Abstract
Nek8 is a serine/threonine kinase that is mutated in the jck (juvenile cystic kidneys) mouse, a model of autosomal recessive juvenile polycystic kidney disease, but its function is poorly understood. We used the jck mouse to study the functional relationship between Nek8 and other proteins that have been implicated in polycystic kidney diseases. In the collecting tubules and collecting ducts of wild-type mice, we found that Nek8 was localized to the proximal portion of primary cilia and was weakly detected in the cytosol. In the jck mutant, however, Nek8 was found along the entire length of cilia. Coimmunoprecipitation experiments demonstrated that Nek8 interacted with polycystin-2, but not with polycystin-1, and that the jck mutation did not affect this interaction. Western blot analysis and real-time reverse transcriptase PCR revealed that the protein and mRNA expression of polycystin-1 (PC1) and polycystin-2 (PC2) were increased in jck mouse kidneys. The jck mutation also led to abnormal phosphorylatin of PC2, and this was associated with longer cilia and ciliary accumulation of PC1 and PC2. Our data suggests that Nek8 interacts with the signal transduction pathways of the polycystins and may control the targeting of these ciliary proteins. Dysfunction Nek8 may lead to cystogenesis by altering the structure and function of cilia in the distal nephron.
NIMA (never in mitosis, gene A) is a serine/threonine kinase in Aspergillus nidulans that has been shown to have a role in the progression of mitosis. Defects of NIMA cause cells to arrest in G2, whereas overexpression of NIMA results in the premature onset of mitotic events.1,2 NIMA-related kinase (Nek or Nrk) of Tetrahymena thermophila, Nrk-1 and Nrk-2, localize to the basal bodies and cilia and regulate ciliary length.3 Fa2p is a NIMA homolog from Chlamydomonas reinhardtii and has been shown to have roles in cell cycle progression and microtubule severing during deflagellation.4 Recently, Fa2p was found localized to the proximal end of cilia, in both Chlamydomonas and cultured kidney epithelial cells, and its kinase activity is required for deflagellation.5
To date, 11 Nek family members have been cloned from mouse or human cells, of which Nek2 is considered to be the closest NIMA homolog and therefore has been the most intensively studied. Nek2 is an important player in the coordination of centrosome structure and function with mitotic progression. It is also required for centrosome separation at the G2-M cell-cycle transition.6,7 Nek1, the first family member identified, was found to be mutated in the kat mouse, which develops pleiotropic effects including facial dysmorphism, dwarfing, male sterility, anemia, cystic choroid plexus, and progressive polycystic kidney disease (PKD).8,9 Another Nek family member, Nek8, was found to be mutated in the jck (juvenile cystic kidneys) mouse, which develops autosomal recessive juvenile PKD (ARJPKD).10 Human Nek8 was found overexpressed in primary breast tumors, suggesting that Nek8 is involved in cell proliferation, as are other NIMA family members.11 Nevertheless, the function of Nek1 and Nek8 and their role in cyst formation in PKD remain unclear.
Autosomal dominant PKD (ADPKD) is the major form of PKD, with a frequency of 1 in 400 to 1 in 1000. Polycystin-1 (PC1) and polycystin-2 (PC2) are the two molecules mutated in almost all ADPKD patients. PC1 is an integral membrane glycoprotein of approximately 460 kD that is located in the plasma membrane and cilia of renal epithelia.12–18 PC2, the functional partner of PC1, is an integral membrane protein of approximately 110 kD. PC2 has calcium channel functions in endoplasmic reticulum, plasma membrane, and cilia.17–21 Proteins responsible for autosomal recessive PKD (ARPKD) are also found in cilia of renal epithelia, such as cystin for cpk (congenital polycystic kidney) mouse model,22 polaris for orpk (Oak Ridge polycystic kidney) mouse model,23,24 and fibrocystin in human ARPKD.25,26
In this study, we report that the Nek8 protein is located in the proximal region of the primary cilia in mouse kidney tubules and in the same protein complex with PC2. The ciliary localization of Nek8 is observed only in the collecting tubules and collecting ducts where the cysts develop in the jck mice. The transcription for both Pkd1 and Pkd2 genes are upregulated. An increase in the expression of PC1 and PC2 in the primary cilia and abnormal phosphorylaton for PC2 was seen in the jck mouse kidney. These data suggest that Nek8 modulates the normal expression of PC1 and PC2 and the phosphorylation of PC2. In addition, Nek8 controls or modulates the ciliary localization of PC1 and PC2. Mutations in Nek8 cause abnormal transcription and localization of polycystins, ultimately resulting in cystogenesis in the jck mouse kidney.
