Abstract
T-bet is a transcription factor that is essential for T helper (Th)1 lineage commitment and optimal IFN-γ production by CD4+ T cells. We examined the role of T-bet in the development of experimental crescentic glomerulonephritis, which is induced by Th1-predominant, delayed-type hypersensitivity-like responses directed against a nephritogenic antigen. Anti-glomerular basement membrane (GBM) glomerulonephritis was induced in T-bet−/− and wild-type C57BL/6 mice. Compared with wild-type controls, renal injury was attenuated in T-bet−/− mice with glomerulonephritis, evidenced by less proteinuria, glomerular crescents, and tubulointerstitial inflammation. Accumulation of glomerular CD4+ T cells and macrophages was decreased, and was associated with reduced intrarenal expression of the potent Th1 chemoattractants CCL5/RANTES and CXCL9/Mig. Supporting the pro-inflammatory nature of T-bet signaling, assessment of systemic immunity confirmed that T-bet−/− mice had a reduction in Th1 immunity. The kinetic profile of T-bet mRNA in wild-type mice supported the hypothesis that T-bet deficiency attenuates renal injury in part by shifting the Th1/Th2 balance away from a Th1 phenotype. Expression of renal and splenic IL-17A, characteristically expressed by the Th17 subset of effector T cells, which have been implicated in the pathogenesis of autoimmune disease, was increased in T-bet−/− mice. We conclude that T-bet directs Th1 responses that induce renal injury in experimental crescentic glomerulonephritis.
The initial description of functionally distinct T helper (Th) cell subsets, Th1 and Th2,1,2 has provided a useful framework for understanding various diseases. Th1 cells are characterized by the production of pro-inflammatory cytokines (IFN-γ, IL-2, and lymphotoxin-α), mediate cellular immune responses, and play important roles in autoimmunity and clearance of intracellular organisms. In contrast, Th2 cells produce IL-4, IL-5, IL-10, and IL-13, and play an important role in allergy and clearance of helminth infections. Although the precise molecular mechanisms underlying Th1 development remain incompletely understood, the process is known to involve signals provided by the T cell receptor, costimulatory and chemokine–chemokine receptor signaling pathways3 and the cytokine milieu at the time of antigen presentation (par-ticularly IFN-γ, IL-12, and IL-18).4–8 Most recently, T helper cell subsets have been expanded to include Th17 cells, a distinct T helper cell lineage characterized by the production of IL-179 and implicated in the pathogenesis of autoimmune diseases, such as experimental autoimmune encephalomyelitis,10,11 a model of multiple sclerosis, and collagen-induced arthritis.12
T-bet (Tbx21), a novel member of the T-box family of transcription factors, is a key regulator of Th1 lineage commitment and is required for optimal production of IFN-γ by CD4+ T cells.13 It is primarily induced in response to IFN-γ/Stat1 signaling, up-regulating expression of both IFN-γ and the IL-12 receptor β2 chain (IL-12Rβ2), thereby enabling IL-12-induced stabilization of IFN-γ production and activation of the IL-18 signaling pathway. IL-12,14 IL-15,15 IL-21,15 and IL-2716,17 have also been identified as being able to induce T-bet expression in CD4+ T cells, whereas TGF-β is able to suppress T-bet expression.18 Although its role in CD4+ T cells has been most extensively investigated, T-bet is expressed in other immune cells, including CD8+ T cells,19 B cells,20 dendritic cells,21 and NK cells.22
Glomerulonephritis (GN) is a common cause of end-stage kidney disease worldwide, and crescentic GN is the most injurious form. Evidence from human and experimental forms of crescentic GN suggests that crescent formation is driven by a Th1-predominant nephritogenic immune response.23–25 In patients with systemic lupus erythematosus, T-bet expression in the urine and kidneys of patients correlates with clinically and histologically active lupus nephritis.26 Studies in T-bet-deficient, lupus-prone mice have found that T-bet is dispensable for the development of T cell-mediated autoimmune manifestations (such as salivary gland and hepatic infiltrates) but is required for IgG class switching, pathogenic autoantibody production, and the development of immune-complex GN.27 Intrarenal expression of T-bet in human renal biopsy specimens has been demonstrated to be predictive of functionally significant renal allograft rejection.28 The hypothesis that T-bet plays a pathogenic role, via Th1 polarization of the immune response, in the development of anti-glomerular basement membrane (GBM) GN, a model that is driven by a CD4+ Th1-predominant immune response directed toward a planted nephritogenic antigen, was addressed in T-bet−/− mice and C57BL/6, wild-type (WT) controls. Immune responses and renal injury were compared, and the involvement of Th17 cells was sought by measurement of immune and renal IL-17A mRNA expression.
