Abstract
Activation and expansion of interstitial fibroblasts and myofibroblasts play an essential role in the evolution of renal fibrosis. After obstructive injury, mice lacking tissue-type plasminogen activator (tPA) have fewer myofibroblasts and less interstitial fibrosis than wild-type controls. This suggests that tPA controls the size of the fibroblast/myofibroblast population in vivo, and this study sought to determine the underlying mechanism. In vitro, tPA inhibited staurosporine or H2O2-induced caspase-3 activation, prevented cellular DNA fragmentation, and suppressed the release of cytochrome C from mitochondria into the cytosol in a rat interstitial fibroblast cell line (NRK-49F). tPA also protected TGF-β1–activated myofibroblasts from apoptosis. This antiapoptotic effect of tPA was independent of its protease activity but required its membrane receptor, the LDL receptor–related protein 1 (LRP-1). Deletion or knockdown of LRP-1 abolished tPA-mediated cell survival, whereas re-introduction of an LRP-1 minigene in a mouse LRP-1–deficient fibroblast cell line (PEA-13) restored the cytoprotective ability of tPA. tPA triggered a cascade of survival signaling involving extracellular signal–regulated kinase 1/2 (Erk1/2), p90RSK, and phosphorylation of Bad. Blockade of Erk1/2 activation abrogated the antiapoptotic effect of tPA, whereas expression of constitutively active MEK1 promoted cell survival similar to tPA. In vivo, compared with wild-type controls, apoptosis of interstitial myofibroblasts was increased in tPA−/− mice after obstructive injury, and myofibroblasts were completely depleted 4 wk after relief of the obstruction. Together, these findings illustrate that tPA is a survival factor that prevents apoptosis of renal interstitial fibroblasts and myofibroblasts through an LRP-1–, Erk1/2-, p90RSK-, and Bad-dependent mechanism.
Despite the diverse causes of kidney diseases, tubulointerstitial fibrosis is considered as the inevitable, convergent destiny of a wide variety of nephropathies. Interstitial fibrosis is characterized by florid inflammation, massive activation of fibroblasts, and excessive deposition of extracellular matrix (ECM).1,2 Interstitial fibroblasts, along with the activated, α-smooth muscle actin (α-SMA)-positive myofibroblasts, are the primary matrix-producing cells and considered to be the principal mediator of renal fibrosis associated with progressive renal failure.3–5 It is well documented that the numbers of interstitial fibroblasts and myofibroblasts closely correlate with the magnitude and severity of tubulointerstitial fibrosis and concomitant decline of kidney function in both experimental animal models and patients with chronic kidney disease.4,6 The expanded population of fibroblasts in the diseased kidney could result from an increase of proliferation and/or a decrease in cell death. Although evidence indicates that fibroblasts from the fibrotic kidney display an increased proliferative activity,7,8 regulation of interstitial fibroblast and myofibroblast survival in pathologic conditions remains largely mysterious.
Tissue-type plasminogen activator (tPA), a member of the serine protease family, plays a pivotal role in the homeostasis of blood coagulation and fibrinolysis. In the kidney, the main function of tPA is to convert plasminogen into biologically active plasmin, which in turn participates in the regulation of matrix homeostasis via its proteolytic potential. Earlier studies from our laboratory showed that mice lacking tPA display a reduced myofibroblast mass and are protected against interstitial fibrosis after obstructive injury, although the reasons are incompletely understood.9 tPA is a molecule with dual functions: Its protease activity renders it able to participate in activation of zymogens and certain growth factors,10,11 and its cytokine property endows it with the ability to regulate specific gene expression by triggering cell signaling via binding to cell membrane receptor, the LDL receptor–related protein-1 (LRP-1).12–14 It is interesting that our previous studies demonstrated that the α-SMA–positive myofibroblast population is actually reduced in tPA−/− mice at 7 d after obstructive injury, as compared with an earlier time point (3 d).9 This suggests that under pathologic conditions, tPA may be an endogenous factor that governs the survival and fate of myofibroblasts, thus ultimately controlling the size of the activated fibroblast population.
In this study, we demonstrated that tPA functions as a survival factor that prevents renal interstitial fibroblasts and myofibroblasts from apoptosis induced by staurosporine and oxidative stress. We showed that tPA triggers a cascade of cell survival signaling involving the sequential phosphorylation of extracellular signal–regulated kinase 1/2 (Erk1/2), p90 ribosomal protein S6 kinase (p90RSK), and Bad. It seems that the antiapoptotic effect of tPA is independent of its protease activity but requires its membrane receptor LRP-1. Hence, our data reveal that tPA can act as a cytokine, executing its cytoprotective actions via activation of a survival signaling hierarchy in interstitial fibroblasts.
RESULTS
tPA Protects Renal Interstitial Fibroblasts from Staurosporine- and H2O2-Induced Apoptosis
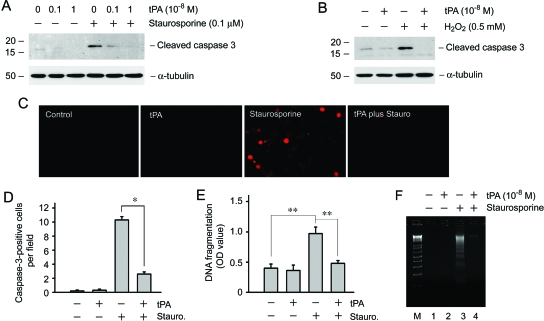
For evaluation of the effects of tPA on renal interstitial fibroblast survival after injury, normal rat kidney interstitial fibroblasts (NRK-49F) were treated with staurosporine, a well-established proapoptotic death inducer.15 As shown in Figure 1A, staurosporine at 0.1 μM markedly induced caspase-3 cleavage and activation in interstitial fibroblasts, as demonstrated by Western blot analysis using an antibody specific for cleaved caspase-3. Addition of tPA dramatically inhibited caspase-3 activation induced by staurosporine in a dosage-dependent manner; tPA at 10−8 M almost completely abolished the generation of cleaved caspase-3 in NRK-49F cells. Immunofluorescence staining also revealed that tPA significantly prevented caspase-3 activation in interstitial fibroblasts (Figure 1, C and D). Using an independent assessment of apoptosis, we could show that addition of tPA prevented the staurosporine-induced DNA fragmentation in interstitial fibroblasts (Figure 1, E and F). Furthermore, tPA protected NRK-49F cells against H2O2-induced apoptosis, as illustrated by the lack of activated caspase-3 in tPA-treated cells (Figure 1B).