RESULTS
Nek8 Is Located in the Proximal Segment of the Primary Cilia of Renal Tubules
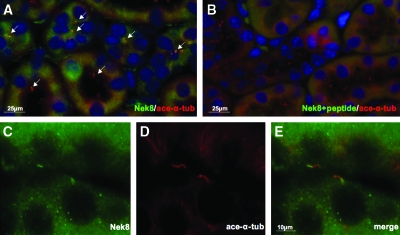
The affinity-purified antibody against Nek8 was used for immunohistochemistry of kidney from 3-mo-old wild-type mice.10 In kidney tissue, Nek8 colocalized with the primary cilia (Figure 1A). A weak intracellular signal of Nek8 was observed as well. Both cilia and intracellular signals of Nek8 could be blocked by preincubation of Nek8 antibody with its antigen peptide, whereas the signal of cilium marker remained (Figure 1B). Immunohistochemical image at high magnification revealed that Nek8 localization on the primary cilia is limited to the proximal segment (Figure 1, C to E).
Figure 1.
Nek8 localized at proximal region of primary cilia in kidney tubules in vivo. (A) Immunofluorescence of kidney tubules with anti-Nek8 antibody (green) revealed its ciliary localization and colocalization with a cilia marker, acetylated-α-tubulin (red). Arrows indicate Nek8 signal. (B) Both ciliary and cytoplasmic signals of Nek8 could be blocked by preincubation of Nek8 antibody with its antigen, whereas the signals of cilia marker remained. (C to E) An enlarged image of two cilia shows Nek8 localization at the proximal segment of the primary cilia, which overlaps the signals of acetylated-α-tubulin.
Nek8 Is Absent from Primary Cilia of Proximal Tubules
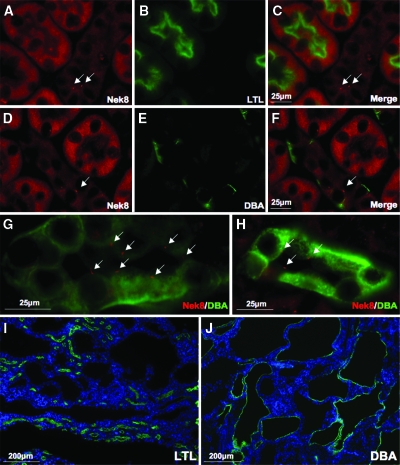
To investigate the characteristic of the ciliary localization of Nek8, we double-stained kidney tissue with Nek8 and Lotus teragonolobus lectin (LTL), a proximal tubule marker or Dolichos biflorus agglutinin (DBA), a collecting tubule and collecting duct marker. Cytoplasmic signals of Nek8 were most obvious in the LTL-positive tubules that represent proximal tubules (Figure 2, A to C). However, ciliary Nek8 was not detected in those tubules. On the contrary, clear ciliary localization of Nek8 is seen in the DBA-positive tubules that represent collecting tubules and collecting ducts, where the cytosolic signal is weak (Figure 2, D and G). Ciliary localizations of Nek8 were also observed in the collecting ducts of medulla (Figure 2H). To investigate whether there is any correlation between ciliary Nek8 expression and cyst formation in the jck mouse, we stained kidney tissue from jck mice with DBA and LTL (Figure 2, I and J). Most cysts in jck mice were positive for DBA staining and none of the cysts was stained with LTL, suggesting that ciliary Nek8 in collecting tubules and collecting ducts are responsible for cyst formation in jck mice.
Figure 2.
Ciliary Nek8 are found only in collecting tubules and collecting ducts. (A to C) Ciliary localization of Nek8 (red) was observed only in the tubules negative for Lotus teragonolobus lectin (LTL), a proximal tubule marker, although cytosol signal is obvious in the LTL-positive tubules (green). Arrows indicate ciliary signal of Nek8. (D to F) Ciliary localization of Nek8 (red) was observed only in the tubules positive for Dolichos biflorus agglutinin (DBA), a collecting tubule marker (green), indicating that ciliary Nek8 only expressed in cilia of collecting tubules to collecting duct. Enlarged immunofluorescence images of Nek8 staining (red) in DBA-positive (green) tubules in cortex (G) and medulla (H). LTL (I) and DBA (J) staining (green) of kidney section from jck mouse. Cysts in jck mice are stained only with DBA. Blue DAPI staining indicates the cell nucleus.