Results
T-bet Deficiency Confers Protection Against Renal Injury
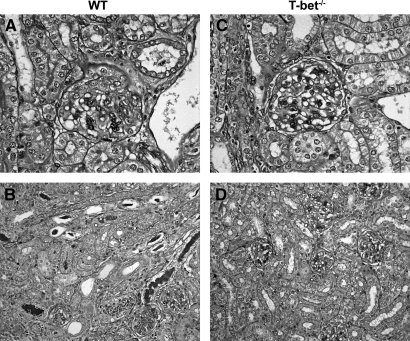
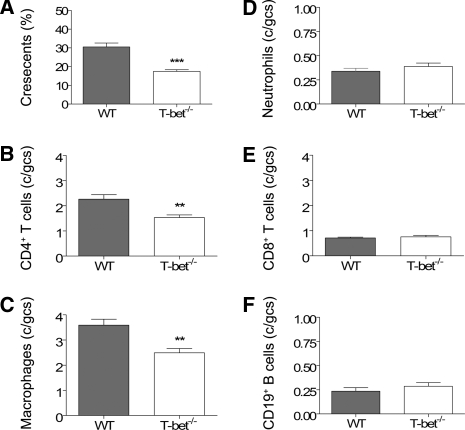
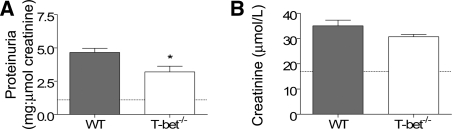
Twenty-one days after intravenous injection with sheep anti-mouse GBM globulin, WT mice developed diffuse proliferative and crescentic GN with moderately severe tubulointerstitial damage (Figure 1, A and B) characterized by elevated serum creatinine (35 ± 2 μmol/L) and abnormal proteinuria (4.7 ± 0.3 mg protein:μmol creatinine). Renal injury was attenuated in T-bet−/− mice with GN (Figure 1, C and D) as indicated by fewer glomerular crescents, CD4+ T cells, and macrophages (Figure 2, A through C) and less interstitial CD4+ T cells (21.16 ± 1.78 versus 29.94 ± 2.67 c/hpf, P < 0.05). There were no differences in glomerular accumulation of neutrophils, CD8+ T cells, or CD19+ B cells (Figure 2, D through F). Semiquantitative analysis of periodic acid-Schiff (PAS)-stained sections indicated that T-bet−/− mice had a lower tubulointerstitial inflammation score than WT controls (1.8 ± 0.1 versus 2.2 ± 0.1, P < 0.05) but tubulointerstitial injury score was unchanged (1.4 ± 0.1 versus 1.4 ± 0.1). T-bet−/− mice also had less proteinuria (Figure 3, A) and a trend toward lower serum creatinine (Figure 3, B).
Figure 1.
Histologic renal injury 21 d after intravenous administration of sheep anti-mouse GBM globulin. WT mice with experimental crescentic GN developed diffuse proliferative and crescentic GN (A) and moderately severe tubulointerstitial injury and inflammation (B), which was diminished in T-bet−/− mice (C and D). Representative photomicrographs from WT (original magnifications: A, ×400; B, ×200) and T-bet−/− mice (original magnifications: C, ×400; D, ×200), respectively.
Figure 2.
Glomerular crescent formation and accumulation of cellular effectors of injury in WT and T-bet−/− mice. At day 21, T-bet−/− mice had fewer glomerular crescents (A) and reduced glomerular infiltration with CD4+ T cells (B) and macrophages (C), compared with WT controls. There were no differences in glomerular accumulation of neutrophils (D), CD8+ T cells (E), or CD19+ B cells (F). **P < 0.01, ***P < 0.001.
Figure 3.
Functional markers of renal injury in mice with autologous phase anti-GBM GN. WT mice with experimental crescentic GN developed renal injury characterized by elevated proteinuria (A) and serum creatinine (B). Proteinuria was less in T-bet−/− mice compared with WT controls, and there was a trend toward lower serum creatinine (P = 0.11). Dotted lines represent mean values from normal WT mice without GN. *P < 0.05.
Systemic Immune Responses Are Polarized Away From a Th1 Phenotype in T-bet−/− Mice
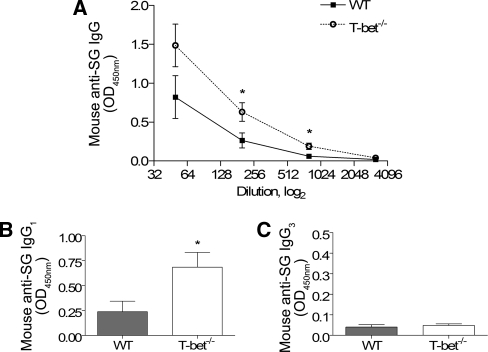
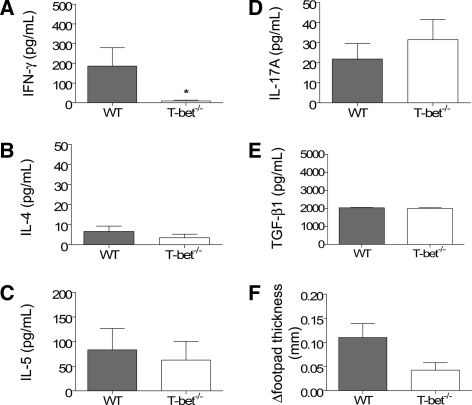
As the nature and direction of the systemic immune response can influence the expression of glomerular disease, the systemic immune response to the nephritogenic antigen (i.e. sheep globulin) was measured. WT mice with GN developed significant antigen-specific humoral immune responses as assessed by circulating antibody levels. T-bet−/− mice with GN had higher titers of antigen-specific IgG and IgG1, but not IgG3 (Figure 4). Antigen-stimulated splenocyte production of IFN-γ was markedly reduced in T-bet−/− mice, but there were no differences in IL-4, IL-5, IL-17A, or TGF-β1 production (Figure 5, A through E). T-bet deficiency was associated with a trend toward reduction in dermal delayed-type hypersensitivity (DTH) to the nephritogenic antigen (P = 0.05; Figure 5, F). These results are consistent with a reduction in Th1 immunity.
Figure 4.
Humoral immune responses in WT and T-bet−/− mice. Serum antigen-specific titers of IgG (A) and IgG1 (B) in T-bet−/− mice were increased but not IgG3 (C), compared with WT controls. *P < 0.05.
Figure 5.
Systemic immune responses in WT and T-bet−/− mice. Antigen-stimulated splenocyte production of IFN-γ (A) was reduced in T-bet−/− mice; however, there were no differences in IL-4 (B), IL-5 (C), IL-17A (D), or TGF-β1 (E) production, compared with WT controls. Dermal delayed-type hypersensitivity (DTH) responses to sheep globulin (F) were reduced in the absence of T-bet but not to the level of significance (P = 0.05). *P < 0.05.
