Abstract
Autosomal recessive polycystic kidney disease is caused by mutations in PKHD1, which encodes the membrane-associated receptor-like protein fibrocystin/polyductin (FPC). FPC associates with the primary cilia of epithelial cells and co-localizes with the Pkd2 gene product polycystin-2 (PC2), suggesting that these two proteins may function in a common molecular pathway. For investigation of this, a mouse model with a gene-targeted mutation in Pkhd1 that recapitulates phenotypic characteristics of human autosomal recessive polycystic kidney disease was produced. The absence of FPC is associated with aberrant ciliogenesis in the kidneys of Pkhd1-deficient mice. It was found that the COOH-terminus of FPC and the NH2-terminus of PC2 interact and that lack of FPC reduced PC2 expression but not vice versa, suggesting that PC2 may function immediately downstream of FPC in vivo. PC2-channel activities were dysregulated in cultured renal epithelial cells derived from Pkhd1 mutant mice, further supporting that both cystoproteins function in a common pathway. In addition, mice with mutations in both Pkhd1 and Pkd2 had a more severe renal cystic phenotype than mice with single mutations, suggesting that FPC acts as a genetic modifier for disease severity in autosomal dominant polycystic kidney disease that results from Pkd2 mutations. It is concluded that a functional and molecular interaction exists between FPC and PC2 in vivo.
Autosomal dominant polycystic kidney disease (ADPKD) and autosomal recessive polycystic kidney disease (ARPKD) are common human genetic disorders that are characterized by numerous, expanding fluid-filled cysts in both kidneys and other duct/tubule-containing organs.1,2 ADPKD is inherited as a dominant trait, occurring relatively late in life, and is characterized by focal outpouchings of spherical cysts in the renal tubules. In contrast, ARPKD is inherited as a recessive trait, usually presents during perinatal life, and is characterized by numerous spindle-shaped renal cysts. Because of these differences, ADPKD and ARPKD are usually considered two distinct diseases in clinical practice.3
ADPKD affects 1 in every 500 to 1000 people and results from mutations in either of at least two causal genes, PKD1 and PKD2, leading to nearly identical clinical manifestations. Mutations in PKD2, which maps to chromosome 4q21–23, are responsible for approximately 15% of familial ADPKD cases.4,5 PKD2 has an approximately 5.4-kb transcript and encodes the 968–amino acid gene product polycystin-2 (PC2). PC2 is predicted to be an integral membrane protein with six putative transmembrane domains and intracellular NH2- and COOH-termini.4 PC2 has been reported to be a receptor-operated, nonselective cation channel; it is also referred to as TRPP2, because it is considered a member of the trp superfamily.6,7 Conversely, mutations in PKD1 are responsible for approximately 85% of ADPKD cases. PKD1 lies in an approximately 53-kb region on chromosome 16p13.3 and yields a 14-kb transcript that encodes a 4303–amino acid integral membrane protein.8 The gene product of PKD1, polycystin-1 (PC1), was reported to interact with PC2 and regulate Pkd2-channel activity.9–12
ARPKD is one of the common hereditary renal cystic diseases in infants and children.13 The estimated incidence of ARPKD is approximately 1 in 20,000 live births.14 The clinical characteristics of ARPKD include ectasia of the renal collecting ducts and hepatic biliary ducts with associated renal and hepatic fibrosis.15 Approximately 50% of patients with ARPKD present with their disease as neonates16 and are born with two very large kidneys with 60 to 90% of the renal tubules being ectatic.15 These neonates suffer a 30% mortality rate as a result of respiratory and/or renal dysfunction.17 Patients who have ARPKD and survive their first year of life have a more optimistic prognosis: 75% reach age 5, and only half develop ESRD.16–18 In rare cases, patients with ARPKD survive beyond age 60.19
The most common complications of ARPKD include hypertension (60 to 100%), portal hypertension owing to severe hepatic fibrosis or Caroli disease (30 to 75%), and chronic lung disease (approximately 11%).17 In addition, growth retardation,15 intracranial aneurysms,20,21 and adrenal insufficiency22 can be seen in patients with ARPKD.
ARPKD is caused by mutations in PKHD1. This gene consists of at least 86 exons spanning 470 kb on chromosome 6p12 and produces a 16-kb transcript. The longest open reading frame is predicted to include 66 exons and to encode the 4074–amino acid membrane-associated receptor-like protein fibrocystin/polyductin (FPC).23–26 It was shown that FPC is associated with the basal bodies/primary cilia of epithelial cells27–30 and co-localizes with PC2 within the cell.31 These observations suggest the possibility that FPC and PC2 may function in a common molecular pathway in vivo.
To investigate a potential functional relationship between FPC and PC2, we generated and characterized a mouse model with a gene-targeted mutation in Pkhd1. We discovered that lack of FPC was associated with the downregulation of PC2 expression in vivo. Renal cyst formation is more severe in mice that are transmutant in Pkhd1 and Pkd2 (Pkd2tm2Som)32,33 than in mice that are heterozygous for the Pkd2 mutation alone. In addition, we found that Pkd2-channel activities were dysregulated in primary cultures of renal epithelial cells derived from Pkhd1 mutant mice. The in vivo observations demonstrated that FPC and PC2 functionally interacted and resided in a common molecular pathway.
RESULTS
Generation of Pkhd1-Deficient Mutant Mice
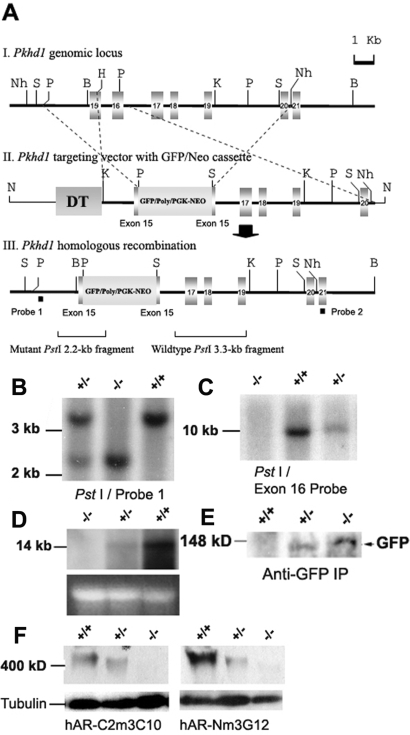
Because the Northern probe containing exons 15 to 16 of PKHD1 displayed highly mRNA expression signal in the kidney tissue, we designed a targeting construct that not only disrupted exon 15 but also deleted exon 16, resulting in a mutant allele designated Pkhd1e15GFPΔ16 (Pkhd1−) (Figure 1, A through D). Because our gene-targeting construct included an in-frame green fluorescence protein (GFP) reporter gene with a predicted fusion protein of 130 kD (Figure 1A), anti-GFP immunoprecipitation assays showed that only the mutant mice expressed the GFP reporter protein (Figure 1E). In addition, with the use of a panel of previously generated anti-FPC antibodies,31 Western blot analyses were able to detect the expected immunoreactive bands at >400 kD in the wild-type (WT) and heterozygous mouse kidneys (Figure 1F). These immunoreactive bands were not detected in protein lysate from Pkhd1−/− mouse kidneys, suggesting that the homozygous mice lacked full-length, functional FPC.
Figure 1.
Pkhd1 gene-targeting construct and molecular analysis of the specific targeting event at the Pkhd1 locus. (A) Schematic representation of the gene-targeting strategy. (I) Partial genomic map showing exons 15 to 21 of Pkhd1. (II) Pkhd1e15GFPΔ16 targeting vector in which exon 16 was deleted and exon 15 was disrupted. (III) Partial map of the mutant Pkhd1 allele. Nh, NheI; S, SphI; P, PstI; B, BamHI; K, KpnI. (B) Tail biopsy DNA from mice with the germline targeted mutation in Pkhd1 were digested with PstI and hybridized with probe 1 from outside the targeted region (AIII). The expected 3.3-kb WT band was observed in WT and Pkhd1 heterozygous mice. A mutant 2.2-kb band was seen in Pkhd1 heterozygous and homozygous mice. (C) Tail biopsy DNA were digested with BamHI and were hybridized with the Pkhd1 exon 16 probe. The radioactive signal was not observed in Pkhd1 homozygous mice. (D) A 3.8-kb cDNA fragment containing exons 37 to 43 of Pkhd1 served as a Northern probe to detect Pkhd1 in the total RNA of adult kidneys. Pkhd1−/− mice showed an absence of Pkhd1 mRNA (D, top). The 28S rRNA band images provided a total RNA loading control (D, bottom). (E) Because the Pkhd1 mutant alleles contained an in-frame GFP reporter gene, we used an anti-GFP antibody to detect GFP expression. GFP immunoreactivity was detected at the expected size of 130 kD in the adult kidneys of Pkhd1−/− and Pkhd1+/− mice. (F) Western blot detection of FPC. Antibodies hAR-Cm3C10 and hAR-Nm3G12, which recognize the COOH- and NH2-portions of FPC, respectively, were used to detect immunoreactivity to FPC in the adult kidneys of WT, Pkhd1+/−, and Pkhd1−/− mice. FPC expression was significantly reduced in Pkhd1−/− kidneys compared with WT and Pkhd1+/−.