Nek8 Protein Complex Contains PC2
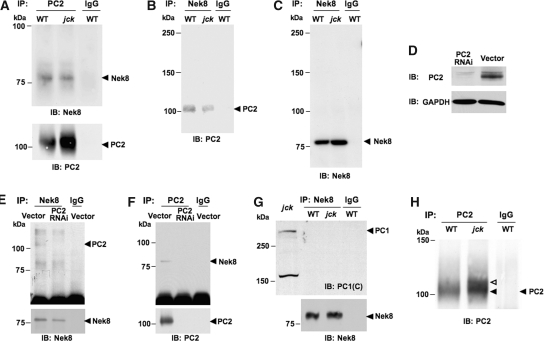
To determine whether polycystins play a role in cyst formation in jck mice, we tested the interaction of Nek8 protein with PC1 or PC2 in vivo. Co-immunoprecipitation experiments of Nek8 and PC1 or PC2 were performed in tissue extracts from kidneys of 3-mo-old wild-type and jck mice. PC2 co-immunoprecipitated Nek8 in the lysates of the wild-type mouse kidneys (Figure 3A), demonstrating that Nek8 was in the same protein complex with PC2 in vivo. A similar amount of Nek8 protein co-immunoprecipitated by PC2 in tissue lysates from the jck and the wild-type mice (Figure 3A) suggested that Nek8-PC2 interaction was preserved in the jck mouse. The interaction of PC2 with Nek8 was confirmed by co-immunoprecipitation with Nek8 antibody (Figure 3, B and C). Nek8 antibody recognizes a single band in Nek8 immunoprecipitates (Figure 3C). To further verify the interaction between PC2 and Nek8, we knocked down PC2 in inner medullary collecting duct (IMCD) cells using the lentiviral system (Figure 3, D to F). Infection of lentiviral particles that express PC2 shRNA into IMCD cells resulted in significant downregulation of PC2 protein as revealed by Western analysis with a PC2 antibody (Figure 3D). Nek8 antibodies were able to co-immunoprecipitate PC2 in vector-infected control cells but not PC2 knockdown cells (Figure 3E), although Nek8 was present in both cell lines. Moreover, PC2 could co-immunoprecipitate Nek8 only in control cells but not in PC2 knockdown cells (Figure 3F). Interestingly, Nek8 was unable to co-immunoprecipitate PC1 in tissue lysate from either jck or wild-type mice (Figure 3G). These data suggest that Nek8 interacts with PC2 but not PC1, and that the missense mutation in Nek8 in jck mice does not disrupt its interaction with PC2. The interaction between PC1 and PC2 was present in tissue lysates of both jck and wild-type mice (data not shown).
Figure 3.
Interaction between PC2 and Nek8. (A) Immunoprecipitation of kidney homogenates with PC2 antibodies. Top, Both wild-type (WT) and mutant Nek8 was co-immunoprecipitated by PC2. Bottom, PC2 was present in PC2 but not in IgG immunoprecipitation samples. (B and C) Co-immunoprecipitation of kidney homogenates with Nek8 antibodies: (B) PC2 was co-immunoprecipitated with Nek8 in both WT and jck mice (arrowhead points to PC2); (C) Nek8 antibody detected only one band in Nek8 immunoprecipitates (left, same blot as shown in B). (D to F) Verification of PC2-Nek8 interaction using lentiviral-based PC2 knockdown system inner medullary collecting duct (IMCD) cells. (D) After puromycin selection, PC2 band disappeared in IMCD infected with Pkd2 knockdown viruses. On the contrary, PC2 remained in IMCD infected with control lentiviruses. GAPDH is used as loading control. (E) Co-immunoprecipitation of cell lysate with Nek8 antibodies. PC2 was co-immunoprecipitated with Nek8 in control, but not in PC2 knockdown IMCD cells. IgG was used as an immunoprecipitation control (top). Nek8 antibodies could immunoprecipitate itself in both PC2 knockdown and control cells (bottom). (F) Co-immunoprecipitation of cell lysate with PC2 antibodies. Nek8 was co-immunoprecipitated with PC2 in control, but not in PC2 knockdown IMCD cells or in IgG-immunoprecipitaes. (G) PC1 was not co-immunoprecipitated with Nek8 antibodies (top) although Nek8 antibodies could immunoprecipitate itself (bottom). Top left lane shows PC1 is present in the tissue homogenates from jck mouse kidney. (H) Abnormal modification of PC2 protein was seen in jck mouse kidney (open arrowhead). IgG was used as a control for immunoprecipitation.
Increased Phosphorylation of PC2 in jck Mice
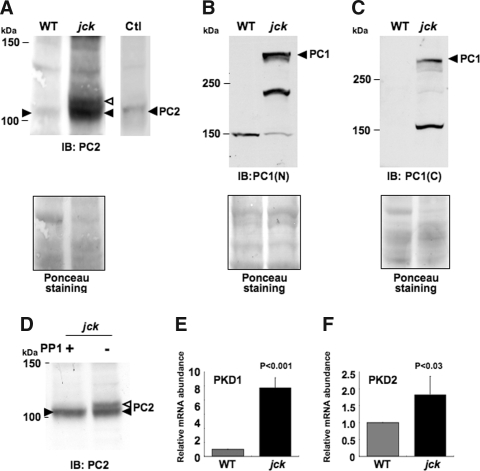
Immunoprecipitation with a PC2 antibody detected an additional band just above the normal PC2 band in the jck mouse kidney, but not in wild-type mouse kidney (Figure 3H). Western analyses confirmed the presence of an additional form of PC2 in jck mice but not in the wild-type mice (Figure 4A) and an increase in PC1 expression levels (Figure 4, B and C). To determine which type of protein modification is responsible for this additional band of PC2, we performed dephosphorylation assay (Figure 4D). Treatment with protein phosphatase 1 resulted in the loss of the additional PC2 in the jck samples, indicating that PC2 is abnormally phosphorylated in jck mice. The increase of PC1 and PC2 expression was also seen in kidney samples from 15 d old mice (Supplemental Figure I).