Intrarenal mRNA Expression of Cytokines and Chemokines
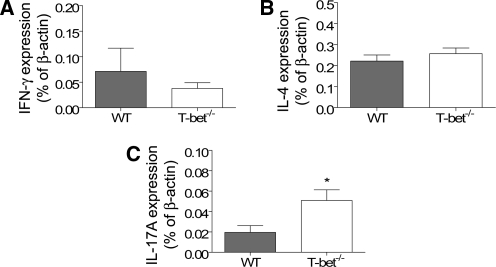
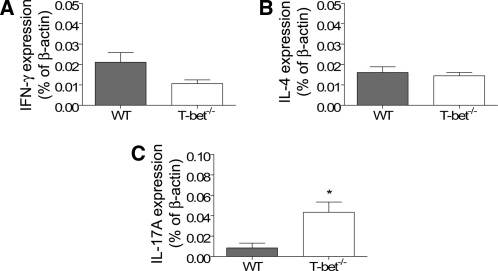
To investigate mechanisms by which T-bet deficiency confers protection against renal injury in experimental crescentic GN, we analyzed the intrarenal mRNA expression of cytokines and chemokines by real-time polymerase chain reaction (PCR) and RNase protection assay. Compared with WT controls, expression of CCL5/RANTES and CXCL9/Mig was significantly reduced (Table 1). These are potent Th1 chemoattractants, and their reduction was associated with reduced CD4+ T cell accumulation. IFN-γ and IL-4 mRNA expression in T-bet−/− mice was not significantly different, but IL-17A expression was increased (Figure 6). In addition, expression of MIP-1α and −1β was increased (Table 1).
Table 1.
Intrarenal chemokine mRNA expression in mice 21 days after injection of sheep anti-mouse GBM globulin
| WT (mRNA, % of housekeeping gene) | T-bet−/− (mRNA, % of housekeeping gene) | |
|---|---|---|
| CCL5/RANTES | 7.76 ± 1.08 | 2.87 ± 0.17a |
| CXCL9/Mig | 4.86 ± 1.43 | 1.73 ± 0.31b |
| XCL1/Lymphotactin | 0.17 ± 0.04 | 0.12 ± 0.02 |
| CXCL10/IP-10 | 3.54 ± 0.32 | 3.04 ± 0.42 |
| CCL1/TCA-3 | 0.79 ± 0.11 | 0.88 ± 0.13 |
| CCL3/MIP-1α | 0.34 ± 0.03 | 0.87 ± 0.09c |
| CCL4/MIP-1β | 0.28 ± 0.07 | 0.51 ± 0.06d |
| CXCL2/MIP-2 | 2.28 ± 0.42 | 3.58 ± 1.45 |
| CCL2/MCP-1 | 6.89 ± 1.26 | 11.98 ± 3.11 |
Data are percentage of housekeeping gene (β-actin for CXCL9/Mig and L32 for other chemokines). T-bet−/− mice had reduced intrarenal mRNA expression of CCL5/RANTES and CXCL9/Mig and increased CCL3/MIP-1α and CCL4/MIP-1β.
P = 0.0003.
P = 0.04.
P = 0.0002.
P = 0.03.
Figure 6.
Intrarenal mRNA expression of prototypical Th1 (A), Th2 (B), and Th17 (C) cytokines at day 21. Kidney mRNA expression of IFN-γ and IL-4 was unchanged in T-bet−/− mice, but expression of IL-17A was increased. *P < 0.05.
Temporal Expression of T-bet
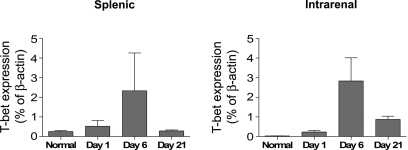
Given the central role of T-bet in directing Th1 lineage commitment, we examined the nature of the immune response in experimental crescentic GN by analyzing the splenic mRNA expression of T-bet in WT mice throughout the course of the disease. T-bet expression in the spleen increased after administration of sheep anti-mouse GBM globulin, peaking at day 6 (Figure 7). The kinetic profile of T-bet mRNA expression in the kidney was similar (Figure 7).
Figure 7.
Kinetic profile of splenic and intrarenal T-bet mRNA expression in experimental crescentic GN. T-bet mRNA expression in both the spleen and kidney increases after injection of sheep anti-mouse GBM globulin in WT mice, peaking on day 6.
Effect of T-bet Deficiency on CD4+ T Cell and CD19+ B Cell Proliferation, Apoptosis and Activation, and Splenic mRNA Expression of Cytokines and Chemokine Receptors
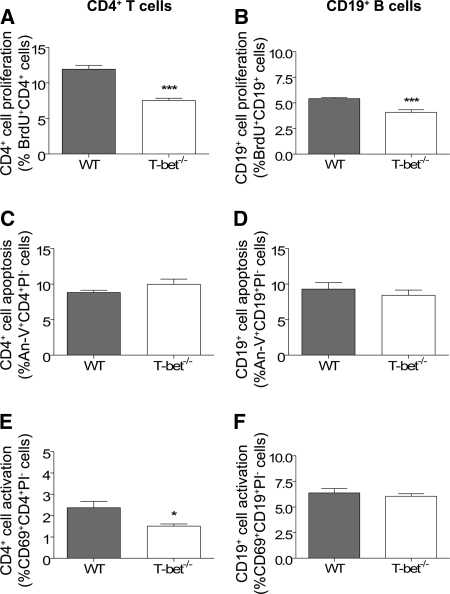
To further dissect the mechanisms by which T-bet−/− mice are relatively protected from renal injury, we next examined immune responses at an earlier time point when adaptive immune responses are being established and when T-bet expression is greatest. Six days after administration of anti-GBM globulin, splenic CD4+ T cell and CD19+ B cell proliferation were diminished in T-bet−/− mice (Figure 8, A and B) with no effect on apoptosis (Figure 8, C and D). T-bet−/− mice also had less activated CD4+ T cells, but not CD19+ B cells (Figure 8, E and F). Analyses of cytokine and chemokine mRNA receptor expression in the spleen were performed by real-time PCR and RNase protection assay, respectively. T-bet deficiency was associated with a trend toward lower IFN-γ mRNA expression, no difference in IL-4 expression, and increased expression of IL-17A (Figure 9). Expression of CXCR3, a chemokine receptor that is preferentially expressed on Th1 cells, was diminished in T-bet−/− mice compared with WT controls, while expression of CCR1 was augmented (Table 2).
