Abstract
The immunosuppressive agent sirolimus exerts an antiproliferative effect by inhibiting mammalian target of rapamycin (mTOR). Because excessive proliferation of the biliary epithelium is a prominent feature of the polycystic liver that accompanies autosomal dominant polycystic kidney disease (ADPKD), we hypothesized that sirolimus may benefit patients with this disorder. We retrospectively measured the volumes of polycystic livers and kidneys in ADPKD patients who had received kidney transplants and had participated in a prospective randomized trial that compared a sirolimus-containing immunosuppression regimen to a tacrolimus-containing regimen. Sixteen subjects (seven with sirolimus, nine with tacrolimus) had received abdominal imaging studies within 11 mo before and at least 7 mo after transplantation, making them suitable for our analysis. Treatment with the sirolimus regimen for an average of 19.4 mo was associated with an 11.9 ± 0.03% reduction in polycystic liver volume, whereas treatment with tacrolimus for a comparable duration was associated with a 14.1 ± 0.09% increase. A trend toward a greater reduction in native kidney volume was also noted in the sirolimus group compared with the nonsirolimus group. Regarding mechanism, the epithelium that lines hepatic cysts exhibited markedly higher levels of phospho-AKT, phospho-ERK, phospho-mTOR, and the downstream effector phospho-S6rp compared with control biliary epithelium. In summary, treatment with sirolimus was associated with decreased polycystic liver volume, perhaps by preventing aberrant activation of mTOR in epithelial cells lining the cysts.
Autosomal dominant polycystic kidney disease (ADPKD) is a life-threatening monogenic disease with a prevalence of 1 in 400 to 1000 live births. Polycystic liver disease (PLD) is its most common extrarenal manifestation in the majority of ADPKD patients by age 60 yr.1,2 ADPKD is caused by mutations to the genes PKD1 (approximately 85% of the cases) or PKD2 (the remaining 15%), encoding polycystin-1 (PC1) and polycystin-2 (PC2), respectively. PC1 is a putative, cell-surface, receptor-like protein with yet-to-be-identified ligand(s), and PC2 is a channel protein with a high conductance of Ca2+ (reviewed in Torres et al.3). PC1 and PC2 are expressed in multiple cellular systems including renal and biliary epithelia. Interaction of PC1 and PC2 in renal epithelial cells inhibits cell-cycle progression.4 PKD mutations induce a change in renal epithelial cell phenotype associated with an activation in cAMP/Ras/Raf/ERK signaling.5–8 Downstream to this aberrant signaling, mammalian target of rapamycin (mTOR) is found to be activated and may contribute to excessive tubular epithelial cell proliferation and renal cyst expansion.9
Sirolimus (rapamycin) is a macrocyclic lactone derived from a strain of Streptomyces hygroscopicus. It inhibits cell growth and proliferation and promotes apoptosis by inhibiting mTOR-mediated signaling.10,11 Sirolimus has been used in renal transplant patients as a part of an alternative long-term immunosuppressive regimen with a comparable or superior allograft outcome compared to that of calcineurin-containing immunosuppression.12,13 In recent years, its usage has been extended, experimentally and clinically, to the treatment of immunomediated glomerulonephritis,14 an array of tumors,15–17 refractory uveitis, and coating stents to prevent coronary artery restenosis.18,19 Sirolimus has recently been shown to reduce cystic renal enlargement in animal models of PKD and the native end-stage cystic kidneys in ADPKD patients with a functional allograft after renal transplantation.9,20,21
Liver cysts in ADPKD originate from biliary microhematomas or focal proliferations of biliary ductules and from peribiliary glands. Excessive proliferation of biliary epithelial cells, combined with neovascularization, altered cell–extracellular matrix (ECM) interaction/ECM remodeling, and cAMP-mediated fluid secretion, is required for the development and expansion of PLD liver cysts.22–25
PLD may become symptomatic with acute complications such as cyst hemorrhage, rupture, and infection. Chronic symptoms are frequently associated with massively enlarged PLD, including abdominal distension and pain; dyspnea; gastroesophageal reflux and early satiety, which may lead to malnutrition; mechanical lower back pain; obstructions of the inferior vena cava, hepatic and portal veins (leading to dialysis associated hypotension, hepatic venous outflow obstruction, and portal hypertension); and biliary obstruction. Currently, apart from invasive interventions such as cyst aspiration with sclerosis, cyst fenestration, combined hepatic resection and cyst fenestration, liver transplantation, and, rarely, selective hepatic artery embolization, no medical treatment is available.26
We have retrospectively examined the effect of sirolimus on PLD in patients who participated in a prospective, randomized trial after kidney transplantation comparing sirolimus-mycophenolate mofetil-prednisone to tacrolimus-mycophenolate mofetil-prednisone, and had computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen before and after renal transplantation. The sirolimus-containing regimen for an average duration of 19.4 months (mo) was associated with a reduction in total liver volume, whereas the liver volumes continue to increase in patients received nonsirolimus regimen. Consistent with this observation, mTOR and its downstream effector, S6 ribosomal protein (S6rp), were highly activated in PLD cyst-lining epithelia but not in non-ADPKD liver sections.