Figure 4.
Upregulation of PC1 and abnormal phosphorylation of PC2 in jck mice kidney. (A to C) Western blot of kidney homogenates with PC2 (A) or PC1 (B and C) antibody. (A) Abnormal modification of PC2 protein was seen in jck mouse kidney (open arrowhead) in comparison with the WT kidneys. PC2 overexpressed IMCD lysate was used as a positive control (Ctl) with a shorter exposure time. (B and C) PC1 protein levels were upregulated in the jck mouse kidney (arrowhead), as detected with antibodies against either the N-terminus (B) and C-terminus (C) of PC1. Bottom panels of A to C are loading controls shown by Ponceau staining of the membrane. (D) Dephosphorylation assay with protein phosphatase 1 (PP1). Kidney homogenates from jck mouse kidneys were incubated with or without PP1. Abnormal modified form of PC2 disappeared from the sample incubated with PP1. (E and F) Real-time reverse transcriptase PCR analysis of Pkd1 (I) and Pkd2 (J). Total RNA from whole mouse kidney was used as a starting material. Pkd1 expression was 9.8-fold increased in jck mice (P < 0.001, n = 4). Pkd2 expression was 1.7-fold increased in jck mice (P < 0.03, n = 4).
To determine whether the increased expression of PC1 and PC2 protein was caused by an increase in gene transcription or a decrease in protein degradation, we performed real-time RT-PCR of Pkd1 and Pkd2 genes. Pkd1 mRNA levels in jck mice is 9.8 ± 0.15-fold greater than in wild-type mice even after correction by GAPDH mRNA level (P < 0.001, n = 4) (Figure 4E), indicating that increased PC1 expression is caused by increased transcription of Pkd1. This increased transcriptions of Pkd1 and Pkd2 is consistent with previous data.27 On the other hand, mRNA levels of Pkd2 in jck mice were only 1.9 ± 0.5-fold increased compared with wild-type mice (P < 0.03, n = 4) (Figure 4F). These results were consistent with Western blotting results of PC1 and PC2 expression in jck mouse kidneys.
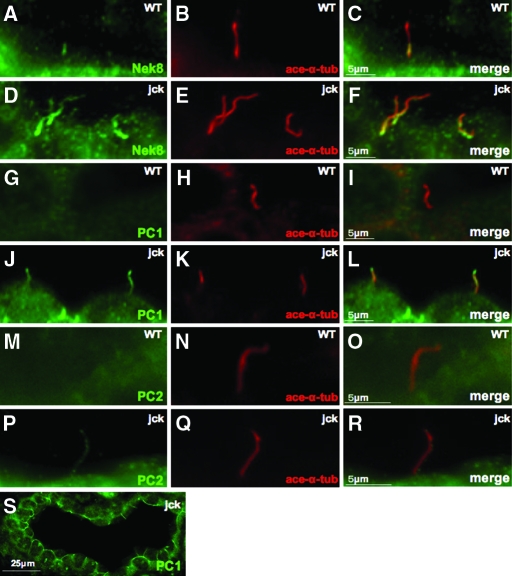
Aberrant Ciliary Localization of Nek8, PC1, and PC2 in jck Mouse Kidney
Having observed an increased level of PC1 protein expression and PC2 phosphorylation in the jck mouse kidney, we went on to investigate their expression and subcellular localization in the kidneys by immunohistochemistry. In contrast to its restricted expression in the proximal segment of primary cilia in wild-type kidneys (Figure 5, A to C), Nek8 expression was seen through out the length of primary cilia in jck mice (Figure 5, D to F).
Figure 5.
PC1 and PC2 abnormal expression in the primary cilia in the jck mouse kidney. (A to F) Ciliary localization of Nek8 in WT (A to C) and jck (D to F) mouse kidney. Nek8 (green) localized to the proximal region of the primary cilia in WT mouse kidney and to the full length of cilia in the jck mouse kidney. (G to L) Ciliary localization of PC1 in WT (G to I) and jck (J to L) mouse kidney. PC1 (green) was not detected in cilia of WT mouse kidney but strongly expressed in cilia of jck mouse kidney. (M to R) Ciliary localization of PC2 (green) in WT (M to O) and jck (P to R) mouse kidney. PC2 was not detected in cilia of WT mouse kidney, but clearly expressed in cilia of jck mouse kidney. (S) Localization of PC1 in plasma membrane of cyst-lining epithelia in jck mouse kidney. Acetylated-α-tubulin was used as a cilia marker (red).