Figure 8.
Effect of T-bet deficiency on CD4+ T cell and CD19+ B cell responses. Splenic CD4+ T cell and CD19+ B cell proliferation, apoptosis, and activation were assessed by flow cytometric analysis of in vivo bromodeoxyuridine (BrdU) incorporation, Annexin-V (An-V) staining, and CD69 expression, respectively, 6 d after administration of anti-GBM globulin. In comparison to WT controls, T-bet−/− mice exhibited reduced proliferation of CD4+ T cells (A) and CD19+ B cells (B) with no effect on apoptosis (C and D). There were less activated splenic CD4+ T cells (E), but not CD19+ B cells (F), in T-bet−/− mice compared with WT mice. *P < 0.05, ***P < 0.001.
Figure 9.
Cytokine mRNA expression in spleens of mice 6 d after injection of sheep anti-mouse GBM globulin. T-bet−/− mice had a trend toward lower expression of IFN-γ (A; P = 0.06), no difference in IL-4 expression (B), and higher expression of IL-17A (C) than WT controls. *P < 0.05.
Table 2.
Splenic chemokine receptor mRNA expression 6 days after injection of sheep anti-mouse GBM globulin, showing that T-bet−/− mice had lower mRNA expression of CXCR3 and increased CCR1
| WT (mRNA, % of L32) | T-bet−/− (mRNA, % of L32) | |
|---|---|---|
| Preferentially expressed on Th1 cells | ||
| CXCR3 | 0.91 ± 0.11 | 0.27 ± 0.02a |
| CCR5 | 0.80 ± 0.14 | 0.90 ± 0.11 |
| Preferentially expressed on Th2 cells | ||
| CCR3 | 2.40 ± 0.54 | 3.30 ± 0.59 |
| CCR4 | 0.18 ± 0.05 | 0.23 ± 0.06 |
| Not preferentially expressed | ||
| CCR1 | 0.78 ± 0.18 | 2.49 ± 0.45b |
| CCR2 | 0.94 ± 0.14 | 1.43 ± 0.25 |
P = 0.0005.
P = 0.003.
Histologic Renal Injury at Earlier Time Points
We also examined histologic renal injury 1 d (during the heterologous phase of nephritis) and 6 d following intravenous injection with sheep anti-mouse GBM globulin. Compared with WT controls, T-bet−/− mice had similar numbers of abnormal glomeruli, glomerular and interstitial CD4+ T cells, and glomerular macrophages, and a similar degree of tubulointerstitial injury and inflammation (Table 3).
Table 3.
Histologic renal injury in WT and T-bet−/− mice 1 and 6 days after injection of sheep anti-mouse GBM globulin
| Day 1 | Day 6 | |
|---|---|---|
| Abnormal glomeruli (%) | ||
| WT | 27.0 ± 2.5 | 54.3 ± 2.9 |
| T-bet−/− | 27.5 ± 2.9 | 52.6 ± 1.6 |
| Glomerular CD4+ T cells (c/gcs) | ||
| WT | 0.13 ± 0.01 | 0.23 ± 0.05 |
| T-bet−/− | 0.16 ± 0.03 | 0.16 ± 0.02 |
| Interstitial CD4+ T cells (c/hpf) | ||
| WT | 0.85 ± 0.06 | 5.38 ± 0.59 |
| T-bet−/− | 0.73 ± 0.08 | 3.73 ± 0.30a |
| Macrophages (c/gcs) | ||
| WT | 0.25 ± 0.02 | 0.46 ± 0.04 |
| T-bet−/− | 0.28 ± 0.03 | 0.49 ± 0.03 |
| Tubulointerstitial inflammation (0–4) | ||
| WT | 0.78 ± 0.05 | 0.78 ± 0.06 |
| T-bet−/− | 0.70 ± 0.04 | 0.73 ± 0.06 |
| Tubulointerstitial injury (0–4) | ||
| WT | 0.60 ± 0.07 | 0.61 ± 0.06 |
| T-bet−/− | 0.55 ± 0.10 | 0.74 ± 0.06 |
At the 6-day time point, T-bet−/− mice had less infiltrating interstitial CD4+ T cells than WT controls. At both the 1-day and 6-day time points, T-bet−/− mice had similar numbers of abnormal glomeruli, infiltrating glomerular leukocytes, and tubulointerstitial damage, compared with WT controls.
P = 0.03.
Discussion
In planted antigen models of GN, CD4+ T helper cells play a critical role in both the initiation of the nephritogenic immune response29 and the effector phase of the disease.25,30 The Th1/Th2 bias of the CD4+ T cell response also affects the pattern and extent of glomerular injury such that deficiency or neutralization of Th1 or Th2 cytokines leads to amelioration or exacerbation of disease, respectively.25,31–35 Significant evidence therefore suggests that crescentic GN is driven by a CD4+, Th1-directed nephritogenic immune response. Transcription factors play a pivotal role in determining immune cell fate and function and most likely exert significant influence on the development of crescentic GN.