RESULTS
Clinical and Laboratory Characteristics of Renal Transplant ADPKD Patients with or without Receiving Sirolimus-Containing Immunosuppression
Sixteen patients, seven on a sirolimus-containing (+ sirolimus) and nine on a tacrolimus-containing (− sirolimus) regimen, met the inclusion criteria. Diagnoses of ADPKD were made on the basis of family history and clinical criteria.27 As shown in Table 1, with the exception of gender, patients in the sirolimus and tacrolimus groups had comparable demographical, clinical, and laboratory characteristics at the time of the first imaging study. None of the female patients were on oral contraceptive or hormone replacement therapy. Total liver volumes in the two groups at the time of the first imaging were not significantly different (3.06 ± 0.47 versus 2.83 ± 0.80 L, P = 0.80).
Table 1.
Patient characteristics at the time of the first abdominal image, the interval between the 2 images, and duration of sirolimus treatment
| Mean (range)
|
||
|---|---|---|
| + Sirolimus, n = 7 | − Sirolimus, n = 9 | |
| Age, yr | 55.4 (41 to 66) | 57.6 (48 to 67) |
| Gender, female/male | 2/5 | 6/3 |
| Systolic BP, mmHg | 134.3 (88 to 180) | 128.1 (121 to 155) |
| Diastolic BP, mmHg | 80.9 (55 to 90) | 76.3 (66 to 97) |
| Total liver size, L | 3.06 (1.75 to 5.35) | 2.83 (1.15 to 9.07) |
| Duration between 2 scans, months | 30 (11 to 58) | 28.7 (17 to 46) |
| Duration of sirolimus treatment, months | 19.4 (7 to 40) | — |
| Serum sirolimus concentration, ng/ml | 14.3 (7.8 to 17.9) | — |
The adverse effects of sirolimus immunosuppression on bone marrow elements and serum lipids were examined. At the time of the second imaging study, five of the seven patients (71%) in the sirolimus group were on one or two agents (four and one patients, respectively) for dyslipidemia compared with five of the nine patients (56%) in the tacrolimus group, all on single agents. As shown in Table 2, LDL level in the sirolimus group was significantly higher than that in nonsirolimus group (122.6 ± 19.6 versus 73.1 ± 6.7, P = 0.048). Average serum triglyceride level was also higher (220.3 ± 40.0 versus 193.2 ± 30.9, P = 0.06). Likewise, platelet counts tended to be lower (154.3 ± 19.9 versus 210.6 ± 44.0, P = 0.27) and total cholesterol concentrations higher (200.9 ± 17.5 versus 168.5 ± 6.1, P = 0.12) with sirolimus-containing immunosuppression.
Table 2.
Laboratory parameters before and after the sirolimus-containing and sirolimus-sparing immunosuppression
| + Sirolimus, mean (range)
|
− Sirolimus, mean (range)
|
|||
|---|---|---|---|---|
| 1st Scan | 2nd Scan | 1st Scan | 2nd Scan | |
| HGB, g/dl | 11.8 (9.4 to 15.4) | 13.3 (11.8 to 16.8) | 12.9 (10.1 to 14.4) | 12.6 (9.7 to 14.7) |
| Platelet, 109/L | 172.1 (151 to 214) | 154.3 (76 to 249) | 200 (89 to 338) | 210.6 (65 to 523) |
| Serum creatinine, mg/dl | 3.59 (2.3 to 9.7) | 1.46 (0.8 to 2.4) | 5.86 (1.3 to 7.5) | 1.48 (1.1 to 2.3) |
| Total cholesterol, mg/dl | 173.9 (149 to 195) | 200.9 (128 to 265) | 196.7 (121 to 337) | 168.5 (145 to 204) |
| LDL, mg/dl | 101.6 (80 to 135) | 122.6 (81 to 209)* | 90.8 (60 to 117) | 73.1 (41 to 105)* |
| HDL, mg/dl | 43.9 (29 to 87) | 46.7 (12 to 70) | 48.3 (35 to 60) | 51.6 (25 to 77) |
| Triglyceride, mg/dl | 168.9 (53 to 441) | 220.3 (127 to 434) | 264.2 (55 to 687) | 193.2 (102 to 392) |
| Albumin g/dl | 4.1 (3.7 to 4.5) | 3.9 (3.4 to 4.4) | 3.9 (3.3 to 4.8) | 4.1 (3.7 to 4.3) |
Statistically significant difference between + sirolimus group and − sirolimus group at the time of the second scan.