Although unable to detect PC1 in primary cilia of kidney from 3-mo-old wild-type mouse kidney (Figure 5, G to I), we observed striking ciliary expression in 3-mo-old jck mice (Figure 5, J to L). PC1 localization in plasma membrane was also detected in the kidneys of jck mice (Figure 5W). We and others have previously shown that PC2 is localized to primary cilia in a variety of cultured cell lines17–21 and in the kidney.21 To our surprise, we did not detect PC2 in the cilia of tubular epithelia from wild-type mouse kidney, using either snap-frozen or perfusion-fixed kidney sections with nicely dilated lumens under our experimental conditions (Figure 5, M to O), although Nek8 was easily detectable in cilia of kidney tubules (Figure 5A). In jck mice, however, PC2 became easily detectable in the cilia (Figure 5, P to R). Consistent with a recent report,27 the cilia in jck kidney also appear to be longer than those in wild-type kidneys (Figure 5). The increased ciliary localizations of polycystins in jck mice were also observed in 15-d-old mice (Supplemental Figure I-D). These data suggest that abnormality in Nek8 results in increased PC1 and PC2 ciliary expression.
DISCUSSION
Although several proteins of the Nek family have been shown to play a role in regulating G2/M cell-cycle progression, the function of Nek8 is still not known. The finding that Nek8 is the protein mutated in the jck mouse linked Nek8 with PKD,10 which is believed to involve abnormal cell proliferation and differentiation. A recent study found that Nek8 as measured by RT-PCR is overexpressed in primary human breast tumors,11 suggesting that Nek8 is important in cell growth control.
In this study, we provide the first evidence of Nek8 localization to primary cilia in vivo. Although the ciliary expression of Nek8 was recently reported in a cultured cell line and primary culture of kidney cells,27,28 a previous study of Nek8 in kidney reported cytosolic but not ciliary Nek8 expression in collecting ducts.10 Therefore, it was unknown whether ciliary or cytosolic Nek8 correlates with cyst formation in jck mice. We found the ciliary localization of Nek8 only in the collecting tubules and collecting ducts, which suggests that Nek8 has a function in those locations. Furthermore, DBA and LTL staining revealed that cysts in jck mice are derived from collecting tubules and collecting ducts, correlating ciliary Nek8 expression with cyst formation in these segments in jck mice. This observation is highly significant, given the evidence that a number of genes shown to be mutated in PKD are similarly localized to cilia and that defects in ciliary proteins can cause PKD.12,13,17,18,20–26 We could detect the Nek8 signal in the cytosol, as previously reported.10 This antibody also recognizes ciliary Nek8 in IMCD cells28 (Y.L. and J.Z., unpublished observations).
Immunoprecipitation analysis revealed that PC2 but not PC1 is in the same protein complex with Nek8. Although Nek8 could not co-immunoprecipitate PC1, PC1 was able to co-immunoprecipitate PC2 in both the wild-type kidney and the jck mouse kidney. These results suggest that PC2 forms a distinct protein complex with either Nek8 or PC1.
We also observed an abnormal posttranslational modification of PC2 in the jck mouse kidney. Dephosphorylation assays suggested that it is likely a result of abnormal phosphorylation of PC2 as a result of the jck mutation. The mechanisms that regulate ciliary trafficking of PC2 in mammals are unknown. A few lines of evidence supported a role of PC2 phosphorylation on its trafficking. In Caenorhabditis elegans, the phosphodefective mutant Pkd2 (S534A) localized to cilia, whereas a phospho-mimicking Pkd2 (S534D) mutant was largely absent from cilia.29 On the contrary, in mammalian cells both wild-type PC2 and phosphodefective mutant S812A localized to cilia, indicating phosphorylation of Serine at position 812 does not affect the trafficking of PC2 to cilia.19 Recent study has revealed that another phosphorylation site at Serine 76 in PC2 regulated cell-surface localization of PC2 in mammalian cells but not ciliary localization.30 The increased phosphorylation and ciliary localization of PC2 in jck mice suggest that protein phosphorylation may serve as a mechanism for the control of ciliary targeting of proteins. However, we cannot exclude the possibility that this ciliary localization of polycystins in jck mice is simply caused by an overflow resulting from an increased amount of polycystins. Further investigation, especially on fine characterization of phosphorylation site(s) of PC2 in jck mice, is required in future.
An accumulation of polycystins in cilia has been reported in other PKD animal models. A study of osm-5, an ortholog of the mouse ARPKD gene Tg737, revealed that the Caenorhabditis elegans ADPKD gene products accumulated in stunted cilia in the absence of osm-5.31 The study of the orpk mouse model revealed that the ciliary expression of PC2 is elevated in Tg737 mutant polycystic mouse kidneys.21 However, increased phosphorylation of polycystins has not been reported in these animal models, indicating that the aberrant phosphorylation of PC2 in jck mice may be caused by specific loss of Nek8 function.