These studies investigated the role of T-bet in autologous phase, anti-GBM GN, a Th1-driven model of crescentic GN. T-bet−/− mice were protected from renal injury, both functionally (proteinuria) and histologically (glomerular crescents, tubulointerstitial inflammation and infiltration with glomerular CD4+ T cells and macrophages and interstitial CD4+ T cells). These results indicate that T-bet plays an important pathogenic role in the development of autologous phase, anti-GBM GN.
T-bet plays a key role in the development and maintenance of Th1 cells, the signature cytokine of which is IFN-γ. In our study, T-bet deficiency was associated with a marked reduction in splenocyte production of IFN-γ but with no effect on IL-4, IL-5, or TGF-β1 production. There was a trend toward diminished dermal DTH to the nephritogenic antigen (P = 0.05). Humoral responses were augmented in T-bet−/− mice, as indicated by elevated titers of circulating IgG and IgG1 with no difference in IgG3 production. Supporting the pro-inflammatory nature of the T-bet signaling pathway, T-bet deficiency was associated with reduced CD4+ T cell proliferation and activation. We also analyzed the kinetic profile of T-bet mRNA expression in WT mice and found that splenic and intrarenal mRNA expression of T-bet was increased throughout the course of experimental crescentic GN, peaking at the 6 d time point. These findings are consistent with the central role of T-bet in polarizing naïve CD4+ T cells to a Th1 phenotype, a process that tends to occur relatively early in the adaptive immune response and supports the evidence that experimental crescentic GN is directed by Th1 nephritogenic immunity. Taken together, these results suggest that one mechanism by which T-bet deficiency abrogates renal injury is by skewing the Th1/Th2 balance away from a Th1 phenotype.
Chemokines play an important role in the recruitment of injurious CD4+ T cells into the inflamed kidney.36 We analyzed the mRNA expression of chemokines in the kidney and chemokine receptors in the spleen to investigate mechanisms of leukocyte recruitment and renal injury in the absence of T-bet. There was a significant reduction in intrarenal mRNA expression of the CD4+ Th1 cell chemoattractants, CCL5/RANTES and CXCL9/Mig. Reduced splenic mRNA expression of CXCR3 and reduced CD4+ T cell proliferation and activation suggests that T-bet deficiency led to reduced Th1 effectors. These 2 observations are likely to explain the reduced accumulation of Th1 effectors in the kidneys of T-bet−/− mice. T-bet deficiency was also associated with increased intrarenal mRNA expression of CCL3/MIP-1α and CCL4/MIP-1β. The biologic significance of this is uncertain but, given that CCL3/MIP-1α and CCL4/MIP-1β are neutrophil chemoattractants, their up-regulation could conceivably explain the persistence of glomerular neutrophils despite a reduction in CD4+ T cells and macrophages.
Recent evidence indicates that T-bet plays a pathogenic role in experimental models of other Th1-type diseases, including inflammatory bowel disease37,38 and experimental autoimmune encephalomyelitis.39 Th17 cells, a recently identified subset of T helper cells, are characterized by the production of IL-17A (in addition to IL-6 and granulocyte macrophage colony stimulating factor) and have also been implicated in the pathogenesis of autoimmune disease.9 Development of Th17 cells occurs in the presence of IL-6 and TGF-β9,40 while their growth and survival are dependent on IL-23. In our study, T-bet−/− mice had enhanced mRNA expression of the pro-inflammatory cytokine, IL-17A, both locally (in the kidney) and systemically (in the spleen), suggesting that its expression is counter-regulated by T-bet signaling in this form of GN. In GN, the precise role of IL-17A and Th17 cells remains to be defined.
In summary, these studies indicate that endogenous T-bet directs injurious, nephritogenic, Th1 responses and promotes the development of renal injury in experimental crescentic GN.
Concise Methods
Experimental Design
T-bet−/− mice on a C57BL/6 background were obtained from the Jackson Laboratory (Bar Harbor, ME) and bred at Monash Medical Centre (Melbourne, Australia). C57BL/6, WT mice were obtained from Monash Animal Services (Melbourne, Australia). Sheep anti-mouse GBM globulin was prepared as described previously.31 Nonaccelerated, autologous phase, anti-GBM GN was induced in age-matched, 10- to 12-wk-old, male mice by intravenous administration of 20 mg sheep anti-mouse GBM globulin (day 0). Immune responses, renal injury, and mRNA expression of T-bet were assessed on days 1, 6, and 21 in T-bet−/− (n = 4, 7, and 8, respectively) and WT (n = 4, 8, and 7, respectively) mice. Studies were approved by the Monash University Animal Ethics Committee and performed in accordance with the National Health and Medical Research Council of Australia guidelines. Results are expressed as means ± SEM. Unpaired t test was used for statistical analysis (GraphPad Prism; GraphPad Software, San Diego, CA). Differences were considered to be statistically significant if P < 0.05.
Assessment of Renal Injury
Glomerular abnormalities were assessed on PAS-stained, Bouin's-fixed, 3-μm-thick, paraffin-embedded sections. Abnormalities included crescent formation (considered to be apparent when glomeruli exhibited 2 or more layers of cells in Bowman's space), segmental proliferation, necrosis, hyalinosis, or capillary wall thickening. The proportion of glomeruli affected was determined by examining a minimum of 50 glomeruli per mouse for abnormalities, as previously published.41 Urine was collected, using metabolic cages, during the 24-h period before death, and serum was obtained from blood collected on the day of death. Serum and urine creatinine concentrations were measured by an enzymatic creatininase assay. Proteinuria was determined by a modified Bradford method42 and expressed as milligrams per micromols of creatinine.