Pkhd1-Deficient Mice Exhibit the Phenotypic Characteristics of Human ARPKD
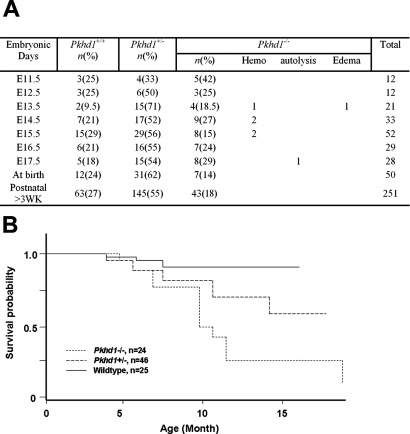
To determine whether the Pkhd1-deficient mice would be a suitable model for ARPKD, we intercrossed heterozygous Pkhd1 mice to produce homozygous (Pkhd1−/−) progeny. Pkhd1−/− mice were born at a frequency of 18%, lower than the expected Mendelian ratio, indicating a loss of approximately 5 to 10% of the Pkhd1−/− mice during the embryonic or perinatal period (Figure 2A). This suggests that the Pkhd1−/− genotype may lead to embryonic lethality. Mice that reached adulthood were used in a cohort study. Kaplan-Meier analysis showed that only 25% of Pkhd1−/− mice survived beyond 12 mo compared with 60% of Pkhd1+/− mice (P < 0.001) and 90% of WT mice (P < 0.001), suggesting that some adult Pkhd1−/− mice died as a result of their disease phenotypes (Figure 2B). Although there was a trend toward increased mortality in Pkhd1+/− mice compared with WT mice, this did not reach statistical significance (P = 0.075).
Figure 2.
Survival analyses of Pkhd1 mutant mice. (A) Genotype and survival rate of Pkhd1 mutant mice from embryo to adulthood. (B) Kaplan-Meier survival curves for WT, Pkhd1+/−, and Pkhd1−/− mice are shown. The mice in these cohorts were observed for >1 yr. Survival of the Pkhd1−/− mice differed significantly from that of the WT and Pkhd1+/− cohorts (P < 0.001).
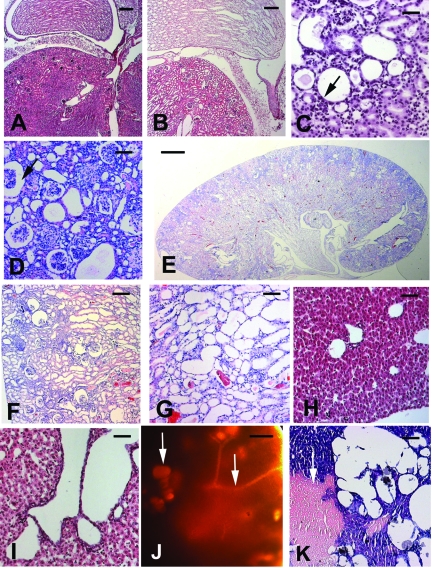
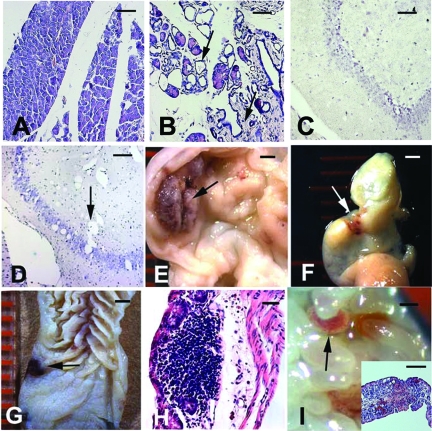
Pkhd1−/− mice that escaped embryonic lethality and survived into adulthood exhibited mild to severe tubular dilation or cyst formation in the kidney and liver accompanied by fibrosis and necrosis (Figure 3). The severity of cysts and the age at the onset of disease varied among individual Pkhd1−/− mice. Approximately 10% of the mice had early-onset, microscopic malformations in the renal tubules (Figure 3, B through G), whereas others (approximately 60%) survived beyond 1 yr and had late-onset cystic phenotypes. The extent of cystic change in the liver of Pkhd1−/− mice was generally more severe than that observed in the kidneys (Figure 3, H through K). These observations indicate that the targeted mutation in Pkhd1 induces cystogenesis in the kidneys and livers of the mice. Cystic or dilated-duct phenotypes were also seen in the pancreas (Figure 4, A versus B) and brain (Figure 4, C versus D) of Pkhd1−/− mice. In the gastrointestinal tract, hemorrhagic, and ulcer-like lesions were observed (Figure 4, E through I).
Figure 3.
Hepatorenal cysts and tubular ectasia in Pkhd1−/− mice. (A) A kidney tissue section shows normal glomeruli and a normal tubulointerstitial compartment in a 2-wk-old WT mouse. (B) Patchy dilation of proximal tubules and focal dilation of papillary collecting ducts were seen in a Pkhd1−/− littermate. (C) There was a marked increase in the degree of tubular dilation with flattening of the tubular epithelial cells (arrow) in the kidney of a 4-wk-old Pkhd1−/− mouse. (D) At 2 mo, there was a further increase in the severity of tubular dilation, in both the cortical proximal tubules and the medullary collecting duct tubules. Moreover, there was an increased expansion of Bowman's space (arrow), an increase in mesangial cellularity, and a reduction of glomerular capillary loop formation. (E) At 4 mo, in the whole-mount Pkhd1−/− kidney, there was a dramatic increase in tubular dilation, involving >80% of the cortex and medulla. (F) A high-power view of E at the cortical region of the kidney shows persistent expansion of Bowman's space with a mild decrease in glomerular size and segmental mesangial hypercellularity. The proximal tubules were dilated. (G) The medullary sections of a higher-power view of E also showed occasional small cysts lined by a single layer of epithelium. Red blood cells were seen within the tubular lumen. (H) Liver section showed normal histology of the 2-wk-old WT mouse. (I) Liver section of the homozygous littermates showed dilation of the biliary ducts. (J) Transillumination of an affected liver in the 2-mo-old Pkhd1−/− mice showed cyst formation (left arrow) and dilated ducts (right arrow). (K) There were focally enlarged cysts with areas of hemorrhage and necrosis (arrow) within the liver parenchyma in the same mouse. Bar = 20 μm in A, B, H, and I; 10 μm in C, D, F, G, and K; and 1 mm in E and J.
Figure 4.
Abnormal phenotypes were seen in the extrahepatorenal organs of Pkhd1−/− mice. (A) Pancreatic sections from a 6-mo-old WT mouse showed normal histology. (B) In the same homozygous littermates, there was dilation of both the small and large pancreatic ducts (upper arrow) with a marked increase in interstitial fibrosis (lower arrow). (C) Normal brain section from a 2-mo-old WT mouse. (D) Brain tissue sections showed multiple, diffuse, small- to medium-sized vacuoles in a homozygous littermate (arrow). (E) The stomach mucosa of a 2-mo-old homozygous mouse showed an ulcerated lesion (arrow) that was raised with beaded borders. (F) The colon of the same mouse showed small areas of subserosal hemorrhage. (G) The mucosal aspect of the colon from a 2-mo-old homozygous mouse showed a small, ulcerated lesion with hemorrhage (arrow). (H) The microscopic colon section of the sample in G revealed a submucosal lymphocytic infiltrate with focal necrosis and erosion of the overlying epithelium. (I) Gross view of the small intestinal mucosa with mild superficial hemorrhage (arrow). Histologic sections of the sample in I showed submucosal edema, lymphocytic infiltrate, hemorrhage, and erosion of the overlying mucosa. Bar = 25 μm in A through D; 5 μm in H; 1 mM in E through G and I; and 10 μm in I inset.
