferentiation. J Am Soc Nephrol 12: 261–274, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Dijkman H, Smeets B, Van der Laak J, Steenbergen E, Wetzels J: The parietal epithelial cell is crucially involved in human idiopathic focal segmental glomerulosclerosis. Kidney Int 68: 1562–1572, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Dijkman H, Weening J, Smeets B, Verrijp K, van Kuppevelt T, Assmann K, Steenbergen E, Wetzels J: Proliferating cells in HIV and pamidronate-associated collapsing focal segmental glomerulosclerosis are parietal epithelial cells. Kidney Int 70: 338–344, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Sjoberg G, Jiang W, Ringertz N, Lendahl U, Sejersen T: Colocalization of nestin and vimentin/desmin in skeletal muscle cells demonstrated by three-dimensional fluorescence digital imaging microscopy. Exp Cell Res 214: 447–458, 1994 [DOI] [PubMed] [Google Scholar]