PC1 and PC2 were clearly localized to the cilia of cyst-lining epithelia in the jck mouse kidneys, consistent with the recent report on cultured kidney cells.27 To our surprise, we could not detect ciliary expressions of PC1 and PC2 in wild-type kidneys. This may be a result of the age of mice we analyzed and a tightly regulated entry of PC1 and PC2 to cilia by proteins such as Nek8. It is known that PC1 expression is developmentally regulated, its expression level significantly decreases in wild-type mouse kidney after birth,32 and it is barely detectable by immunofluorescence in the adult kidney, although it was easily detected in the fetal kidney.14,32 Because we did observe very clear ciliary PC1 and PC2 expression in cultured cells with the same antibody,17,20 we cannot exclude the possibility that the amount of ciliary PC1 and PC2 in the wild-type kidney is below the limit of immunohistochemical detection.
We found the ciliary Nek8 to be restricted to the proximal segment of cilia in the kidney. In jck mouse, Nek8 expression was seen throughout the length of primary cilia. This finding is different from the recent report in which the authors found the loss of Nek8 in primary cilia of cultured kidney cells from jck mouse.27 This difference may be the result of differences in cell type and cell differentiation status between tissues and cultured cells. The characteristics of the proximal segment of cilia are not well investigated. It has been reported, however, that the proximal segment of olfactory cilia appears to express a small fraction of cyclic nucleotide–gated channels, whereas the ciliary distal segment contains the majority,33 indicating the existence of different characteristics between proximal and distal segments of cilia. Fa2p, a NIMA-related kinase important for cilia disassembly, localized to proximal segment of the cilia in Chlamydomonas.5 Our data showed that Nek 8 regulates cilium length and restricts polycystins entering cilia. Together with the specific localization of Nek8 to the proximal segment of primary cilia, it is tempting to hypothesize that the proximal segment may be an important site for gating the entry of proteins to the shaft of cilia. Our previous work showed that PC1 and PC2 cooperate in cilia as the machinery for sensing fluid flow, and regulating a signaling cascade induced by calcium influx.17 In the jck mouse kidney, PC1 and PC2 accumulated in cilia of renal epithelia. Although we do not know at present whether the cilia are functional in the jck mice, the longer than normal length of cilia and the identification of abnormal levels and phosphorylation of the ciliary sensory machinery in cystic kidney suggest that ciliary defects are responsible for the uncontrolled tube lumen size and abnormal kidney development in the jck mice.
CONCISE METHODS
Mice
Three-month-old wild-type or jck mice in a C57 background were used for immunostaining, Western blot analysis, and real-time RT-PCR of PC1 and PC2. Fifteen-day-old mice were used for immunostaining.
Antibodies
Affinity-purified polyclonal antibody was raised against the fusion protein of the C-terminal RCC domain (285 to 698 amino acids) of mouse Nek8 (1:200 dilution for immunofluorescence and 1:1000 for Western blotting).10 Affinity-purified polyclonal antibody MR3 raised against 2938 to 2956 amino acids of PC1 (1:200 dilution)14 was used for immunohistochemistry. For Western blotting of PC1, monoclonal antibody 7e12 to the N-terminus region of PC1 (1:600 dilution)34 and polyclonal PC1 antibody to the C-terminus region of PC1 (1:200 dilution) (sc-25570, Santa Cruz Biotechnology, Santa Cruz, CA) were used. Affinity-purified polyclonal antibodies 96525 were raised against 44 to 62 amino acids of mouse PC2 (1:200 dilution for immunofluorescence and 1:1000 for western blotting).17,20 Commercial antibodies anti–α-acetylated-tubulin antibody (Sigma-Aldrich, St. Louis, MO) was used as marker for immunofluorescence (1:5000 dilution). Fluorescent DBA and LTL were purchased from Vector Laboratories (Burlingame, CA) and used as markers for immunofluorescence (1:500 dilution).
Immunofluorescence of Tissue Sections
Formalin-fixed tissue sections were deparaffinized and rehydrated, and antigen retrieval was performed with Antigen Unmasking Solution (Vector Laboratories) according to the manufacturer's protocol, except that we substituted 30-min boiling without high pressure for the prescribed 1-min boiling under high pressure. After antigen retrieval, the sections were preincubated with 10% goat serum in phosphate-buffered saline (PBS) and incubated with specific primary antibody in 10% goat serum in PBS for 2 h at 37°C. After washing, secondary antibodies were incubated for 1 h. Vectershield mounting medium with DAPI (Vector Laboratories) was used for DAPI staining and protection of immunofluorescence signals from fading. A Nikon TE2000-U microscope (Nikon Inc., Melville, NY) and a Hamamatsu ORCA-ER camera (Hamamatsu, Shizuoka, Japan) were used to capture images when noted.