CD4+ T cells, CD8+ T cells, macrophages, neutrophils, and B cells were demonstrated by immunoperoxidase staining of 6-μm-thick, acetone-fixed, frozen kidney sections. The primary monoclonal antibodies used were GK1.5 for CD4+ T cells (anti-mouse CD4; American Type Culture Collection [ATCC], Manassas, VA), 53-6.7 for CD8+ T cells (anti-mouse CD8; ATCC), FA/11 for macrophages (anti-mouse CD68; generously provided by Dr. Gordon L Koch, Medical Research Council Laboratory of Molecular Biology, Cambridge, England), RB6–8C5 for neutrophils (anti-Gr-1; DNAX, Palo Alto, CA), and 1D3 for B cells (anti-mouse CD19; BD Biosciences, North Ryde, Australia), and the secondary antibody used was rabbit anti-rat biotin (BD Biosciences). Endogenous biotin was blocked using a biotin-blocking system (Dako, Botany, Australia), and CD4+ T cells and macrophages were detected using an avidin-biotin complex detection system (Dako). A minimum of 20 consecutively viewed glomeruli and a minimum of 10 high-power cortical interstitial fields (excluding perivascular regions) were assessed per animal, and results expressed as cells per glomerular cross section (c/gcs) or cells per high-power filed (c/hpf), as described previously.35
Semiquantitative analysis of tubulointerstitial damage was performed on PAS-stained sections and scored according to previously described criteria.43 Ten randomly selected cortical areas from each animal were examined at ×200 magnification using a 10-mm2 graticule. Tubulointerstitial injury was defined as tubular dilation, tubular atrophy, sloughing of tubular epithelial cells, or thickening of the basement membrane. Interstitial inflammation was defined according to the degree of leukocytic infiltration in the interstitium. Tubulointerstitial injury and interstitial inflammation were each assessed separately and graded according to a scale of 0 to 4: grade 0, no tubulointerstitial injury, no interstitial inflammation; grade 1, less than 25% of the tubulointerstitium injured, minimal tubulointerstitial inflammation; grade 2, 25% to 50% of the tubulointerstitium injured, mild tubulointerstitial inflammation; grade 3, 51% to 75% of the tubulointerstitium injured, moderate tubulointerstitial inflammation; grade 4, more than 75% of the tubulointerstitium injured, diffuse tubulointerstitial inflammation.
Splenocyte Cytokine Production and Dermal DTH
Spleens were aseptically removed from mice and single-cell suspensions obtained. Splenocytes (4 × 106 cells/ml per well) were cultured in RPMI/10% FCS with protein G-purified normal sheep IgG (10 μg/ml) for 72 h at 37°C. Concentrations of IFN-γ, IL-4, and IL-5 in splenocyte supernatants were measured by ELISA, as described previously.35,44 The antibodies used were rat anti-mouse IFN-γ (R4–6A2; BD Pharmingen, San Diego, CA), biotinylated rat anti-mouse IFN-γ (XMG1.2; BD Pharmingen), rat anti-mouse IL-4 (11B11; ATCC), biotinylated rat anti-mouse IL-4 (BVD6; DNAX Research Institute, Palo Alto, CA), rat anti-mouse IL-5 (TRFK5; R&D systems, Minneapolis, MN), and biotinylated rat anti-mouse IL-5 (TRFK4; R&D systems). Concentrations of IL-17A and TGF-β1 were measured by ELISA using an IL-17 DuoSet and TGF-β1 ELISA kit (R&D Systems), respectively, as per the manufacturer's protocol. To measure dermal DTH, mice were challenged by intradermal injection of 0.5 mg of sheep globulin into the left plantar footpad or the same dose of horse globulin as an irrelevant control antigen into the contralateral footpad, 24 h before the end of experiments. DTH was quantified 24 h later by measurement of the difference in footpad thickness (Δmm) using a micrometer (Mitutoyo, Kawasaki-shi, Japan).
Circulating Antigen-Specific Antibody Levels
Circulating serum antigen-specific Ig titers were assessed by ELISA as described previously,44 using horseradish peroxidase-conjugated sheep anti-mouse IgG (1:50 to 1:3200 dilutions, Amersham Biosciences, Rydalmere, Australia), goat anti-mouse IgG1 (1:100 dilution, Silenus, Boronia, Australia), and biotinylated rat anti-mouse IgG3 (1:50 dilution, BD Pharmingen). Results are expressed as the mean OD450 ± SEM.
Proliferation, Apoptosis, and Activation of CD4+ T Cells and CD19+ B Cells
For assessment of proliferation, apoptosis, and activation by flow cytometry, spleens were obtained from T-bet−/− and WT mice 6 d after injection of sheep anti-mouse GBM globulin. For measurements of proliferation, mice were administered an intraperitoneal injection of 1 mg of bromodeoxyuridine (BrdU; Sigma-Alrdrich, St. Louis, MO) 48, 36, 24, and 12 h before day 6. Proliferation, apoptosis, and activation were assessed by analysis of intracellular BrdU incorporation (results expressed as percentage of CD4+ or CD19+ cells that are BrdU+), Annexin-V staining (results expressed as percentage of CD4+ or CD19+ cells that are Annexin-V+ and propidium iodide negative), and CD69 expression (results expressed as percentage of CD4+ or CD19+ cells that are CD69+), respectively, as described previously.45,46 Antibodies used were allophycocyanin-Cy7 (APC-Cy7)-conjugated anti-CD4, phycoerythrin (PE)-conjugated anti-CD4, FITC (FITC)-conjugated anti-CD4, PE-conjugated anti-CD19, APC-conjugated anti-CD19, FITC-conjugated anti-BrdU with DNase, PE-conjugated anti-CD69 mAb (BD Pharmingen), and fluorescence-conjugated Annexin-V (Roche Diagnostics, Penzberg, Germany).