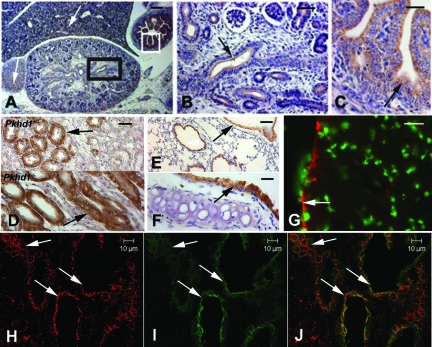
Because the Pkhd1 mutant allele carries an in-frame GFP reporter gene in exon 15, we used immunohistochemistry (IHC) and immunofluorescence (IF) staining with anti-GFP antibodies to detect GFP distribution in the organs of homozygous mutant mice. GFP-positive signals, defining the expression pattern of Pkhd1, were observed in the tubular epithelia of the kidneys (Figure 5, A, B, and D), gastrointestinal tract (Figure 5, A and C), bronchioles/trachea (Figure 5, E and F), ependymal cells lining the ventricles of the brain (Figure 5G), and hepatobiliary epithelial cells (Figure 5, H through J).
Figure 5.
GFP expression in Pkhd1 mutant mice. (A) E15.5 Pkhd1−/− kidney, liver, adrenal gland, and gastrointestinal tract stained with IHC using an anti-GFP polyclonal antibody. Positive signals were seen in the cortical adrenal cells (left arrow), periportal liver cells (right arrow), gastrointestinal tract (white box), and weakly positive staining was seen in the renal tubules (black box). (B) Higher magnification of the black box in A shows positive staining of the renal epithelia (arrow). (C) Higher magnification of the white box in A shows strong positive staining in the mucosal cells of the colon (arrow). (D) The differential interface contrast view showed GFP-positive staining (arrows) in the renal tubule epithelia of 4-mo-old Pkhd1+/− (D, top) and Pkhd1−/− (D, bottom) littermates. (E) Positive GFP staining was detected in an alveolar bronchiole of the lung in a 2-mo-old homozygous mouse (arrow). (F) Positive GFP was also seen in the tracheal epithelium of the same mouse (arrow). (G) GFP-positive staining (red) appeared in the ependymal cells lining the ventricles of the brain in a 2-mo-old homozygous mouse (arrow). Yo-pro (green) was used to stain the nuclei of cells. (H) GFP-positive staining (red) was observed in the diseased liver of a 2-mo-old homozygous mouse (arrows). (I) Cytokeratin 7–positive staining (green), a marker for epithelial cells, outlined the biliary epithelial structures (arrows). (J) The merged confocal image showed that GFP co-localized with cytokeratin 7. Bar = 30 μm in A; 15 μm in B and C; and 10 μm in D through J.
Lack of FPC Exhibits Aberrant Ciliogenesis in the Renal Epithelial Cells
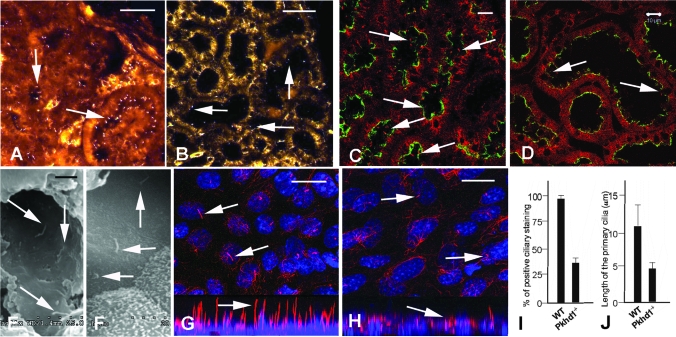
FPC was demonstrated to localize to the primary cilium and/or basal bodies of renal tubular epithelia,27–31 and malformation of the cilia was shown to induce cyst formation in the kidneys34,35; therefore, we began to determine whether the lack of FPC also disrupts ciliogenesis in Pkhd1-deficient mice. We used IF with an anti-acetylated α-tubulin antibody to examine the number and morphology of renal primary cilia in 6-mo-old littermates. Compared with WT mice, there were far fewer primary cilia in the renal tubular epithelia of Pkhd1−/− mice (Figure 6, A versus B), indicating that lack of FPC might reduce ciliogenesis in the kidneys. The ciliary defects seem to be most severe in the cortical proximal tubules of the Pkhd1−/− kidneys. In addition, confocal images also displayed similar ciliary changes in corresponding regions of littermate kidneys (Figure 6, C versus D). Scanning electron microscopy confirmed these results in 3-mo-old littermates with or without the targeted Pkhd1 mutation (Figure 6, E versus F). For further validation of these findings, primary culture of renal epithelial cells derived from 2-mo-old Pkhd1−/− and WT littermates was used to determine whether the ciliary malformation could be observed in vitro. Compared with the cultured WT cells, a shortened ciliary structure and decreased ciliary staining were seen in the Pkhd1−/− cells (Figure 6, G versus H). The cilia stained in approximately 94% of WT cells and in fewer than 34% of Pkhd1−/− cells (P < 0.001; Figure 6I). The mean length of primary cilia was 10 μm in cultured WT cells and was <5 μm in Pkhd1−/− littermate cells (P < 0.001; Figure 6J). That short or absent cilia were observed in Pkhd1−/− cells but not in WT cells suggests that lack of FPC causes defects in ciliogenesis in renal epithelial cells in vivo.
Figure 6.
Lack of FPC induces aberrant ciliogenesis in the Pkhd1−/− kidneys. (A) A common ciliary marker, anti-acetylated α-tubulin antibody, was used for IF staining of kidney sections from 6-mo-old WT and Pkhd1−/− littermates. Ciliary structures (arrows) were abundantly observed in the WT kidneys. (B) Decreased ciliary staining (arrows) was seen in the corresponding cortical region of Pkhd1−/− mouse kidneys. (C) The confocal images also showed normal ciliary structures in the 4-mo-old WT kidneys (arrows). Lotus Tetragonolobus Lectin (green) was used to stain renal proximal tubules. (D) The ciliary structures in the Pkhd1−/− littermates were reduced in number and shorter (arrow) than WT controls. (E) Scanning electron microscopy showed the normal primary cilia (arrows) of a 3-mo-old WT kidney. (F) The ciliary structures in the Pkhd1−/− kidney are shorter than those in the WT littermates, in the similar regions of the kidney. (G) Primary cultures of renal epithelia derived from the 2-mo-old WT and Pkhd1−/− kidneys were stained with an anti-acetylated α-tubulin antibody. The confocal images showed normal ciliary structures (arrows) in both top (G, top) and lateral views (G, bottom). Blue To-pro was used to stain nuclei. (H) In the primary cultured epithelial cells from the Pkhd1−/− littermate, confocal images showed ciliary structures that were shorter and fewer in number than those in control cells (arrows). The confocal top and lateral views were composed of multiple sections (approximately 0.5 μm thick and up to 16 layers) that were projected onto one plane to present the ciliary staining patterns. (I) One hundred individual primary cultured cells from five random high-power fields (×1000) were numbered; the cell number and positive cilium-staining rates are shown in I. In WT cells, 94% of cells stained positive for cilia, compared with 34% of Pkhd1−/− littermate cells (P < 0.001). (J) The length of 50 individual primary cilia of cultured cells from three random high-power fields was measured using lateral views of the confocal images; the average length of the primary cilia was calculated and showed in J. The primary cilium length is approximately 10 μm in WT and <5 μm in Pkhd1−/− littermate cells (P < 0.005). Bar = 10 μm in A through D; 2 μm in E and F; and 5 μm in G and H.