Figure 1.
tPA protects renal interstitial fibroblasts from apoptotic death. (A) tPA inhibited staurosporine-induced caspase-3 activation in a dosage-dependent manner. NRK-49F cells were treated with 0.1 μM staurosporine in the absence or presence of various doses of tPA as indicated for 4 h. Cell lysates were immunoblotted with antibodies against cleaved caspase-3 or α-tubulin, respectively. (B) tPA also protected NRK-49F cells from H2O2-triggered caspase-3 activation. NRK-49F cells were incubated with 0.5 mM H2O2 for 16 h. (C and D) Immunofluorescence staining showed that tPA reduced active caspase-3–positive cells after staurosporine treatment. Representative images from various groups (C) as well as quantitative data on active caspase-3–positive cells per ×400 field (D) were presented. *P < 0.05 (n = 5). (E) tPA prevented renal interstitial fibroblasts from DNA fragmentation after staurosporine treatment. DNA fragmentation was assayed at 4 h after various treatments by an ELISA and expressed as OD value at 450 nm. **P < 0.01 (n = 3). (F) tPA prevented NRK-49F cell DNA fragmentation after staurosporine treatment as shown using a DNA laddering assay. M, DNA size marker; 1, control; 2, tPA (10−8 M); 3, staurosporine (0.1 μM); and 4, tPA plus staurosporine.
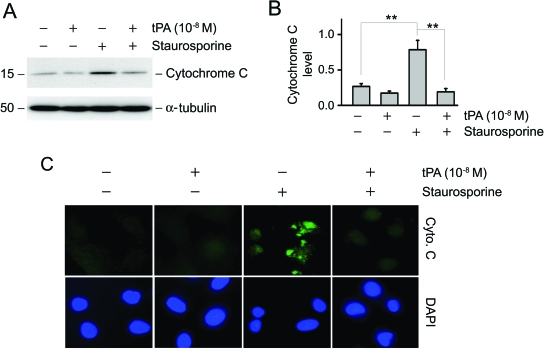
We next examined the potential effect of tPA on cytochrome C release from mitochondria, a critical, early event in cell apoptosis triggered by a variety of different injuries. As shown in Figure 2, staurosporine disrupted mitochondrial membrane integrity, resulting in the release of cytochrome C from the mitochondria to cytosol. This step is known to precede caspase activation and subsequent apoptosis.16,17 As shown in Figure 2, A and B, treatment with tPA effectively prevented the release of cytochrome C into the cytosol. These results were confirmed using immunostaining for cytochrome C in treated NRK-49F cells (Figure 2C). Thus, tPA is able to stabilize the mitochondrial membrane and prevent the release of cytochrome C to the cytosol and therefore leads to an increase in cell survival.
Figure 2.
tPA stabilizes mitochondrial membranes and prevents cytochrome C release from mitochondria. (A) Mitochondria and cytosol of NRK-49F cells were separated after staurosporine treatment in the absence or presence of tPA. Cytosolic proteins were subjected to Western blot analysis for cytochrome C. (B) Quantitative determination of the relative abundance of cytochrome C release among different groups. Data are means ± SEM of three experiments. **P < 0.01. (C) Immunofluorescence staining for cytochrome C (green) in NRK-49F cells after various treatments as indicated. DAPI (blue) was used to visualize the nuclei.
tPA also Protects Renal Interstitial Myofibroblasts from Apoptosis
In the fibrotic kidney, interstitial fibroblasts are often activated and transformed into α-SMA–positive myofibroblasts1,3; therefore, we next sought to test whether tPA also protects activated myofibroblasts from apoptosis. To this end, NRK-49F cells were preincubated with 0.5 ng/ml TGF-β1 for 24 h to induce myofibroblastic activation, which was confirmed by positive staining for α-SMA (Figure 3A). These activated fibroblasts were treated with staurosporine in the absence or presence of tPA. As shown in Figure 3, tPA was able to protect activated myofibroblasts from staurosporine-triggered apoptosis; Western blot analysis demonstrated that tPA abolished the induction of cleaved caspase-3. Double immunostaining for α-SMA and cleaved caspase-3 confirmed the cytoprotective role of tPA in preventing myofibroblast apoptosis (Figure 3A).
Figure 3.
tPA protects renal interstitial myofibroblasts from apoptosis in vitro. NRK-49F cells were preincubated with 0.5 ng/ml TGF-β1 for 24 h to induce the activated myofibroblast phenotype, as confirmed by positive staining for α-SMA. Cells were then treated with staurosporine (0.1 μM) and/or tPA as indicated for 4 h. (A) Double immunofluorescence staining for α-SMA (green) and active caspase-3 (red). Nuclei were stained with DAPI (blue). (B and C) Western blot analysis demonstrated the presence of active, cleaved caspase-3. A representative picture (B) and quantitative determination (C) of relative cleaved caspase-3 levels are presented. *P < 0.05 (n = 3).
Antiapoptotic Effect of tPA Is Independent of Its Protease Activity
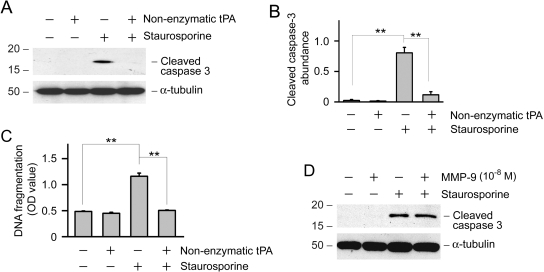
tPA is a unique protein with dual functions, possessing both protease and cytokine properties.12 For determination of whether the protease activity of tPA is involved in mediating its antiapoptotic effects, NRK-49F cells were treated with a nonenzymatic mutant of tPA at comparable concentrations to wild-type tPA. Mutant tPA has the serine within the active site of the enzyme mutated to alanine, rendering it catalytically inactive.18,19 As shown in Figure 4, A through C, nonenzymatic tPA retained its ability to inhibit staurosporine-induced caspase-3 activation and DNA fragmentation, suggesting that the prosurvival action of tPA is independent of its protease activity. Earlier studies showed that tPA induces matrix metalloproteinase-9 (MMP-9) expression in NRK-49F fibroblasts.12 To determine whether tPA promotes cell survival through its induction of MMP-9, we examined the effect of MMP-9 on NRK-49F apoptosis. As shown in Figure 4D, MMP-9 seemed to have no effect on staurosporine-induced apoptosis of fibroblasts.