Sirolimus-Containing Immunosuppression Is Associated with a Reduction in PLD Liver Volume
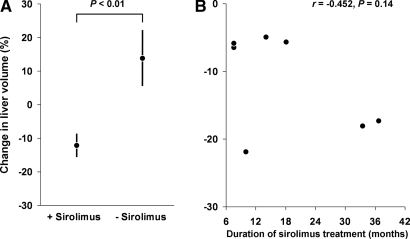
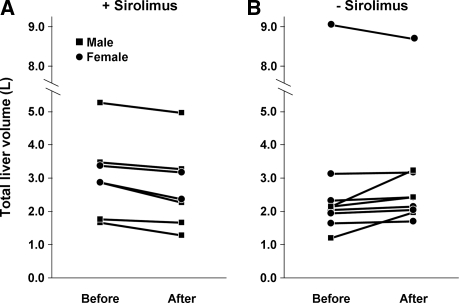
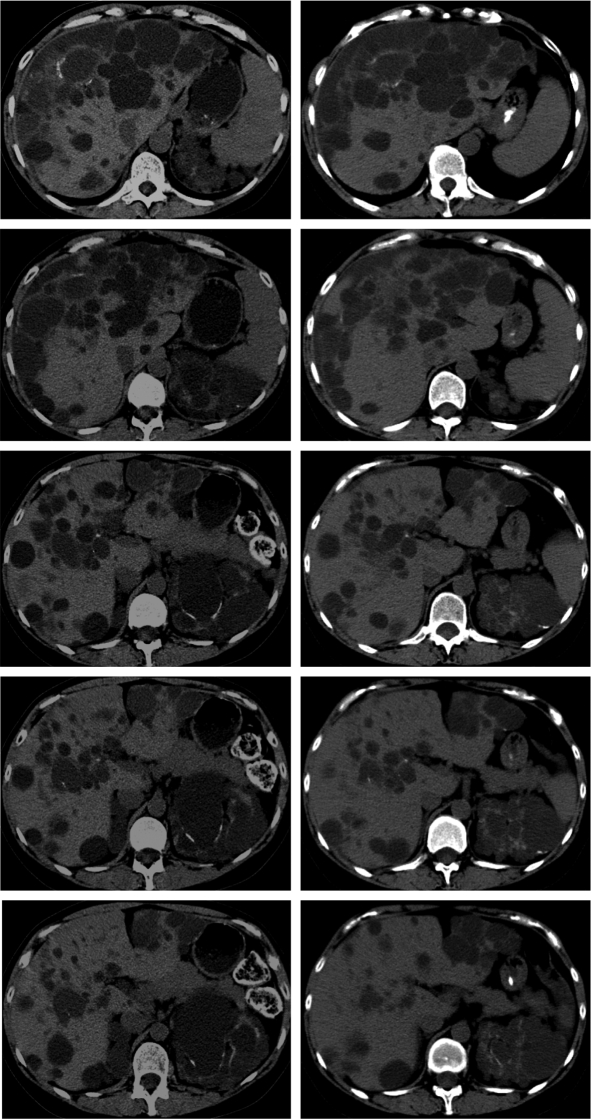
At the time of the first imaging study, total liver volumes ranged from 1148 to 9072 ml (Table 1). Five of the seven patients in the sirolimus group and six of the nine patients in the tacrolimus group had total liver volumes >2000 ml. As shown in Figure 1A, the sirolimus group showed a decrease (−11.85% ± 0.03) in total liver volume, whereas the tacrolimus group showed an increase (+14.13 ± 0.09, P = 0.009) in total liver volume. A suggestive but not statistically significant correlation between the duration of sirolimus exposure and reduction in liver volume was observed (r = −0.452, P = 0.12) (Figure 1B). The changes in liver volume for each individual patient are shown in Figure 2. Representative series of transaxial CT scans from an ADPKD patient before and after 15.5 mo of sirolimus-containing immunosuppression (Figure 3) illustrate the reduction in the size and number of liver cysts. Because the mean interval (30 mo) between the two scans in the sirolimus treated patients was greater than the mean duration of treatment (19.4 mo), it is possible that sirolimus effect might have been underestimated.
Figure 1.
(A) Average changes in the liver volume in autosomal dominant polycystic kidney disease (ADPKD) patients with or without receiving sirolimus. (B) Reduction in total liver volume plotted as a function of the duration of sirolimus treatment.
Figure 2.
Total liver volume in each individual patient at the first and second imaging studies. Each circle or square represents a single subject.
Figure 3.