Trans-Mutant Mice in Pkhd1 and Pkd2 Accelerate Renal Cyst Disease Progression
Several in vitro studies from our group and others have shown that FPC and PC2 co-localize to the basal bodies/primary cilia of renal epithelial cells and are able to form a molecular complex and function in the same signaling pathway.31,36 For further validation of this finding in vivo, the phenotypic effects of transheterozygosity for Pkhd1 and Pkd2 were examined. We intercrossed Pkhd1+/− and Pkd2+/− mutant mice to produce cohorts of age-matched littermates. The four genotypes of interest (Pkhd1−/−, Pkhd1−/−/Pkd2+/−, and WT) were obtained from live-born progeny. Cohorts were killed at 1 mo. Gross inspection did not reveal cysts on the surface of the kidneys in any of the genotypes.
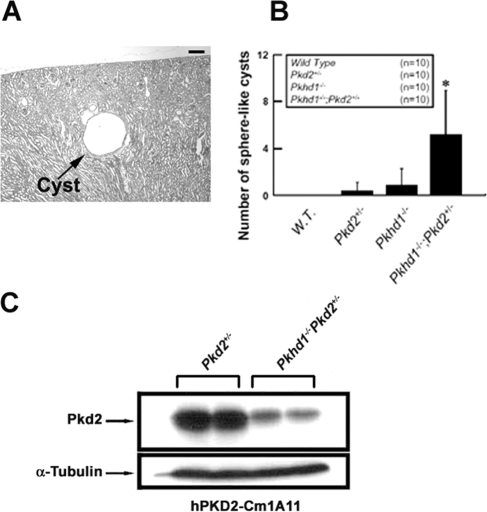
Because spherical renal cysts (at least three times the normal diameter of the proximal tubule) are characteristic of ADPKD and massive, dilated, spindle-shaped renal tubules are more typical of the ARPKD phenotype, we used sphere-shape cysts to represent ADPKD-like renal cysts.1–3 In mice with Pkhd1−/−/Pkd2+/− alleles, spherical cysts were distributed at the medullary and cortical regions on a background of Pkhd1−/−-specific dilated tubules (Figure 7A). By statistical analysis, Pkhd1−/−/Pkd2+/− mice had significantly greater numbers of spherical cysts than other genotypes (P < 0.05; Figure 7B). Western blot analyses of lysates from Pkd2+/− and Pkhd1−/−/Pkd2+/− littermates demonstrated that loss of FPC in the adult mouse kidney reduced PC2 expression in vivo (Figure 7C). These studies indicate that lack of FPC exacerbates the severity of ADPKD.
Figure 7.
The cystic phenotype of mice trans-mutant for Pkhd1 and Pkd2. (A) In a representative hematoxylin- and eosin-stained section, spherical renal cysts (arrows; diameter >50 μm was considered as renal cyst) were identified in a 1-mo-old Pkhd1−/−/Pkd2+/− double-mutant mouse. (B) Numbers of cysts are presented as means ± SD for four genotypes at the age of 1 mo (n = number of animals in each group). The increase in spherical renal cysts in Pkhd1−/−/Pkd2+/− trans-mutant mice was significantly higher than other genotypes (*P < 0.05). (C) Western blot of protein lysates from Pkd2+/− 1-mo-old mouse kidney with or without the Pkhd1−/− mutation was performed using the anti-PC2 antibody hPKD2-Cm1A11. A significant decrease in immunoreactivity in lysates from the Pkhd1−/− mutant mice indicates that lack of FPC reduces PC2 expression in vivo. Bar = 30 μm in A.
FPC and PC2 Interact In Vivo and Form a Molecular Complex in Renal Epithelia
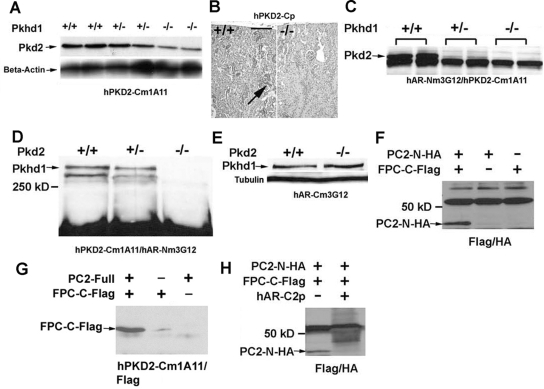
Because a lack of FPC inhibited the expression of PC2 and induced severe cystic phenotypes in the Pkhd1−/−/Pkd2+/− mouse model (Figure 7), we decided to analyze the molecular relationship between FPC and PC2. We used our mAb hPKD2-Cm1A11, which is directed against the intracellular COOH-terminus of PC2, to examine protein level in tissue lysates from WT, Pkhd1+/−, and Pkhd1−/− littermates at embryonic day 13.5 (E13.5). Compared with WT embryos, PC2 expression was decreased in the Pkhd1−/− embryos, suggesting that lack of FPC downregulates PC2 expression in vivo (Figure 8A). In addition, IHC staining with the anti-PC2 polyclonal antibody hPKD2-Cp was used to examine expression level of PC2 in the Pkhd1−/− 1-mo-old kidneys. In the renal cortex, significantly less staining was observed in Pkhd1−/− kidneys than in WT littermates (Figure 8B), giving further evidence that lack of FPC disrupts normal PC2 expression in vivo; however, quantitative PCR did not reveal a significant difference in Pkd2 expression among Pkhd1−/−, Pkhd1+/−, and WT 1-mo-old littermate kidneys (data not shown), suggesting that lack of FPC may affect only the synthesis and/or stability of PC2 in vivo.
Figure 8.
Molecular relationship between FPC and PC2. (A) Using the anti-PC2 mAb hPKD2-Cm1A11, Western blot of duplicate protein lysates from WT, Pkhd1+/−, and Pkhd1−/− E13.5 littermates showed a significant downregulation of PC2 in Pkhd1−/− embryos, indicating that lack of FPC inhibits PC2 expression in vivo. An anti–β-actin antibody was used for a protein-loading control. (B) In comparison with the WT littermate (left), IHC staining with the anti-PC2 polyclonal antibody hPKD2-Cp showed a significant decrease in PC2 expression in the cortical region of the 1-mo-old Pkhd1−/− kidney (right). (C) Duplicate lysates from E13.5 WT, Pkhd1+/−, and Pkhd1−/− littermates were used to perform a co-IP Western using the anti-FPC antibody hAR-Nm3G12 to IP and the anti-PC2 antibody hPKD2-Cm1A11 to detect PC2 expression. Positive immunoreactivity was seen in the WT embryo, and progressively reduced immunoreactivities were seen in the Pkhd1+/− and Pkhd1−/− littermates, suggesting that FPC binds to PC2 in vivo. (D) Lysates from E13.5 WT, Pkd2+/−, and Pkd2−/− littermates were used to perform a co-IP Western using the anti-PC2 antibody to IP and the anti-FPC antibody to detect FPC expression. Positive immunoreactivity was seen in the WT and Pkd2+/− littermates, but no immunoreactivity was observed in the Pkd2−/− littermate, providing further evidence that FPC physically interacts with PC2 in vivo. (E) There was no change in FPC expression in Western blot analysis among the WT and Pkd2−/− littermates, indicating that the downregulation of PC2 does not affect FPC expression. (F) HA- and Flag-tagged expression vectors, in which the COOH-terminus of FPC (FPC-C-Flag) and the NH2-terminus of PC2 (PC2-N-HA) were constructed in-frame, were transiently co-transfected into HEK293 cells. Using an anti-Flag antibody to IP and an anti-HA antibody to detect the NH2-terminus of PC2, positive immunoreactivity was seen only in the co-transfected sample, indicating that the COOH-terminus of FPC physically interacts with the NH2-terminus of PC2 in vitro. (G) The same FPC-C-Flag expression vector was transiently co-transfected into HEK293 cells with an expression vector containing the human full-length PKD2 cDNA (PC2-Full). The anti-PC2 antibody hPKD2-Cm1A11 was used for IP and an anti-Flag antibody was used to detect the COOH-terminus of FPC. Strong positive immunoreactivity was seen only in the co-transfected sample, and weak immunoreactivity was detected in the FPC-C-Flag single-transfected sample, indicating that either exogenously transfected or endogenously expressed PC2 immunoprecipitates with FPC-C-Flag construct. This further confirms that the COOH-terminus of FPC physically interacts with the NH2-terminus of PC2. (H) Using the hAR-C2p antibody against the COOH-terminus of FPC to preincubate with FPC-C-Flag single-transfected protein lysates, positive immunoreactivity was seen only in the nonpreincubated co-IP sample, whereas the immunoreactivity was missing in the preincubated co-IP sample. Bar = 30 μm in B.