Figure 4.
Antiapoptotic effect of tPA is independent of its protease activity. (A) NRK-49F cells were incubated with staurosporine (0.1 μM) and/or nonenzymatic tPA (10−8 M) for 4 h. Cell lysates were immunoblotted with antibodies against active, cleaved caspase-3 and α-tubulin, respectively. (B) Quantitative determination of relative abundance of cleaved caspase-3. Data are means ± SEM of three experiments. **P < 0.01. (C) Nonenzymatic tPA prevented DNA fragmentation in NRK-49F cells after staurosporine treatment for 4 h. DNA fragmentation was assayed by an ELISA and expressed as OD value at 450 nm. **P < 0.01 (n = 3). (D) MMP-9 did not affect fibroblast apoptosis after staurosporine treatment. NRK-49F cells were treated with staurosporine (0.1 μM) and/or MMP-9 (10−8 M) for 4 h. Cell lysates were immunoblotted with antibodies against cleaved caspase-3 and α-tubulin, respectively.
Cytoprotective Action of tPA Is Mediated by Its Membrane Receptor LRP-1
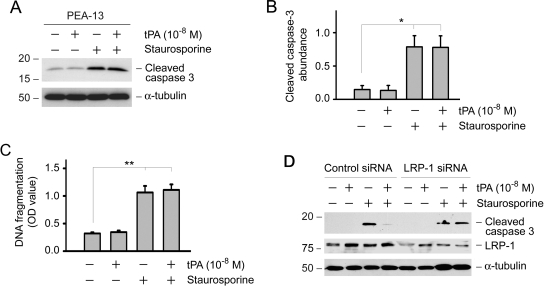
Our previous studies suggested that the cytokine function of tPA is mediated by the membrane receptor LRP-1 because tPA specifically binds to LRP-1 and triggers its tyrosine phosphorylation.12 To test whether the antiapoptotic action of tPA also occurs via LRP-1, we assessed the ability of tPA to promote cell survival in a mouse embryonic fibroblast cell line that is LRP deficient (PEA-13). As shown in Figure 5, A through C, tPA was unable to prevent caspase-3 activation and DNA fragmentation after staurosporine treatment in PEA-13 cells, suggesting that LRP-1 is required for tPA to elicit its antiapoptotic actions. Furthermore, knockdown of LRP-1 expression in NRK-49F cells by small interference RNA (siRNA) strategy abolished the ability of tPA to promote cell survival (Figure 5D). Of note, LRP-1 downregulation in NRK-49F cells was confirmed by Western blot after transfection with LRP-1–specific siRNA (Figure 5D).
Figure 5.
Antiapoptotic effect of tPA is mediated by its cell membrane receptor LRP-1. (A) Knockout of LRP-1 abolished the prosurvival action of tPA. LRP-1–deficient mouse embryonic fibroblast cells (PEA-13) were incubated with staurosporine (0.1 μM) and/or tPA (10−8 M) for 6 h. Cell lysates were immunoblotted with antibodies against cleaved caspase-3 and α-tubulin, respectively. (B) Quantitative determination of relative abundance of cleaved caspase-3. Data are means ± SEM of three experiments. *P < 0.05. (C) DNA fragmentation was assayed by an ELISA and expressed as OD 450 nm. **P < 0.01 (n = 3). (D) Knockdown of LRP-1 in NRK-49F cells by siRNA inhibition abolished the cytoprotective effect of tPA. NRK-49F cells were transfected with either control siRNA or LRP-1–specific siRNA, followed by treatment with staurosporine and/or tPA as indicated. Cell lysates were immunoblotted with antibodies against cleaved caspase-3, LRP-1, and α-tubulin.
We further addressed this issue by re-introducing the LRP-1 gene into PEA-13 cells. Because the cDNA that encodes LRP-1 is >15 kb in size and is difficult to manipulate, we used a LRP-1 minigene in which the ligand-binding domain II of LRP-1 is linked to its β subunit (Figure 6A). This LRP-1 minireceptor (mLRP2) retains the binding site for tPA and is biologically functional.14,20 As shown in Figure 6, B and C, ectopic expression of LRP-1 minigene largely restored the ability of tPA to prevent apoptosis induced by staurosporine in LRP-1–deficient fibroblasts. Hence, LRP-1 is essential for mediating the prosurvival activity of tPA in fibroblasts.
Figure 6.
mini-LRP-1 gene restores the cytoprotective effect of tPA in LRP−/− fibroblasts. (A) Graphic illustration of the structure of full-length and mini-LRP-1. mLRP2 consists of the ligand-binding domain II, which binds to tPA, and the entire 85-kD β unit of LRP-1. (B) Overexpression of pHA-mLRP2 into LRP-1−/− fibroblast (PEA-13) restored the antiapoptotic effect of tPA. (C) Quantitative illustration of relative abundance of cleaved caspase 3. **P < 0.01 (n = 3).
tPA Activates a Cascade of Survival Signaling that Involves Sequential Phosphorylation of Erk1/2, p90RSK, and Bad
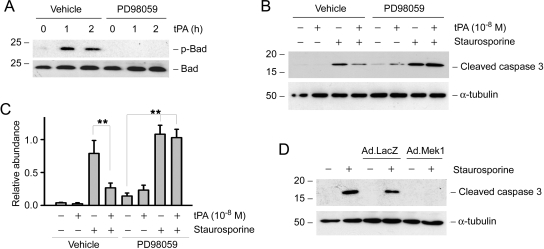
To explore the mechanism by which tPA inhibits apoptosis, we next investigated cell survival–related signaling in interstitial fibroblasts after tPA stimulation. We found that tPA did not affect Akt/protein kinase B phosphorylation and activation (data not shown) but rapidly induced Erk1/2 activation in NRK-49F cells, as demonstrated by Western blot analysis using an antibody specific for phosphorylated Erk1/2 (Figure 7A). Erk1/2 phosphorylation took place at 2 min after tPA stimulation and returned to baseline by 30 min. We also found that p90RSK, a downstream effector kinase of Erk1/2, was dramatically and transiently phosphorylated at 5 min (Figure 7B), which was followed by a sustained phosphorylation of Bad that began between 10 and 30 min after tPA stimulation (Figure 7C). Because tPA-mediated LRP-1 phosphorylation occurs at 0.5 to 1 min after tPA treatment,12 it seems that in interstitial fibroblasts, after tPA stimulation, there is a cascade of cell survival signaling that involves the sequential phosphorylation of Erk1/2, p90RSK, and Bad (Figure 7D).