Co-Immunoprecipitation and Western Blotting
Fresh mouse kidneys were homogenized in T-PER Tissue Protein Extraction Reagent (Pierce, Rockford, IL) containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). IMCD cells were homogenized in M-PER Tissue Protein Extraction Reagent (Pierce) containing protease inhibitor cocktail (Roche Diagnostics). The lysate was centrifuged at 10,000 × g at 4°C for 15 min, and the supernatant was collected. Co-immunoprecipitation and Western blotting were performed as described previously.20
For immunoblotting, we used 7e12 antibody for PC1 (1:600 dilution), C-terminus antibody for PC1 (1:200 dilution), and 96525 antibody for PC2 (1:1000 dilution). Next, filters were washed and incubated for 1 h in secondary antibodies (anti-mouse or anti-rabbit IgG horseradish peroxidase conjugated; Amersham Bioscience, Buckinghamshire, UK). Proteins were visualized with the ECL system (Hyperfilm, Amersham Bioscience). Dephosphorylation of kidney proteins was performed using a protein phosphatase 1 as recommended by the manufacture (New England Biolabs, Beverly, MA). Kidney homogenate from jck mouse kidney were incubated with or without protein phosphatase 1 at 30°C for 2 h before denaturing. For quantitative analysis of Western blotting band, we used ImageJ software.
Real-Time RT-PCR
Primers for real-time RT-PCR of mouse Pkd1, Pkd2, and GAPDH were prepared as follows:
mouse Pkd1: 5′ TAT CTG CAG TAC CGA CTG TGT TAC C 3′
mouse Pkd1: 5′ TTG AAC TGG CGG AGT ACC TG 3′
mouse Pkd2: 5′ TGT GTG GTC AGG TTA TTG GCG 3′
mouse Pkd2: 5′ GCC AGG AAG AAA TCA AAG GC 3′
This primer set was designed to amplify cDNA from 3368 to 3500 of Pkd1 (U70209) and 1303 to 1415 of Pkd2 (NM008861). Total RNA isolated from whole kidneys of 3-mo-old mice was reverse transcribed with M-MLV Reverse Transcriptase (Invitrogen, Carlsbad, CA) and Random Hexamers (QIAGEN, Valencia, CA). Real-time PCR was carried out in a LightCycler (Roche Dignostics) using QuantiTect SYBR Green PCR Kit (QIAGEN). Quantitative data were corrected with GAPDH mRNA expression level.
Short Hairpin RNA for PC2
pLKO.1-Based lentiviral vectors that contain stem-loop cassettes encoding shRNAs targeted to the coding sequence (TRCN88790) of the mouse Pkd2 were obtained from the MISSION TRC shRNA library through Sigma-Aldrich. The oligonucleotide sequences of the shRNAs were as follows (21-nt stem sequences matching the target transcript are underlined; noncomplementary 6-nt loop sequences are italicized): MISSION shRNA TRCN88790, 5′-CCGGCGTGATTGTCAAGCTGGAGATCTCGAGATCTCCAGCTTGACAATCACGTTTTT-3′. We used MISSION pLKO.1-puro Control Vector (Sigma-Aldrich) as control. VSV-G lentiviral particles were produced by cotransfection of 293T cells with pLKO.1 constructs, the packaging plasmid pCMVΔR8.91, and the VSV-G-expressing envelope plasmid pMD.G.35 All of the experimental procedure was performed according to instructions from The RNAi Consortium. Transfections were carried out using FuGENE 6 (Roche Diagnostics), and virus was harvested 48 and 72 h after transfection. Lentiviral supernatants were used to transduce cells in the presence of 8 μg/ml polybrene, and infected cells were selected with 2 μg/ml puromycin (Sigma-Aldrich).
DISCLOSURES
None.
Supplementary Material
Acknowledgments
The authors thank Q. Xi and P. Finnerty for technical assistance, P. Starremans for providing primer set for real-time RT-PCR, Dr. C. Ward for 7e12 antibody, Dr. S. Nauli for technical assistance, Dr. I.A. Drummond for helpful discussion and providing some of the kidney sections used in this study, and Dr. F. S. David for assistance with lentiviral shRNA screening and production. This work was supported by grants from the National Institutes of Health (DK53357, DK40703, and DK51050) to J.Z., and fellowships from Study Group on Molecular and Cellular Pathogene.sis of Kidney Diseases and JSPS Postdoctoral Fellowships for Research Abroad to E.S. and from the PKD Foundation to Y.L.
Published online ahead of print. Publication date available at www.jasn.org.
E.S. and Y.L. contributed equally to this work.