To investigate whether FPC and PC2 physically interact, we used tissue lysates from E13.5 WT, Pkhd1+/−, and Pkhd1−/− littermates to perform a co-immunoprecipitation assay (co-IP) with antibodies against FPC and PC2. We found that FPC immunoprecipitated with PC2 in tissue from WT and Pkhd1+/− littermates but not from the negative control Pkhd1−/− littermates (Figure 8C). In addition, we used tissue lysates from an E13.5 Pkd2-mutant set in another co-IP assay. Similar to the previous result, FPC immunoprecipitated with PC2 in tissue from WT and Pkd2+/− littermates but not in tissue from Pkd2−/− littermates (Figure 8D). Immunoreactivity was stronger in WT than in heterozygous littermates and was not detected in homozygous littermate controls, providing strong evidence that FPC may physically interact with PC2 in vivo. It is interesting that no reduction of FPC expression was seen in Pkd2−/− littermates by Western analysis, suggesting that lack of PC2 does not affect the level FPC in vivo (Figure 8E).
We constructed serial Hemagglutinin (HA) and Flag-tagged expression vectors that contain intracellular portions of FPC and PC2, respectively. Positive immunoreactivity was seen in co-IP assays between the COOH-terminal portion of FPC (FPC-C-Flag) and the NH2-terminal portion of PC2 (PC2-N-HA; Figure 8F). Immunoreactivity was not observed between COOH-terminal portions of FPC (FPC-C-Flag) and PC2 (PC2-C-HA; data not shown). This suggests that the interaction between FPC and PC2 occurs via the intracellular COOH-terminal tail of FPC and NH2-terminal portion of PC2. For confirmation of this, HEK293 cells were transiently co-transfected with both an expression vector containing the full-length human PKD2 cDNA (PC2-Full) and the aforementioned FPC-C-Flag vector. With the use of an anti-PC2 antibody to immunoprecipitate and an anti-Flag antibody for detection by Western blot, a strong band was seen in lysate from the co-transfected cells. Weak immunoreactivity was observed in lysate from cells that were transfected only with FPC-C-Flag. These data suggest that FPC-C-Flag can be co-immunoprecipitated with either PC2 introduced exogenously or PC2 endogenous to HEK293 cells (Figure 8G). In addition, when lysates from FPC-C-Flag–transfected HEK293 cells were preincubated with an antibody against the COOH-terminal tail of FPC (hAR-C2p), the detection of PC2-N-HA and FPC-C-Flag co-IP was blocked (Figure 8H). This result provides further evidence of a specific interaction between the C-terminal tail of FPC and the N-terminal portion of PC2.
Lack of Pkhd1 Disrupts Pkd2-Induced Cation Channel Activities
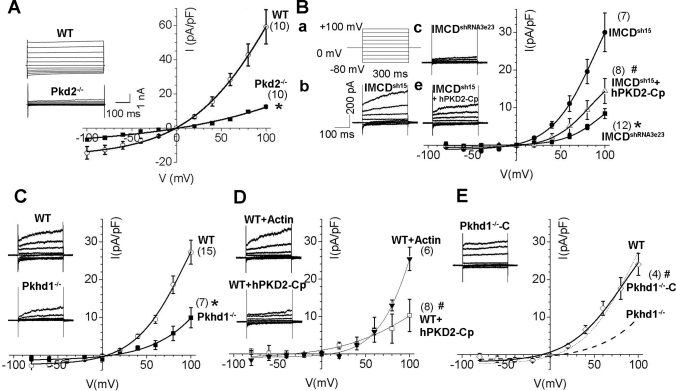
To study further whether FPC and PC2 functionally interact, we used whole-cell patch clamp recordings to characterize Pkd2-channel activities in Pkhd1-deficient cells. We first tested whether Pkd2-induced whole-cell current could be detected under published conditions37,38 and then investigated whether the recorded channel-like current is Pkd2-specific. First, primary cultured fibroblasts derived from E13.5 WT and Pkd2−/− embryos were tested to determine whether whole-cell current densities were dysregulated by the lack of Pkd2. The recorded current from the Pkd2−/− fibroblasts was significantly lower than that from the WT fibroblasts at all voltages tested, indicating that reduction of functional channel activities is caused by missing Pkd2 expression (P < 0.005; Figure 9A). Next, we used the same approach to test the Pkhd1-silenced inner medullary collecting duct (IMCD) cells (IMCDshRNA3e23) and WT control cells (IMCDsh15) that we had generated previously.39 It is interesting that Pkhd1-silenced IMCD cells exhibited a similar channel reduction, indicating that inhibition of FPC also reduces Pkd2-channel activities (P < 0.001; Figure 9, Bb versus Bc). For verification that the functional channel changes were induced by missing Pkd2, the anti-PC2 polyclonal antibody hPKD2-Cp, which recognizes the intracellular COOH-terminal region of PC2, was transferred into WT control IMCDsh15 cells via pipette solution. The current density was significantly inhibited in IMCDsh15 cells that were treated with hPKD2-Cp antibody (P < 0.02; Figure 9, Bb versus Be), suggesting that the current change is Pkd2 specific. To validate our findings further, we produced primary cultures of renal epithelia from 2-mo-old WT and Pkhd1−/− kidneys. Whole-cell current densities were significantly decreased in Pkhd1−/− cells compared with WT cells (P < 0.003; Figure 9C), indicating that lack of FPC also inhibits Pkd2-specific channel activities. For clarification that the downregulated current in Pkhd1−/− primary cultured cells was due to the Pkd2-specific channel, the whole-cell current of WT primary cultured cells was recorded in the presence of either the anti-PC2 antibody hPKD2-Cp or an anti-actin antibody. As representative current records and I-V curves in Figure 9D show, the anti-PC2 antibody reduced the current density, but the anti-actin antibody did not, confirming that the whole-cell current was conducted through Pkd2 channels.
Figure 9.
Analysis of whole-cell current recordings in Pkhd1-deficient cells. (A) Average current density (pA/pF) and voltage relation (I-V curve) of primary cultured fibroblast cells from E13.5 embryos show a statistically significant difference between WT and Pkd2−/− littermates, suggesting that the cation channel activity is induced by lack of PC2 (n = 10; *P < 0.005). (B) A Pkhd1-knockdown stable cell line IMCDshRNA3e23 and its WT–controlled cell line IMCDsh15 were used to perform the whole-cell current recording assays under the same conditions. Currents were elicited by stepping from a holding potential of 0 mV to various test potentials (Ba). The current densities between IMCDshRNA3e23 (n = 12) and IMCDsh15 (n = 7) cells exhibited a statistically significant difference (Bb versus Bc; *P < 0.001). The control IMCDsh15 cells displayed a steep inwardly rectifying current, which was significantly reduced by the intracellular introduction of the anti-PC2 C-terminal antibody hPKD2-Cp via pipette solution at a dilution of 1:200 (n = 8; Bb versus Be; #P < 0.02), suggesting that the current changes are PC2 specific. (C) Primary cultured renal epithelial cells derived from 2-mo-old WT (WT) and Pkhd1−/− littermates were used to perform the same whole-cell current recording assays. The control WT cells (n = 15) displayed a steep inwardly rectifying current, which was substantially reduced in Pkhd1−/− cells (n = 7). This indicates that the whole-cell current density in cells from the Pkhd1−/− mice is significantly lower than in those from the WT littermates (*P < 0.003). (D) hPKD2-Cp and a negative control anti-actin antibody were each added intracellularly into separate WT cells via pipette solution. Representative current traces were not clearly reduced in the cells with anti-actin antibody (n = 6), but a substantially smaller current amplitude was seen in the cells with hPKD2-Cp antibody (n = 8; #P < 0.01), further suggesting that the current alteration between WT and Pkhd2−/− cells in C is Pkd2 specific. (E) For unequivocal validation that the inhibited current density seen in the Pkhd1−/− cells is due to lack of FPC expression, a human full-length PKHD1 cDNA was in-frame constructed into a GFP-tagged expression vector and was transiently transfected into the Pkhd1−/− cells. GFP-positive cells were chosen to perform the whole-cell current recording (n = 4), and current density-voltage plots showed that the re-expression of FPC rescues the inhibited current density seen in the Pkhd1−/− cells (Pkhd1−/−-C versus Pkhd1−/−; #P < 0.01). The dashed line represents the mean I-V curve from the Pkhd1−/− cells in C, and the dotted line shows the means I-V curve from the WT cells in C. Currents were measured 220 ms after stepping to the test potential. Data are means ± SEM from the tested independent cells. Statistical difference at *+80 and #+100 mV between the tested cell groups. The data were fitted with standard Boltzmann equation (I = Imax/1 − e(V50 − V)/k) + C).