Figure 7.
tPA activates cell survival signaling via sequential phosphorylation of Erk1/2, p90RSK, and Bad. NRK-49F cells were incubated with 10−8 M tPA for various periods of time as indicated and then subjected to Western blotting for phospho-Erk1/2 (A), phospho-p90RSK (B), and phospho-Bad (C). The samples were also probed with antibodies against total Erk1/2, p90RSK1/2/3, and Bad, respectively. (D) Schematic illustration of the cytoprotective effects of tPA in interstitial fibroblast. After binding to its membrane receptor LRP-1, tPA induces its tyrosine phosphorylation, which in turn stimulates Erk1/2 and p90RSK phosphorylation, leading to Bad phosphorylation. Phosphorylation of Bad suppresses cytochrome C release from mitochondria and protects the cells from apoptosis. *Tyrosine or serine/threonine phosphorylation.
Activation of Erk1/2 Is Necessary for Interstitial Fibroblast Survival
Next we tested whether Erk1/2-mediated signaling is required for promoting the tPA-induced fibroblast survival. To this end, NRK-49F cells were incubated with tPA in the absence or presence of PD98059, a specific inhibitor of the Erk1/2 upstream kinase mitogen-activated protein kinase/Erk kinase-1 (MEK-1). Of note, PD98059 at the concentrations used was able fully to suppress Erk1/2 activation in NRK-49F cells, as previously reported.21 As shown in Figure 8A, inhibition of Erk1/2 activation blocked tPA-mediated Bad phosphorylation. Similarly, PD98059 abrogated the prosurvival actions of tPA and restored the staurosporine-induced caspase-3 activation in interstitial fibroblasts (Figure 8, B and C), suggesting that Erk1/2-triggered signaling is necessary for mediating the prosurvival activity of tPA. Ectopic expression of constitutively activated MEK1 via an adenoviral vector mimicked the effect of tPA, abolishing staurosporine-induced caspase-3 activation in NRK-49F cells (Figure 8D). Together, these data suggest that Erk1/2 activation induced by tPA is indispensable for promoting fibroblast survival after injury.
Figure 8.
Erk1/2 activation is indispensable for mediating interstitial fibroblast survival induced by tPA. (A) Pretreatment with the MEK1 inhibitor PD98059 (20 μM) for 0.5 h abrogated the Bad phosphorylation induced by tPA. (B) PD98059 also abolished the tPA-mediated inhibition of caspase-3 activation in NRK-49F cells. (C) Quantitative determination of relative abundance of cleaved caspase-3. Data are means ± SEM of three experiments. **P < 0.01. (D) Overexpression of constitutively active MEK1 by an adenoviral vector (Ad.MEK1) was sufficient to prevent caspase-3 activation after staurosporine treatment. NRK-49F cells were infected with adenovirus for 16 h, followed by incubation with staurosporine (0.1 μM) for 4 h. An adenoviral vector containing the β-galactosidase gene (Ad.LacZ) was used as a control.
tPA Promotes Renal Interstitial Myofibroblast Survival In Vivo
To examine whether tPA promotes interstitial fibroblast and myofibroblast survival in vivo, we studied myofibroblast apoptosis in fibrotic kidneys induced by unilateral uretal obstruction (UUO) in tPA+/+ and tPA−/− mice. The overall abundance of α-SMA at 7 d after UUO in tPA−/− kidney was significantly less than that in the wild-type controls (Figure 9, A and B), as previously reported.9 Kidney sections were stained for active caspase-3. As shown in Figure 9, C and D, the number of apoptotic interstitial cells, as indicated by positive cleaved caspase-3 staining, was significantly higher in tPA-deficient mice compared with their wild-type controls (Figure 9, C and D). Double immunofluorescence staining confirmed that the cells that underwent apoptosis were largely the α-SMA–positive myofibroblasts (Figure 9C, right). These data strongly suggest that, in vivo, tPA is also able to protect interstitial myofibroblasts from apoptosis in diseased kidneys.
Figure 9.
Increased apoptosis of interstitial myofibroblasts in mice lacking tPA after obstructive injury. (A) Western blot analyses show a reduced α-SMA expression in tPA−/− mice after obstructive injury. Kidney homogenates from both tPA+/+ and tPA−/− mice at 7 d after UUO were assayed for α-SMA expression. Contralateral intact kidneys (CLK) served as controls. (B) Quantitative determination of relative abundance of α-SMA protein among various groups. Data are means ± SEM of four to five animals per group. *P < 0.05. (C) Representative micrographs show immunohistochemical staining for cleaved caspase-3 (red, left). (Middle, inset) Enlarged image of the cleaved caspase-3–positive cell. (Right) Double immunofluorescence staining confirmed the co-localization of α-SMA (green) and cleaved caspase-3 (red). Paraffin-embedded kidney sections were prepared from tPA+/+ or tPA−/− mice at day 7 after UUO. Arrowheads indicate the cells with positive staining in the obstructed kidneys. (D) Interstitial cells undergoing apoptosis (cleaved caspase-3 positive) were calculated and expressed as the percentage in total interstitial cell population. Data were obtained from five animals per group. **P < 0.01 (n = 5). (E) Experimental design for reversible UUO model. (F) Western blot analyses show α-SMA expression at 4 wk after relief of obstruction in the reversible UUO model. Complete loss of α-SMA expression was observed only in tPA−/− mice but not in tPA+/+ controls. Numbers 1 through 5 denote each individual animal. (G) Quantitative determination of α-SMA expression at 4 wk after relief of obstruction in reversible UUO model. *P < 0.05 (n = 5).