Renal and Splenic mRNA Expression of T-bet, Cytokines, Chemokines, and Chemokine Receptors
Spleens and kidneys were obtained from T-bet−/− and WT mice before and on days 1, 6, and 21 after administration of sheep anti-mouse GBM globulin to assess the kinetic profile of T-bet mRNA expression. Six days after injection of sheep anti-mouse GBM globulin, spleens were obtained from T-bet−/− and WT mice to assess mRNA expression of cytokines and chemokine receptors. Twenty-one days after administration of sheep anti-mouse GBM globulin, kidneys were obtained from T-bet−/− and WT mice to assess mRNA expression of cytokines and chemokines.
Splenic and intrarenal RNA was prepared and assessed for expression of chemokine receptors and chemokines using the RiboQuant RNase protection assay system (BD Pharmingen; template sets mCR-5, including template for CXCR3, and mCK-5c, respectively), as described previously.47 Results are expressed as percentage, relative to the housekeeping gene, L32, as previously published.47 For measurement of splenic and intrarenal mRNA expression of IFN-γ, IL-4, IL-17A, Mig, T-bet, and β-actin by real-time PCR, 500 ng of RNA from each sample was treated with 1 unit of amplification-grade DNase I (Invitrogen, Mount Waverley, Australia), primed with 500 ng of oligo(dT)12 to 18 (Roche, Mannheim, Germany) and reverse transcribed using Superscript III RT (Invitrogen). Gene-specific oligonucleotide primers were designed with Primer3 software (Whitehead Institute for Biomedical Research, Cambridge, MA)48 and synthesized by Invitrogen (Table 4). Real-time PCR was performed on a Rotor Gene RG-3000 (Corbett Research, Mortlake, Australia) using FastStart DNA master, Sybr Green I (Roche). PCR products were confirmed by melt-curve analysis, and mRNA expression was quantified using serial dilutions of an exogenous standard. Results are expressed as percentage, relative to the housekeeping gene, β-actin.
Table 4.
Primers used for analysis of mRNA expression of cytokines and chemokines
| Forward Primer | Reverse Primer | |
|---|---|---|
| IFN-γ | TGAAAGACAATCAGGCCATC | TTGCTGTTGCTGAAGAAGGT |
| IL-4 | TCAACCCCCAGCTAGTTGTC | TGTTCTTCGTTGCTGTGAGG |
| IL-17A | GGCTACAGTGAAGGCAGCAG | TCTTCATTGCGGTGGAGAGT |
| T-bet | CCTGGACCCAACTGTCAACT | AACTGTGTTCCCGAGGTGTC |
| CXCL9/Mig | TTGCTACACTGAAGAACGGAGA | TCCCATTCTTTCATCAGCTTC |
| β -actin | AGGCTGTGCTGTCCCTGTAT | AAGGAAGGCTGGAAAAGAGC |
Disclosures
None.
Acknowledgments
This study was supported by a National Health and Medical Research Council (NHMRC) Postgraduate Medical Scholarship (ID 284499) (R.K.S.P.). Program grants from the NHMRC are gratefully acknowledged.
Parts of this work were previously published in abstract form (program and abstracts of the American Society of Nephrology 2006 Annual Meeting, November 14–19, 2006; San Diego, CA; abstract SA-PO1080).
The authors thank A. Wright for technical assistance.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL: Two types of murine helper T cell clone: I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136: 2348–2357, 1986 [PubMed] [Google Scholar]
- 2.Romagnani S: Biology of human TH1 and TH2 cells. J Clin Immunol 15: 121–129, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F: The role of chemokines and chemokine receptors in T cell priming and Th1/Th2-mediated responses. Haematologica 84(Suppl EHA4): 28–31, 1999 [PubMed] [Google Scholar]
- 4.Glimcher LH, Murphy KM: Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev 14: 1693–1711, 2000 [PubMed] [Google Scholar]
- 5.Murphy KM, Reiner SL: The lineage decisions of helper T cells. Nat Rev Immunol 2: 933–944, 2002 [DOI] [PubMed] [Google Scholar]
- 6.O'Garra A, Arai N: The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol 10: 542–550, 2000 [DOI] [PubMed] [Google Scholar]
- 7.O'Garra A, Murphy K: Role of cytokines in development of Th1 and Th2 cells. Chem Immunol 63: 1–13, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH: Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol 21: 713–758, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Weaver CT, Hatton RD, Mangan PR, Harrington LE: IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25: 821–852, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD: Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421: 744–748, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ: IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201: 233–240, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lubberts E, Koenders MI, van den Berg WB: The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther 7: 29–37, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH: A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100: 655–669, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Smits HH, van Rietschoten JG, Hilkens CM, Sayilir R, Stiekema F, Kapsenberg ML, Wierenga EA: IL-12-induced reversal of human Th2 cells is accompanied by full restoration of IL-12 responsiveness and loss of GATA-3 expression. Eur J Immunol 31: 1055–1065, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S: IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J Immunol 169: 3600–3605, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA: IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res 23: 513–522, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H: Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol 170: 4886–4890, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Park IK, Shultz LD, Letterio JJ, Gorham JD: TGF-beta1 inhibits T-bet induction by IFN-gamma in murine CD4+ T cells through the protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1. J Immunol 175: 5666–5674, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH: Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A 100: 15818–15823, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durali D, de Goer de Herve MG, Giron-Michel J, Azzarone B, Delfraissy JF, Taoufik Y: In human B cells, IL-12 triggers a cascade of molecular events similar to Th1 commitment. Blood 102: 4084–4089, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C, Glimcher LH: T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells. Proc