To obtain unequivocal evidence that the whole-cell current changes were induced by downregulation of FPC, we further investigated whether re-expression of FPC could rescue the reduced channel activities seen in Pkhd1−/− cells (Figure 9C). We transiently transfected primary cultured Pkhd1−/− cells with a mammalian expression vector, pcDNA3.1/Hygro, containing the full-length ORF cDNA of human PKHD1 with GFP fused in-frame. GFP-positive Pkhd1−/− cells, named Pkhd1−/−-C, were chosen for the whole-cell current recording assay. As shown in Figure 9E, both the current density and I-V relation of Pkhd1−/−-C cells were used to compare the currents recorded from WT (dotted line) and Pkhd1−/− cells (dashed line; Figure 9C). Unlike Pkhd1−/− cells, the current density of Pkhd1−/−-C cells was similar to WT cells (Figure 9E), indicating that the current changes between WT and Pkhd1−/− cells are due to downregulation of FPC. That inhibition of Pkhd1 expression disrupts Pkd2-specific channel activities gives further evidence to demonstrate FPC and PC2 function in the same molecular pathway.
DISCUSSION
Although the gene responsible for ARPKD, PKHD1, has been identified23–25 and its gene product, FPC, has been initially characterized,27,28,30,31,39,40 the mechanisms by which PKHD1 causes disease phenotypes remain largely unknown. To study the disease mechanism and pathogenesis of ARPKD, we created a mouse that allows manipulation of Pkhd1, an animal model that recapitulates the human ARPKD phenotype. Through genetic and biochemical studies, we demonstrated that the COOH-terminus of FPC physically interacts with the NH2-terminus of PC2 and that lack of FPC leads to downregulation of PC2 expression in vivo. Transmutant mice for Pkhd1 and Pkd2 displayed a significantly more severe renal cystic phenotype than single-mutant mice, suggesting that Pkhd1 serves as a disease modifier for ADPKD. For functional validation of these findings, Pkd2-channel activities were examined both in primary cultures of renal epithelia derived from Pkhd1 mutant mice and in Pkhd1-silenced IMCD cells.39 Pkd2-channel activities were significantly dysregulated in Pkhd1-deficient cells, indicating that FPC and PC2 reside in the same molecular pathway. During submission of our article, another group reported that there are genetic interactions between Pkhd1 and Pkd1.41 Because the gene product of PKD1, PC1, was reported to interact with PC2 and regulate Pkd2-channel activity,9–11 our findings combined with theirs draw the conclusion that ADPKD and ARPKD may reside in the same pathogenic pathway.
In this study, the Pkhd1−/− genotype seemed to cause embryonic lethality; however, a phenotypic analysis of these Pkhd1−/− embryos did not show any significant cardiac defects and only mild edema, which was prevalent in the Pkd2 mutant mice. Because Pkd2−/− mice are embryonically lethal, the finding that a lack of FPC promotes a significant downregulation of PC2 expression raises the possibility that partial embryonic lethality in Pkhd1−/− mice may be caused by this reduction of PC2.
We previously reported that FPC and PC2 co-localize to the same subcellular organelles, the basal bodies/primary cilia of renal epithelial cells,31 suggesting that these two cystoproteins may form a molecular complex. Recent reports have shown that the same chemical molecule (a vasopressin V2 receptor antagonist, OPC31260) can inhibit cyst progression in both a rat genetic model of ARPKD and a mouse genetic model of ADPKD, suggesting that both causal gene products for ADPKD and ARPKD may reside in a common molecular pathway.42 Recently, our company study demonstrated that FPC and PC2 indirectly interact via their COOH-termini and that this is mediated by KIF3B, a motor subunit of the heterotrimer kinesin-2.36 Pkd2-channel activities were significantly altered when the FPC–PC2 complex was disrupted. This in vitro study provides a molecular basis for a functional link between FPC and PC2. Another recent report that supports a functional link suggests that FPC regulates mechanotransduced Ca2+ responses, which may be induced by PC2, in cultured Pkhd1-knockdown cells.43 In this study, the evidence that the COOH-terminus of FPC directly interacts with the NH2-terminus of PC2 suggests that FPC and PC2 are able physically to form a heterodimeric complex in vivo. Lack of FPC downregulates Pkd2-channel activities in either the Pkhd1-knockout renal epithelial cells in primary culture or the Pkhd1-knockdown IMCD cells that we generated previously.39 Given that FPC and PC2 physically interact and that the lack of FPC downregulates PC2 expression in vivo but PC2 does not downregulate FPC, we speculate that PC2 may function immediately downstream of FPC.
The disruption of ciliary formation in renal epithelia induces cystogenesis in the kidneys.34,35 We recently reported that downregulation of Pkhd1 significantly decreases ciliary formation in cultured Pkhd1-silenced IMCD cells, suggesting that lack of FPC might disrupt ciliogenesis in renal epithelial cells. This result is consistent with studies in which transient small interference RNA–mediated inhibition of Pkhd1 in cholangiocytes resulted in shortening and decreased formation of cilia,29 but spatial and environmental differences between in vivo tissues and in vitro cell culture may lead to different results. Mouse models with a deletion of Pkhd1 exon 4044 and gene-targeted mutations in Pkd2 that cause distinct liver and/or kidney cysts33,45,46 do not exhibit defects in ciliary structure in the affected epithelial cells, suggesting that the failure of renal epithelia to assemble primary cilia may not be the only factor leading to cyst formation in the kidneys. For example, a recent study showed that disruption of the extracellular matrix protein laminin α5, which is a major component of the tubular and glomerular basement membranes, also produces cystic kidneys in Lama5 mutant mice.47
By carefully examining ciliogenesis in our Pkhd1 mutant kidneys, we found that primary cilia of the cortical tubular epithelia were reduced in number and were shorter than controls, documenting that disruption of FPC expression induces malformation of the primary cilia in the kidneys in vivo. Recently, an elegant study demonstrated that disruption of FPC expression causes defective planar cell polarity in another Pkhd1 genetic model, the pck rat. Spindle orientation in the renal epithelial cells of the pck rat is aberrant, suggesting that cell polarity is disrupted during mitosis of renal epithelial cells.48 Our previous in vitro study also demonstrated that renal epithelial IMCD cells with downregulated FPC exhibit aberrant migratory polarity and lose the ability to drive collective cell migration, suggesting that the planar cell polarity also might be disrupted.39 Aberrant planar cell polarity seen in cells with Pkhd1 defects suggests that ciliary defects in our Pkhd1 mutant mice might be caused by impeding orientally centriole arrangement and disabling the establishment of epithelial polarity.39,49
Because our Pkhd1 mutant mice bear an in-frame GFP reporter, immunostaining with anti-GFP antibodies provided an FPC expression profile in affected tissues. Positive GFP expression was detected in the apical domain of epithelial derivatives, including the renal, hepatic, pulmonary, and gastrointestinal epithelia as well as ependymal cells lining the ventricles of the brain. These findings are consistent with our previous report31 and indicate that FPC may modulate the morphogenesis and maintenance of tubular/ductal architectures in organs generated from the primary duct system.50 Several recent studies indicated that FPC is involved in notch-like processing and that its extremely large extracellular domain is released from the primary cilia of renal epithelial cells.51 That GFP-positive signals were detected at the microvilli of tubular epithelia in our Pkhd1−/− mice agrees with the report that FPC may be released into the tubular/ductal lumen.