We further examined the consequence of an accelerated myofibroblast apoptosis in tPA−/− mice by using a reversible UUO model. As shown in Figure 9E, both tPA−/− and tPA+/+ mice were subjected to a temporary UUO for 7 d, followed by relief of the obstruction. At 4 wk after relief, renal α-SMA expression was assessed quantitatively by Western blot. As demonstrated in Figure 9, F and G, little α-SMA was detected in tPA−/− kidney at 4 wk after relief of obstruction, whereas α-SMA expression was largely sustained in tPA+/+ kidney under the same conditions. This indicates that tPA deficiency promotes myofibroblast apoptosis, leading to the complete depletion of these cells in the obstructed kidney after the obstruction is relieved.
DISCUSSION
Interstitial fibrosis is generally preceded by a massive activation and expansion of interstitial fibroblasts and myofibroblasts.1–3 Because these cells are the principal matrix-producing effector cells, the size of the activated fibroblast and myofibroblast population in fibrotic kidneys is believed to be a determining factor dictating the progression and prognosis of a wide variety of chronic kidney diseases.22,23 In normal kidneys, interstitial fibroblasts are scarce in number and often quiescent in nature. After chronic injury, these cells become phenotypically activated, with an increased proliferative capacity, de novo expression of the myofibroblast hallmark, α-SMA, and active production of ECM components1,3; however, regulation of the ultimate destiny of these activated fibroblasts under pathologic conditions remains elusive. In this study, we demonstrated that tPA is a key endogenous survival factor controlling the fate of renal interstitial fibroblasts and myofibroblasts. tPA, via its membrane receptor LRP-1, transduces a cascade of signaling that protects fibroblasts from apoptosis induced by a variety of death cues. These findings establish a novel, previously unrecognized function for tPA in controlling the size of the activated fibroblast population in diseased kidneys.
Apoptosis is a tightly regulated active process of programmed cell death that is essential to the homeostasis of cell mass.24 In diseased kidneys, cells often have to make a life or death decision, primarily based on the balance of survival and death signals.25 In this sense, enhanced survival signaling elicited by tPA could render a dramatic shift in this delicate balance, producing a significant impact on the fate of interstitial fibroblasts in response to various death-inducing cues. The cytoprotective role of tPA in preventing fibroblasts from apoptosis is clearly supported by several lines of observation. For instance, tPA prevented the cleavage and activation of caspase-3, a major effector caspase that degrades many physiologically important proteins and causes cells to die in an organized manner. Furthermore, tPA protected fibroblasts from undergoing DNA fragmentation as well as preventing the release of cytochrome C from mitochondria to cytosol, two classic apoptotic hallmarks.17 Notably, the antiapoptotic effect of tPA is not limited to quiescent fibroblasts. tPA was also clearly effective in preventing TGF-β1–activated, α-SMA–positive myofibroblasts from apoptosis after injury. This latter observation is clinically relevant because the majority of the interstitial fibroblasts in diseased kidneys are activated myofibroblasts; however, tPA seemed not to protect renal tubular epithelial cells (HKC-8) from apoptosis (data not shown), consistent with the observation that both tPA and LRP-1 are predominantly expressed in renal interstitial cells but not tubular cells.9,12 Hence, it is conceivable that tPA may selectively protect renal interstitial fibroblasts/myofibroblasts from apoptosis. Furthermore, given that tPA also blocks apoptosis induced by H2O2 (Figure 1B), it seems that the prosurvival action of tPA is operating in a death inducer–independent manner. Together, our data clearly suggest that tPA is a potent, endogenous cell survival factor for interstitial fibroblasts and myofibroblasts, and, by virtue of its ability to inhibit apoptosis, tPA is likely a crucial player in regulating the mass of activated fibroblasts in fibrotic kidneys.
tPA is a widely known serine protease that plays a pivotal role in the homeostasis of blood coagulation and fibrinolysis. Emerging evidence indicates that tPA can also elicit numerous cellular activities by a mechanism independent of its protease activity.26–29 These findings have led to a conceptual advance in our understanding of the biology of tPA. We previously proposed that tPA can function as a cytokine by binding to its cell membrane receptor, LRP-1, triggers its tyrosine phosphorylation, induces intracellular signal transduction, and stimulates expression of specific genes.12 That notion is further strengthened by this study, because mutant tPA that lacks catalytic enzyme activity18,19 still retains its ability to prevent apoptosis (Figure 4), indicating that the prosurvival action of tPA is also independent of its protease activity. Because the prosurvival activity of tPA also depends on LRP-1 (Figures 5 and 6), its function is clearly mediated by a protease activity–independent, receptor-dependent mechanism in interstitial fibroblasts.
By delineating the intracellular signal pathway leading to cell survival, it seems that tPA can initiate a cascade of cell survival signaling involving the sequential phosphorylation of Erk1/2, p90RSK, and Bad (Figure 7). Earlier studies from our laboratory demonstrated that tPA binds to LRP-1, inducing a rapid and transient phosphorylation of its tyrosine residues that, in turn, transactivates the Erk1/2 mitogen-activated protein kinase pathway.12 It is now demonstrated that this Erk1/2 activation is indispensable for mediating the prosurvival actions of tPA. The importance of Erk1/2 in tPA-mediated protection of interstitial fibroblasts is corroborated by both the chemical blockade of its activation and via the use of a MEK1-expressing adenoviral vector to induce constitutive activation (Figure 8). Notably, the observation that Erk1/2 activation is critical for tPA-mediated fibroblast survival is consistent with other reports that Erk1/2 mitogen-activated protein kinase is essential for cell survival under different circumstances.30,31 The downstream effector kinase of Erk1/2 seems to be p90RSK, because tPA rapidly induces its phosphorylation and activation. p90RSK, in turn, can phosphorylate Bad, a pro-death member of the Bcl-2 protein family. Phosphorylation of Bad leads to its inactivation and subsequent degradation,32,33 thereby preventing it from entering the mitochondria after injury. Loss of Bad from the mitochondria leads to the suppression of cytochrome C release into the cytosol, ultimately preventing the cleavage and activation of caspases and, hence, blocking cell apoptosis (Figure 7D). The phosphorylation kinetics of these signaling mediators insinuates a strictly linear pattern, with LRP-1 phosphorylation at 0.5 to 1 min,12 Erk1/2 at 2 min, p90RSK at 5 min, and Bad between 10 and 30 min (Figure 7). Activation of LRP-1, Erk1/2, and p90RSK is transient; however, Bad phosphorylation is sustained after tPA stimulation, suggesting that a transient activation of cellular signaling can lead to sustainable biologic actions.