Natl Acad Sci U S A 100: 7749–7754, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins SH, Tessmer MS, Van Kaer L, Brossay L: Direct effects of T-bet and MHC class I expression, but not STAT1, on peripheral NK cell maturation. Eur J Immunol 35: 757–765, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kitching AR, Holdsworth SR, Tipping PG: Crescentic glomerulonephritis: a manifestation of a nephritogenic Th1 response? Histol Histopathol 15: 993–1003, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Holdsworth SR, Kitching AR, Tipping PG: Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int 55: 1198–1216, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Huang XR, Tipping PG, Shuo L, Holdsworth SR: Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int 51: 94–103, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Chan RW, Lai FM, Li EK, Tam LS, Chow KM, Li PK, Szeto CC: Imbalance of Th1/Th2 transcription factors in patients with lupus nephritis. Rheumatology (Oxf) 45: 951–957, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Peng SL, Szabo SJ, Glimcher LH: T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A 99: 5545–5550, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann SC, Hale DA, Kleiner DE, Mannon RB, Kampen RL, Jacobson LM, Cendales LC, Swanson SJ, Becker BN, Kirk AD: Functionally significant renal allograft rejection is defined by transcriptional criteria. Am J Transplant 5: 573–581, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Tipping PG, Huang XR, Qi M, Van GY, Tang WW: Crescentic glomerulonephritis in CD4- and CD8-deficient mice: requirement for CD4 but not CD8 cells. Am J Pathol 152: 1541–1548, 1998 [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Holdsworth SR, Tipping PG: Antibody independent crescentic glomerulonephritis in mu chain deficient mice. Kidney Int 51: 672–678, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Kitching AR, Holdsworth SR, Tipping PG: IFN-gamma mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J Am Soc Nephrol 10: 752–759, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Kitching AR, Tipping PG, Holdsworth SR: IL-12 directs severe renal injury, crescent formation and Th1 responses in murine glomerulonephritis. Eur J Immunol 29: 1–10, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Kitching AR, Tipping PG, Mutch DA, Huang XR, Holdsworth SR: Interleukin-4 deficiency enhances Th1 responses and crescentic glomerulonephritis in mice. Kidney Int 53: 112–118, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Kitching AR, Tipping PG, Timoshanko JR, Holdsworth SR: Endogenous interleukin-10 regulates Th1 responses that induce crescentic glomerulonephritis. Kidney Int 57: 518–525, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Kitching AR, Turner AL, Wilson GR, Semple T, Odobasic D, Timoshanko JR, O'Sullivan KM, Tipping PG, Takeda K, Akira S, Holdsworth SR: IL-12p40 and IL-18 in crescentic glomerulonephritis: IL-12p40 is the key Th1-defining cytokine chain, whereas IL-18 promotes local inflammation and leukocyte recruitment. J Am Soc Nephrol 16: 2023–2033, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Panzer U, Steinmetz OM, Stahl RA, Wolf G: Kidney diseases and chemokines. Curr Drug Targets 7: 65–80, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Matsuoka K, Inoue N, Sato T, Okamoto S, Hisamatsu T, Kishi Y, Sakuraba A, Hitotsumatsu O, Ogata H, Koganei K, Fukushima T, Kanai T, Watanabe M, Ishii H, Hibi T: T-bet upregulation and subsequent interleukin 12 stimulation are essential for induction of Th1 mediated immunopathology in Crohn's disease. Gut 53: 1303–1308, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, Bhan A, Autschbach F, Sullivan BM, Szabo SJ, Glimcher LH, Blumberg RS: The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J Exp Med 195: 1129–1143, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK: Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med 200: 79–87, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR: Autoimmune inflammation from the Th17 perspective. Autoimmun Rev 6: 169–175, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Dean EG, Wilson GR, Li M, Edgtton KL, O'Sullivan KM, Hudson BG, Holdsworth SR, Kitching AR: Experimental autoimmune Goodpasture's disease: a pathogenetic role for both effector cells and antibody in injury. Kidney Int 67: 566–575, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Huang XR, Tipping PG, Apostolopoulos J, Oettinger C, D'Souza M, Milton G, Holdsworth SR: Mechanisms of T cell-induced glomerular injury in anti-glomerular basement membrane (GBM) glomerulonephritis in rats. Clin Exp Immunol 109: 134–142, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rangan GK, Pippin JW, Coombes JD, Couser WG: C5b-9 does not mediate chronic tubulointerstitial disease in the absence of proteinuria. Kidney Int 67: 492–503, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Tipping PG, Kitching AR, Huang XR, Mutch DA, Holdsworth SR: Immune modulation with interleukin-4 and interleukin-10 prevents crescent formation and glomerular injury in experimental glomerulonephritis. Eur J Immunol 27: 530–537, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Odobasic D, Kitching AR, Semple TJ, Holdsworth SR: Inducible co-stimulatory molecule ligand is protective during the induction and effector phases of crescentic glomerulonephritis. J Am Soc Nephrol 17: 1044–1053, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Odobasic D, Kitching AR, Tipping PG, Holdsworth SR: CD80 and CD86 costimulatory molecules regulate crescentic glomerulonephritis by different mechanisms. Kidney Int 68: 584–594, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Ruth AJ, Kitching AR, Li M, Semple TJ, Timoshanko JR, Tipping PG, Holdsworth SR: An IL-12-independent role for CD40-CD154 in mediating effector responses: studies in cell-mediated glomerulonephritis and dermal delayed-type hypersensitivity. J Immunol 173: 136–144, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Rozen S, Skaletsky H: Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386, 2000 [DOI] [PubMed] [Google Scholar]