In summary, we produced a mouse model for Pkhd1 that recapitulates the phenotypic characteristics of human ARPKD. Using this model along with a Pkd2 mutant mouse, we were able to demonstrate the importance of FPC expression for normal PC2 function. The finding of aberrant ciliogenesis in the kidneys of Pkhd1-deficient mice indicates that FPC disrupts the process of ciliogenesis. That FPC physically interacts with PC2 and that lack of FPC destabilizes PC2 expression in vivo but not vice versa suggest that PC2, also known as TRPP2, functions immediately downstream of FPC. In addition, because inhibition of FPC expression reduces Pkd2-channel activity, we conclude that a functional and molecular interaction exists between FPC and PC2 in vivo. Extracellular biochemical and/or physical signals may activate the receptor-like protein FPC, which then triggers TRPP2 channel to transmit signals that affect intracellular processes. Our in vivo study reveals an intriguing molecular relationship between FPC and PC2.
CONCISE METHODS
Mouse Strains
The gene-targeted mouse model for Pkd2 (Pkd2tm2Som) was previously generated by us.32,33 To produce mutant mice for Pkhd1, we designed a targeting construct disrupting its 15th coding exon (Figure 1). We found 620 embryonic stem cell colonies resistant to G418, with one (W4A5) identified by PCR screening using a pair of outside-construct and cassette-based primers. This cell line was further confirmed by Southern blot analysis and injected into C57Bl/6 blastocysts at the gene targeting and transgenic facility of University of Connecticut Health Center.
Southern and Northern Blotting and Quantitative PCR
Southern analysis was used to genotype Pkhd1 and Pkd2 mutant mice with our published approaches.32,52 For Northern analysis, total RNA was isolated from embryos or kidneys using Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. Probes were labeled using the RadPrime DNA-labeling system (Invitrogen) with α-32P-dCTP (PerkinElmer, Waltham, MA) and were hybridized with total RNA blots (25 μg/lane). Images of 28-S rRNA bands in these same blots were used as a total RNA loading control.
Quantitative PCR was performed using the iCycler iQ Real-Time PCR Detection System with iQ SYBR Green Supermix kit (Bio-Rad, Hercules, CA). Two pairs of primers were designed from each cDNA sequence of Pkhd1 and Pkd2 (Table 1).
Table 1.
Primers for quantitative PCR
| Genes | Primer Names | Location | Forward Primers | Reverse Primers |
|---|---|---|---|---|
| Pkhd1 | Pkhd1-P1 | Exons 18 to 19 | 5′-atgtctccagccaaccagttcc-3′ | 5′-gccttctaaaccttgctcaaatcc-3′ |
| Pkhd1-P2 | Exon 65 | 5′-accttcgttgtcttgcc-3′ | 5′-tctggttttgcttttct-3′ | |
| Pkd2 | Pkd2-P1 | Exons 3 to 4 | 5′-gacagagtcagtctttccatcgttc-3′ | 5′-acgcggcactcctagcag-3′ |
| Pkd2-P2 | Exons 14 to 15 | 5′-tttctaagattgacgccgtg-3′ | 5′-gcttacaccatgacctgtttgc-3′ |
Antibodies
Polyclonal antibodies and mAb against FPC (including hAR-Np, hAR-C2p, hAR-Nm3G12, and hAR-C2m3C10) and antibodies against human PC2 (hPKD2-Cp and hPKD2-Cm1A11, formerly named PKD2A11) were described in our previous studies.31,36 In addition, other polyclonal antibodies and mAb were purchased: Anti-acetylated α-tubulin, anti–γ-tubulin, anti–β-actin, anti-Flag, and anti-HA mAb (Sigma, St. Louis, MO); anti-GFP polyclonal antibodies (ab6556; Abcam, Cambridge, MA); fluorescein lotus tetragonolobus lectin (Vector Laboratories, Burlingame, CA); anti–cytokeratin 7 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA); and the nucleic acid dyes To-pro and Yo-pro (Molecular Probes, Eugene, OR).
Western Blotting and Immunoprecipitation
For Western analysis and immunoprecipitation, the detailed approaches were similar to our previous publications.31,36,39 For performance of co-IP and Western analysis, the entire intracellular termini of FPC and PC2 were constructed into Flag-tagged and HA-tagged pCMV expression vectors53 and were named FPC-C-HA (amino acids 3872 to 4074), PC2-N-HA (amino acids 1 to 221), and PC2-C-Flag (amino acids 682 to 968).
Histology, IF Staining, IHC, Confocal Microscopy, and scanning electron microscopy
To index cystic disease severity, we calculated the total number of cysts using four histologic sections from each kidney. Detailed procedures for histology, IF, and IHC were published previously.31 For confocal microscopy, antibody-stained images were collected as Z-series sections using a Zeiss LSM 510 confocal microscope system with ×40, ×63, and ×100 oil objectives. For scanning electron microscopy, mice were perfused as described previously.31 The kidneys were removed, sectioned longitudinally, washed by 1× PBS three times, and fixed in 2.5% paraformaldehyde. After an ethanol dehydration series, the samples were critical-point dried and sputter-coated with 40% gold/60% palladium microparticles to a thickness of 15 to 17 mm. Images of the samples were obtained using an Electroscan E3 Environmental Scanning Electron Microscope.
Cell Lines and Mouse Kidney Primary Epithelial Cultures
All cell lines used in this study were cultured under previously described conditions.39 To generate primary cultures of renal epithelia, we removed kidneys from 2-mo-old WT or Pkhd1 mutant mice and minced them finely with a scalpel. The minced tissue was incubated with 0.5% collagenase type IV at 37°C for 45 min and pipetted vigorously. The undigested tissue was removed by filtration through a 40-μ mesh filter. The remaining single cells and small organoids were washed three times with PBS containing 5 mM glucose. The cells were incubated with 10 μg/ml biotinylated Dolichos biflorus agglutinin (Vector; B-1035) at 4°C for 60 min. Then the cells were washed again with PBS before incubation with 50 μl of CELLectin Biotin binder Dynabeads (Invitrogen) at 4°C for 30 min. Because Dynabeads are superparamagnetic polystyrene beads, the incubated mixtures were washed twice with PBS containing 5 mM glucose using a magnetic rack. The cells were eluted with release buffer and were plated on 24-well dishes with 10% FCS DMEM under 5% CO2 at 37°C.
Statistical Analyses
The survival rate was determined by observing the cohorts of mice daily. We analyzed the survival using the Kaplan-Meier function in the R Software. Graphical data are presented as means ± SD. Statistical analysis was performed where appropriate using the t test or one-way ANOVA followed by Tukey multiple comparison test. Differences with P < 0.05 were considered statistically significant.
DISCLOSURES
None
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK062373 and DK071090) to G.W.
We kindly thank Dr. Stefan Somlo for allowing us to use the PKD2 mutant model (Pkd2tm2Som) that was generated in his laboratory. We also thank Dr. James P. Smith for excellent advice and suggestions; Dr. Caiying Guo for the embryonic stem cell work at University of Connecticut; Dr. Chun Li for statistical analysis; and Drs. Sae-youll Cho, Shun-Wei Huang, Aijun Zuo, and Hong Wang for technical assistance.
Published online ahead of print. Publication date available at www.jasn.org.
I.K. and Y.F. contributed equally to this work.
See related editorial, “ARPKD and ADPKD: First Cousins or More Distant Relatives?,” on pages 416–418.