The results presented here are in harmony with our previous observation that tPA deficiency protects kidney from developing interstitial fibrosis after obstructive injury.9 One of the interesting findings from our earlier studies was that the level of renal α-SMA at 7 d after UUO actually lessened compared with those found at 3 d in tPA−/− mice.9 That suggested to us that tPA deficiency might accelerate myofibroblast depletion, presumably by an apoptotic mechanism. This study authenticates our hypothesis because a higher percentage of α-SMA–positive myofibroblasts are actively undergoing apoptosis in tPA−/− mice, compared with wild-type controls. Hence, accelerated apoptosis is likely another pathway leading to a decreased myofibroblast population in tPA−/− mice after obstructive injury. This speculation is unambiguously supported by our reversible UUO model, in which tPA deficiency resulted in the complete depletion of α-SMA expression in the kidney at 4 wk after UUO was relieved (Figure 9). Hence, tPA is a major survival factor for interstitial myofibroblasts in vivo.
It should be emphasized that activated fibroblasts in the diseased kidney are heterogeneous and can originate from different sources, including residential quiescent fibroblasts and tubular epithelial cells via epithelial-to-mesenchymal transition (EMT).34–36 Because tPA is also known to induce MMP-9 gene expression that, in turn, degrades and impairs the tubular basement membrane, leading to an increase of EMT,9 this study suggests that tPA controls the size of the activated fibroblast pool in diseased kidneys both by promoting their generation (via EMT) and by preventing them from undergoing apoptosis.
In summary, this study demonstrates that tPA is a potent survival factor that prevents interstitial fibroblast/myofibroblast from apoptosis. After binding to its receptor LRP-1, tPA triggers a sequential phosphorylation of Erk1/2, p90RSK, and Bad, leading to activation of a major cell survival signal pathway in interstitial fibroblasts. By inhibiting apoptosis, upregulated tPA controls the volume of the activated fibroblast population in diseased kidney, thereby having a profound impact on renal interstitial fibrosis.
CONCISE METHODS
Antibodies and Reagents
The rabbit antibodies against cleaved caspase-3, cytochrome C, phospho-specific Bad, phospho-specific p90RSK, phospho-specific Erk1/2, and RSK1/2/3 were purchased from Cell Signaling Technology (Beverly, MA). Mouse anti–α-SMA and anti–α-tubulin and rabbit anti-total Erk1/2 antibodies were obtained from Sigma (St. Louis, MO). Rabbit anti-mouse α-SMA antibody was purchased from Abcam (Cambridge, MA). Rat anti-Bad antibody was purchased from BD Biosciences Pharmingen (Franklin Lakes, NJ). Mouse monoclonal anti–LRP-1 antibody (11H4) was described previously.12 The secondary horseradish peroxidase–conjugated antibodies were obtained from Sigma and Chemicon (Temecula, CA), respectively. Recombinant human single-chain tPA was purchased from American Diagnostica (Stamford, CT). The nonenzymatic tPA was supplied by Molecular Innovations (Southfield, MI). Recombinant human TGF-β1 was provided by R&D Systems (Minneapolis, MN). MEK1 inhibitor PD98059 was obtained from Calbiochem-Novabiochem (La Jolla, CA). Staurosporine, H2O2, and bromodeoxyuridine (BrdU) were obtained from Sigma. Cell culture media, FBS, and supplements were purchased from Invitrogen (Carlsbad, CA). All other chemicals were of analytic grade and were obtained from Sigma or Fisher (Pittsburgh, PA) unless otherwise indicated.
Cell Culture and Treatments
NRK-49F fibroblasts and mouse homozygous LRP-deficient embryonic fibroblasts (PEA-13) were purchased from the American Type Culture Collection (Manassas, VA) and maintained as described previously.12 Cells were seeded onto six-well plates and maintained in complete medium overnight, and then washed with serum-free medium three times. Recombinant human single-chain tPA was added to the serum-free medium at various concentrations for various periods of time, as indicated, in the absence or presence of 0.1 μM staurosporine or 0.5 mM H2O2. For control experiments, cells were treated with vehicle alone. In some experiments, cells were pretreated for 30 min with various chemical inhibitors at the specified concentration, followed by subsequent incubation with vehicle, tPA, staurosporine, or tPA plus staurosporine for an additional 3 to 6 h.
DNA Fragmentation Assay
Apoptosis-associated DNA fragmentation was measured using a cellular DNA fragmentation ELISA kit (Roche Applied Science, Penzberg, Germany) or by a DNA laddering assay. For the DNA fragmentation ELISA assay, BrdU-labeled DNA fragments in the cytoplasm of affected cells were detected according to the manufacturer's instructions.37 Briefly, cells were seeded onto six-well plates and incubated with 10 μM BrdU in complete medium overnight. After washing three times with serum-free medium, cells were treated with staurosporine and/or tPA for 3 h and then incubated in 1× incubation buffer. The resultant supernatant, after centrifugation, was transferred to a microplate precoated with anti-DNA antibody, followed by a standard ELISA assay to detect the BrdU. OD value at 450 nm was measured. DNA laddering was carried out essentially according to the protocols reported previously.38,39
Mitochondrial Isolation
NRK-49F fibroblasts were seeded onto 10-cm dishes and treated with staurosporine and/or tPA. Mitochondria and cytosols were separated as previously reported.40 In brief, cells were lysed in the extraction buffer (50 mM HEPES [pH 7.5], 50 mM KCl, 5 mM EGTA, and 2 mM MgCl2) containing 0.05% digitonin and protease inhibitors. After centrifuging at 700 × g for 10 min, the supernatant was transferred to a new tube, followed by a second centrifugation at 14,000 × g for 20 min at 4°C. The resultant supernatant was the cytosol fraction with the majority of the pellet consisting of mitochondria.