See related editorial, “Too Much of a Good Thing: Does NeK8 Link Polycystic Kidney Disease and Nephronophthisis?” on pages 418–420.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Lu KP, Hunter T: Evidence for a NIMA-like mitotic pathway in vertebrate cells. Cell 81: 413–424, 1995 [DOI] [PubMed] [Google Scholar]
- 2.O'Connell MJ, Norbury C, Nurse P: Premature chromatin condensation upon accumulation of NIMA. EMBO J 13: 4926–4937, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wloga D, Camba A, Rogowski K, Manning G, Jerka-Dziadosz M Gaertig J: Members of the NIMA-related kinase family promote disassembly of cilia by multiple mechanisms. Mol Biol Cell 17: 2799–2810, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahjoub MR, Montpetit B, Zhao L, Finst RJ, Goh B, Kim AC, Quarmby LM: The FA2 gene of Chlamydomonas encodes a NIMA family kinase with roles in cell cycle progression and microtubule severing during deflagellation. J Cell Sci 115: 1759–1768, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Mahjoub MR, Qasim Rasi M, Quarmby LM: A NIMA-related kinase, Fa2p, localizes to a novel site in the proximal cilia of Chlamydomonas and mouse kidney cells. Mol Biol Cell 15: 5172–5186, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fry AM: The Nek2 protein kinase: A novel regulator of centrosome structure. Oncogene 21: 6184–6194, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Fry AM, Meraldi P, Nigg EA: A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. Embo J 17: 470–481, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Upadhya P, Birkenmeier EH, Birkenmeier CS, Barker JE: Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc Natl Acad Sci U S A 97: 217–221, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogler C, Homan S, Pung A, Thorpe C, Barker J, Birkenmeier EH, Upadhya P: Clinical and pathologic findings in two new allelic murine models of polycystic kidney disease. J Am Soc Nephrol 10: 2534–2539, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA Beier DR: A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development 129: 5839–5846, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Bowers AJ, Boylan JF: Nek8, a NIMA family kinase member, is overexpressed in primary human breast tumors. Gene 328: 135–142, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW: The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol 11: 1341–1346, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Barr MM, Sternberg PW: A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401: 386–389, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Geng L, Segal Y, Peissel B, Deng N, Pei Y, Carone F, Rennke HG, Glucksmann-Kuis AM, Schneider MC, Ericsson M, Reeders ST, Zhou J: Identification and localization of polycystin, the PKD1 gene product. J Clin Invest 98: 2674–2682, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, Harris PC: The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet 10: 151–160, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Ibraghimov-Beskrovnaya O, Dackowski WR, Foggensteiner L, Coleman N, Thiru S, Petry LR, Burn TC, Connors TD, Van Raay T, Bradley J, Qian F, Onuchic LF, Watnick TJ, Piontek K, Hakim RM, Landes GM, Germino GG, Sandford R, Klinger KW: Polycystin: In vitro synthesis, in vivo tissue expression, and subcellular localization identifies a large membrane-associated protein. Proc Natl Acad Sci U S A 94: 6397–6402, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J: Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Yoder BK, Hou X, Guay-Woodford LM: The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Cai Y, Anyatonwu G, Okuhara D, Lee KB, Yu Z, Onoe T, Mei CL, Qian Q, Geng L, Wiztgall R, Ehrlich BE, Somlo S: Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J Biol Chem 279: 19987–19995, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J: Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol Cell Biol 23: 2600–2607, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB: Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–R380, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D'Eustachio P, Beier DR, Guay-Woodford LM: Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest 109: 533–540, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NL, Wilkinson JE, Woychik RP: Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science 264: 1329–1333, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG: Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151: 709–718, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Luo Y, Wilson PD, Witman GB, Zhou J: The autosomal recessive polycystic kidney disease protein is localized to primary cilia, with concentration in the basal body area. J Am Soc Nephrol 15: 592–602, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC: Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet 12: 2703–2710, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Smith LA, Bukanov NO, Husson H, Russo RJ, Barry TC, Taylor AL, Beier DR, Ibraghimov-Beskrovnaya O: Development of polycystic kidney disease in juvenile cystic kidney mice: Insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol 17: 2821–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Mahjoub MR, Trapp ML, Quarmby LM: NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J Am Soc Nephrol 16: 3485–3489, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Hu J, Bae YK, Knobel KM, Barr MM: Casein kinase II and calcineurin modulate TRPP function and ciliary localization. Mol Biol Cell 17: 2200–2211, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Streets AJ, Moon DJ, Kane ME, Obara T, Ong AC: Identification of an N-terminal glycogen synthase kinase 3 phosphorylation site which regulates the functional localization of polycystin-2 in vivo and in vitro. Hum Mol Genet 15: 1465–1473, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin H, Rosenbaum JL, Barr MM: An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Curr Biol 11: 457–461, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Geng L, Segal Y, Pavlova A, Barros EJ, Lohning C, Lu W, Nigam SK, Frischauf AM, Reeders ST, Zhou J: Distribution and developmentally regulated expression of murine polycystin. Am J Physiol 272: F451–F459, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Flannery RJ, French DA, Kleene SJ: Clustering of cyclic-nucleotide-gated channels in olfactory cilia. Biophys J 91: 179–188, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ong AC, Ward CJ, Butler RJ, Biddolph S, Bowker C, Torra R, Pei Y, Harris PC: Coordinate expression of the autosomal dominant polycystic kidney disease proteins, polycystin-2 and polycystin-1, in normal and cystic tissue. Am J Pathol 154: 1721–1729, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D: Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 15: 871–875, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.