REFERENCES
- 1.Igarashi P, Somlo S: Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol 13: 2384–2398, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Wilson P: Polycystic kidney disease. N Engl J Med 350: 151–164, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Torres VE, Harris PC: Mechanisms of disease: Autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2: 40–55, quiz 55, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S: PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Sutters M, Germino GG: Autosomal dominant polycystic kidney disease: Molecular genetics and pathophysiology. J Lab Clin Med 141: 91–101, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Montell C, Birnbaumer L, Flockerzi V: The TRP channels, a remarkably functional family. Cell 108: 595–598, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Qamar S, Vadivelu M, Sandford R: TRP channels and kidney disease: Lessons from polycystic kidney disease. Biochem Soc Trans 35: 124–128, 2007 [DOI] [PubMed] [Google Scholar]
- 8.The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. European Polycystic Kidney Disease Consortium. Cell 77: 881–894, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG: PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16: 179–183, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J: Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S: Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG: Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408: 990–994, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Guay-Woodford LM: Autosomal recessive disease: Clinical and genetic profiles. In: Polycystic Kidney Disease, edited by Torres V, Watson M, Oxford, Oxford University Press, 1995, pp 237–267
- 14.Zerres K, Mucher G, Becker J, Steinkamm C, Rudnik-Schoneborn S, Heikkila P, Rapola J, Salonen R, Germino GG, Onuchic L, Somlo S, Avner ED, Harman LA, Stockwin JM, Guay-Woodford LM: Prenatal diagnosis of autosomal recessive polycystic kidney disease (ARPKD): Molecular genetics, clinical experience, and fetal morphology. Am J Med Genet 76: 137–144, 1998 [PubMed] [Google Scholar]
- 15.Zerres K, Rudnik-Schoneborn S, Senderek J, Eggermann T, Bergmann C: Autosomal recessive polycystic kidney disease (ARPKD). J Nephrol 16: 453–458, 2003 [PubMed] [Google Scholar]
- 16.Capisonda R, Phan V, Traubuci J, Daneman A, Balfe JW, Guay-Woodford LM: Autosomal recessive polycystic kidney disease: Outcomes from a single-center experience. Pediatr Nephrol 18: 119–126, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Guay-Woodford LM, Desmond RA: Autosomal recessive polycystic kidney disease: The clinical experience in North America. Pediatrics 111: 1072–1080, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Roy S, Dillon MJ, Trompeter RS, Barratt TM: Autosomal recessive polycystic kidney disease: Long-term outcome of neonatal survivors. Pediatr Nephrol 11: 302–306, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Fonck C, Chauveau D, Gagnadoux MF, Pirson Y, Grunfeld JP: Autosomal recessive polycystic kidney disease in adulthood. Nephrol Dial Transplant 16: 1648–1652, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Neumann HP, Krumme B, van Velthoven V, Orszagh M, Zerres K: Multiple intracranial aneurysms in a patient with autosomal recessive polycystic kidney disease. Nephrol Dial Transplant 14: 936–939, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Lilova MI, Petkov DL: Intracranial aneurysms in a child with autosomal recessive polycystic kidney disease. Pediatr Nephrol 16: 1030–1032, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Yonemura K, Yasuda H, Fujigaki Y, Oki Y, Hishida A: Adrenal insufficiency due to isolated adrenocorticotropin deficiency complicated by autosomal recessive polycystic kidney disease. Ren Fail 25: 485–492, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC: The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30: 259–269, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schoneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG: PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet 70: 1305–1317, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong H, Chen Y, Yi Y, Tsuchiya K, Moeckel G, Cheung J, Liang D, Tham K, Xu X, Chen XZ, Pei Y, Zhao ZJ, Wu G: A novel gene encoding a TIG multiple domain protein is a positional candidate for autosomal recessive polycystic kidney disease. Genomics 80: 96–104, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Bergmann C, Frank V, Kupper F, Schmidt C, Senderek J, Zerres K: Functional analysis of PKHD1 splicing in autosomal recessive polycystic kidney disease. J Hum Genet 51: 788–793, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC: Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet 12: 2703–2710, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Luo Y, Wilson PD, Witman GB, Zhou J: The autosomal recessive polycystic kidney disease protein is localized to primary cilia, with concentration in the basal body area. J Am Soc Nephrol 15: 592–602, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Masyuk TV, Huang BQ, Ward CJ, Masyuk AI, Yuan D, Splinter PL, Punyashthiti R, Ritman EL, Torres VE, Harris PC, LaRusso NF: Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology 125: 1303–1310, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Menezes LF, Cai Y, Nagasawa Y, Silva AM, Watkins ML, Da Silva AM, Somlo S, Guay-Woodford LM, Germino GG, Onuchic LF: Polyductin, the PKHD1 gene product, comprises isoforms expressed in plasma membrane, primary cilium, and cytoplasm. Kidney Int 66: 1345–1355, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Zhang MZ, Mai W, Li C, Cho SY, Hao C, Moeckel G, Zhao R, Kim I, Wang J, Xiong H, Wang H, Sato Y, Wu Y, Nakanuma Y, Lilova M, Pei Y, Harris RC, Li S, Coffey RJ, Sun L, Wu D, Chen XZ, Breyer MD, Zhao ZJ, McKanna JA, Wu G: PKHD1 protein encoded by the gene for autosomal recessive polycystic kidney disease associates with basal bodies and primary cilia in renal epithelial cells. Proc Natl Acad Sci U S A 101: 2311–2316, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu GQ, D'Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H Jr, Kucherlapati R, Edelmann W, Somlo S: Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93: 177–188, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Wu GQ, Markowitz GS, Li L, D'Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H Jr, Kucherlapati R, Edelmann W, Somlo S: Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 24: 75–78, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE Jr, Schafer JA, Balkovetz DF: Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol 282: F541–F552, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P: Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A 100: 5286–5291, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Dai XQ, Li Q, Chen CX, Mai W, Hussain Z, Long W, Montalbetti N, Li G, Glynne R, Wang S, Cantiello HF, Wu G, Chen XZ: Kinesin-2 mediates physical and functional interactions between polycystin-2 and fibrocystin. Hum Mol Genet 15: 3280–3292, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Anyatonwu GI, Ehrlich BE: Organic cation permeation through the channel formed by polycystin-2. J Biol Chem 280: 29488–29493, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Koulen P, Duncan RS, Liu J, Cohen NE, Yannazzo JA, McClung N, Lockhart CL, Branden M, Buechner M: Polycystin-2 accelerates Ca2+ release from intracellular stores in Caenorhabditis elegans. Cell Calcium 37: 593–601, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Mai W, Chen D, Ding T, Kim I, Park S, Cho SY, Chu JS, Liang D, Wang N, Wu D, Li S, Zhao P, Zent R, Wu G: Inhibition of Pkhd1 impairs tubulomorphogenesis of cultured IMCD cells. Mol Biol Cell 16: 4398–4409, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiesberger T, Gourley E, Erickson A, Koulen P, Ward CJ, Masyuk TV, Larusso NF, Harris PC, Igarashi P: Proteolytic cleavage and nuclear translocation of fibrocystin is regulated by intracellular Ca2+ and activation of protein kinase C. J Biol Chem 281: 34357–34364, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Gonzalez MA, Menezes LF, Piontek KB, Kaimori J, Huso DL, Watnick T, Onuchic LF, Guay-Woodford LM, Germino GG: Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet 16: 1940–1950, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH 2nd: Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J: Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol 27: 3241–3252, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moser M, Matthiesen S, Kirfel J, Schorle H, Bergmann C, Senderek J, Rudnik-Schoneborn S, Zerres K, Buettner R: A mouse model for cystic biliary dysgenesis in autosomal recessive polycystic kidney disease (ARPKD). Hepatology 41: 1113–1121, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B: The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol 12: 938–943, 2002 [DOI] [PubMed] [Google Scholar]
- 46.McGrath J, Somlo S, Makova S, Tian X, Brueckner M: Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114: 61–73, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Shannon MB, Patton BL, Harvey SJ, Miner JH: A hypomorphic mutation in the mouse laminin alpha5 gene causes polycystic kidney disease. J Am Soc Nephrol 17: 1913–1922, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M: Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Germino GG: Linking cilia to Wnts. Nat Genet 37: 455–457, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Nagasawa Y, Matthiesen S, Onuchic LF, Hou X, Bergmann C, Esquivel E, Senderek J, Ren Z, Zeltner R, Furu L, Avner E, Moser M, Somlo S, Guay-Woodford L, Buttner R, Zerres K, Germino GG: Identification and characterization of Pkhd1, the mouse orthologue of the human ARPKD gene. J Am Soc Nephrol 13: 2246–2258, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Kaimori JY, Nagasawa Y, Menezes LF, Garcia-Gonzalez MA, Deng J, Imai E, Onuchic LF, Guay-Woodford LM, Germino GG: Polyductin undergoes notch-like processing and regulated release from primary cilia. Hum Mol Genet 16: 942–956, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu G, Tian X, Cai Y, Markowitz G, D'Agati V, Park JH, Yao L, Li L, Geng L, Zhao H, Edelmann W, Somlo S: Trans-heterozygous Pkd1 and Pkd2 mutations modify expression of polycystic kidney disease. Hum Mol Genet 11: 1845–1854, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang CK, Wu D: Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell 114: 215–227, 2003 [DOI] [PubMed] [Google Scholar]