Western Blot Analysis
Cells were treated as indicated and lysed into CHAPS buffer (Cell Signaling Technology) supplemented with protease and phosphatase inhibitor cocktails (Sigma).15 After centrifugation to remove cell debris, supernatant was mixed with SDS loading buffer. Samples were then heated at 100°C for 5 to 10 min before loading, separated through 10% SDS polyacrylamide gels, and subjected to Western blot as described previously.12 Western blot analysis of renal tissue α-SMA abundance was carried out as described previously.9
Indirect Immunofluorescence Staining
Cells were cultured on coverslips and fixed in PBS containing 3% paraformaldehyde and 0.2% Triton X-100 for 10 min at room temperature. Primary antibodies in PBS containing 10% normal donkey serum were applied to cells overnight at 4°C, and, after washing, cells were incubated for 1 h with cyanine (CY2)- or CY3-conjugated, affinity-purified secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Primary antibody replaced with nonimmune IgG served as a negative control. Stained cells were mounted with Vectashield anti-fade mounting media using DAPI (4′, 6-diamidino-2-phenylindole, HCl) to visualize the nuclei (Vector Laboratories, Burlingame, CA). Cells were viewed using an Eclipse E600 epifluorescence microscope equipped with a digital camera (Nikon, Melville, NY).
Plasmid Transfection, siRNA Inhibition, and Adenovirus Infection
The LRP-1 minireceptor expression vector (pHA-mLRP2), in which the ligand-binding domain II of LRP-1 is linked to its β unit, was described previously.20 Transient transfection of pHA-mLRP2 or empty vector control pcDNA3 into PEA-13 fibroblasts was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) reagents, as previously reported.41,42 For knockdown of endogenous LRP-1 expression, NRK-49F cells were transfected with either control siRNA or LRP-1–specific siRNA (Dharmacon, Lafayette, CO), as previously reported.42 Infection of recombinant adenovirus harboring constitutively active MEK1 (Ad.MEK1) was performed as described previously.12,43 Cells were infected by 2 × 107 particles/ml with either control adenovirus (Ad.LacZ) or Ad.MEK1 overnight, followed by incubation with staurosporine for 4 h. Caspase-3 activation was assessed by Western blot analysis.
Animal Model
Homozygous tPA knockout (tPA−/−) and wild-type (tPA+/+) mice were generated from heterozygous crosses using descendents from original breeding pairs obtained from P. Carmeliet (University of Leuven, Leuven, Belgium). Animal studies were performed by using an approved protocol by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Genotype was confirmed by using PCR amplification of genomic DNA from tail snips as described previously.9 The left kidneys of gender-matched mice weighing 20 to 22 g (five animals per group) were subjected to UUO using established procedures described elsewhere.9,12 The right unobstructed kidneys served as controls. At day 7 after UUO, mice were killed and the kidneys were fixed in 10% phosphate-buffered formalin, followed by paraffin embedding for immunohistochemical studies. Kidney homogenates were subjected to Western blot analysis for α-SMA expression. For the reversible (also known as temporary) UUO model,44 the left ureter was exposed through midline abdominal incision. Obstruction was done by placing a 4-mm piece of bisected silicone tubing (ID × wall, 0.5 × 0.8 mm; Daigger, Vernon Hills, IL) around the ureter at a point one third the distance from the bladder to the renal pelvis. The ureter was then occluded by tightening the tubing with a 4-0 suture for 7 d. On the day of relief, the obstructed ureter was decompressed by removal of the tubing and ligature. Relief of obstruction was confirmed by observing the decompression of the dilated ureter. At 4 wk after relief, mice were killed and kidney homogenates were subjected to Western blot analysis for α-SMA expression.
Immunohistochemistry Studies
Paraffin-embedded kidney tissue was sectioned at 4 μm and then subjected to labeling with cleaved caspase-3 antibody.9 Briefly, tissue sections were deparaffinized and hydrated and antigen-retrieved, and endogenous peroxidase activity was quenched by 3% H2O2. Tissue sections were then blocked with 10% normal goat serum, followed by incubating with rabbit anti-mouse α-SMA antibody overnight at 4°C. After incubation with secondary antibody for 1 h, sections were then incubated with ABC reagents for 1 h at room temperature before being subjected to substrate 3-amino-9-ethylcarbazole for red-color staining (Vector Laboratories). Sections were mounted in aqueous mounting medium and viewed using an optic microscope. A total of 500 interstitial cells were counted per slide, and the percentage of interstitial apoptotic (active caspase-3–positive) cells in total interstitial cell population was calculated, five mice per group. Kidney sections of UUO mice were also subjected to double immunofluorescence staining with rabbit anti–α-SMA and cleaved caspase-3 antibodies, and sections were blocked with anti-rabbit IgG Fab fragment between two stainings.
Statistical Analyses
All data were expressed as means ± SEM. Statistical analysis of the data was performed using SigmaStat software (Jandel Scientific Software, San Rafael, CA). Comparison between groups was made using one-way ANOVA followed by the Student-Newman-Keuls test. In some cases, a t test was performed between two groups. P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by National Institutes of Health grants DK061408, DK064005, and DK071040. K.H. and X.T. were supported by postdoctoral fellowships from the American Heart Association.
We thank Dr. Sakae Tanaka for providing the MEK1 adenovirus.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Liu Y: Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Eddy AA: Progression in chronic kidney disease. Adv Chronic Kidney Dis 12: 353–365, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Strutz F, Zeisberg M: Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol 17: 2992–2998, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Qi W, Chen X, Poronnik P, Pollock CA: The renal cortical fibroblast in renal tubulointerstitial fibrosis. Int J Biochem Cell Biol 38: 1–5, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Neilson EG: Mechanisms of disease: Fibroblasts—A new look at an old problem. Nat Clin Pract Nephrol 2: 101–108, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Zeisberg M, Strutz F, Muller GA: Role of fibroblast activation in inducing interstitial fibrosis. J Nephrol 13[Suppl 3]: S111–S120, 2000 [PubMed] [Google Scholar]
- 7.Muller GA, Rodemann HP: Characterization of human renal fibroblasts in health and disease: I. Immunophenotyping of cultured tubular epithelial cells and fibroblasts derived from kidneys with histologically proven interstitial fibrosis. Am J Kidney Dis 17: 680–683, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Rodemann HP, Muller GA, Knecht A, Norman JT, Fine LG: Fibroblasts of rabbit kidney in culture. I. Characterization and identification of cell-specific markers. Am J Physiol 261: F283–F291, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Shultz RW, Mars WM, Wegner RE, Li Y, Dai C, Nejak K, Liu Y: Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest 110: 1525–1538, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mars WM, Zarnegar R, Michalopoulos GK: Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol 143: 949–958, 1993 [PMC free article] [PubMed] [Google Scholar]
- 11.Yee JA, Yan L, Dominguez JC, Allan EH, Martin TJ: Plasminogen-dependent activation of latent transforming growth factor beta (TGF beta) by growing cultures of osteoblast-like cells. J Cell Physiol 157: 528–534, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y: Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem 281: 2120–2127, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH: Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med 9: 1313–1317, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Herz J, Strickland DK: LRP: A multifunctional scavenger and signaling receptor. J Clin Invest 108: 779–784, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai C, Yang J, Liu Y: Transforming growth factor-beta1 potentiates renal tubular epithelial cell death by a mechanism independent of Smad signaling. J Biol Chem 278: 12537–12545, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Yuan J: Divergence from a dedicated cellular suicide mechanism: Exploring the evolution of cell death. Mol Cell 23: 1–12, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Cereghetti GM, Scorrano L: The many shapes of mitochondrial death. Oncogene 25: 4717–4724, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Olson ST, Swanson R, Day D, Verhamme I, Kvassman J, Shore JD: Resolution of Michaelis complex, acylation, and conformational change steps in the reactions of the serpin, plasminogen activator inhibitor-1, with tissue plasminogen activator and trypsin. Biochemistry 40: 11742–11756, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Werner F, Razzaq TM, Ellis V: Tissue plasminogen activator binds to human vascular smooth muscle cells by a novel mechanism: Evidence for a reciprocal linkage between inhibition of catalytic activity and cellular binding. J Biol Chem 274: 21555–21561, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Obermoeller-McCormick LM, Li Y, Osaka H, FitzGerald DJ, Schwartz AL, Bu G: Dissection of receptor folding and ligand-binding property with functional minireceptors of LDL receptor-related protein. J Cell Sci 114: 899–908, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Dai C, Liu Y: Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am J Pathol 163: 621–632, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB: Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 277: C1–C9, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Essawy M, Soylemezoglu O, Muchaneta-Kubara EC, Shortland J, Brown CB, el Nahas AM: Myofibroblasts and the progression of diabetic nephropathy. Nephrol Dial Transplant 12: 43–50, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Ortiz A, Lorz C, Justo P, Catalan MP, Egido J: Contribution of apoptotic cell death to renal injury. J Cell Mol Med 5: 18–32, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chevalier RL: Obstructive nephropathy: Towards biomarker discovery and gene therapy. Nat Clin Pract Nephrol 2: 157–168, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Nassar T, Akkawi S, Shina A, Haj-Yehia A, Bdeir K, Tarshis M, Heyman SN, Higazi AA: In vitro and in vivo effects of tPA and PAI-1 on blood vessel tone. Blood 103: 897–902, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA: Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest 112: 1533–1540, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, Yepes M: Tissue-type plasminogen activator-mediated shedding of astrocytic low density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood 109: 3270–3278, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roelofs JJ, Rouschop KM, Leemans JC, Claessen N, de Boer AM, Frederiks WM, Lijnen HR, Weening JJ, Florquin S: Tissue-type plasminogen activator modulates inflammatory responses and renal function in ischemia reperfusion injury. J Am Soc Nephrol 17: 131–140, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Yoon S, Seger R: The extracellular signal-regulated kinase: Multiple substrates regulate diverse cellular functions. Growth Factors 24: 21–44, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Condorelli G, Trencia A, Vigliotta G, Perfetti A, Goglia U, Cassese A, Musti AM, Miele C, Santopietro S, Formisano P, Beguinot F: Multiple members of the mitogen-activated protein kinase family are necessary for PED/PEA-15 anti-apoptotic function. J Biol Chem 277: 11013–11018, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Liu Y: Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am J Physiol 277: F624–F633, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME: Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Liu Y: Epithelial to mesenchymal transition in renal fibrogenesis: Pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Kalluri R, Neilson EG: Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zavadil J, Bottinger EP: TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 24: 5764–5774, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Li SY, Li Q, Shen JJ, Dong F, Sigmon VK, Liu Y, Ren J: Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: Role of MAP kinase signaling. J Mol Cell Cardiol 40: 283–294, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Wyllie AH: Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284: 555–556, 1980 [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Sun AM, Dworkin LD: Hepatocyte growth factor protects renal epithelial cells from apoptotic cell death. Biochem Biophys Res Commun 246: 821–826, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Wang Y, Zhang J, Kim HP, Ryter SW, Choi AM: FLIP protects against hypoxia/reoxygenation-induced endothelial cell apoptosis by inhibiting Bax activation. Mol Cell Biol 25: 4742–4751, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Li Y, Wen X, Spataro BC, Hu K, Dai C, Liu Y: Hepatocyte growth factor is a downstream effector that mediates the antifibrotic action of peroxisome proliferator-activated receptor-gamma agonists. J Am Soc Nephrol 17: 54–65, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan R, Zhang J, Tan X, Zhang X, Yang J, Liu Y: Downregulation of SnoN expression in obstructive nephropathy is mediated by an enhanced ubiquitin-dependent degradation. J Am Soc Nephrol 17: 2781–2791, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Miyazaki T, Katagiri H, Kanegae Y, Takayanagi H, Sawada Y, Yamamoto A, Pando MP, Asano T, Verma IM, Oda H, Nakamura K, Tanaka S: Reciprocal role of ERK and NF-kappaB pathways in survival and activation of osteoclasts. J Cell Biol 148: 333–342, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chevalier RL, Thornhill BA, Chang AY, Cachat F, Lackey A: Recovery from release of ureteral obstruction in the rat: Relationship to nephrogenesis. Kidney Int 61: 2033–2043, 2002 [DOI] [PubMed] [Google Scholar]