Representative series of transaxial computed tomography sections obtained from an ADPKD patient at the mid-level of the liver before (left column) and after (right column) 15.5 mo of the sirolimus-containing immunosuppressant shows a reduction in the size and number of liver cysts. The top cuts of the two computed tomography series are aligned at the center of a partially calcified cyst.
Sirolimus-Containing Immunosuppression Is Associated with a Trend toward a Greater Reduction in Native Polycystic Kidney Volume
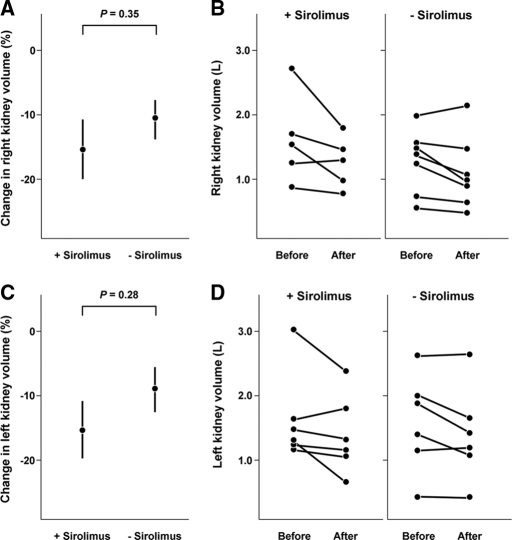
Shillingford et al. have shown that sirolimus-containing immunosuppression, given to ADPKD patients after renal transplantation, is associated with a shrinkage of the native cystic kidney volume.9 The renal volumes of six patients on sirolimus (one patient had bilateral nephrectomy, one had right-sided nephrectomy) and seven on nonsirolimus (two had bilateral nephrectomy, one had left-sided nephrectomy) were measured. As shown in Figure 4, the average renal volume in the sirolimus group was reduced by 14.76 ± 0.08% and 15.03 ± 0.08% versus 10.9 ± 0.06% and 9.0 ± 0.06%, right and left kidneys, respectively, in the nonsirolimus group. Notably, with the exception of a prominent reduction in kidney volume in a sirolimus-treated patient, the changes of kidney volume in both groups appeared broadly similar. Overall, the renal volume changes between the two groups were not statistically different, P = 0.38 and 0.28 for right and left kidneys, respectively.
Figure 4.
The changes of the native polycystic kidneys at the first and second imaging studies. A and C show average changes, mean and SEM, of right and left kidney volumes in patients with or without receiving the sirolimus-containing immunosuppression. B and D show the total right and left kidney volumes in each patient at the time of the first and second imaging studies.
PLD Cyst-Lining Epithelia Show an Elevated mTOR Signaling
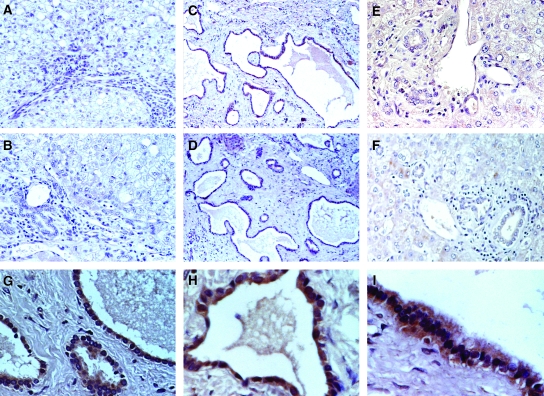
We examined the phosphorylated, activated form of mTOR in the liver sections from two ADPKD patients and two non-ADPKD controls. As shown in Figure 5, compared with normal biliary epithelia and noncystic areas of PLD, the cyst-lining epithelia exhibits intense cytoplasmic staining of active phospho-mTOR. Consistent with this finding, phospho-S6rp, a downstream mTOR effector, was also activated in the cyst epithelium (Figure 6, E to F). The activation of mTOR signaling may occur downstream to the activation of ERK and AKT, both are activated in PLD (Figure 6, G to J).
Figure 5.
Phospho-mammalian target of rapamycin (mTOR) expression is elevated in polycystic liver disease (PLD) cyst-lining epithelia. Liver sections from two normal subjects (A and B) and two PLD patients (C to F) were immunostained with antibody against Ser2448 phospho-mTOR. PLD cyst-lining epithelial cells from both ADPKD patients showed intense staining for phospho-mTOR (C and D, enlarged views in G to I). The phospho-mTOR was nondetectable in the biliary epithelia from normal controls (A and B) and almost nondetectable in the noncystic biliary epithelia at portal triads (E and F) of the same PLD sections as in C and D.
Figure 6.
The expression of effectors of phospho-mTOR, phospho-S6 ribosomal protein and phospho-AKT are elevated in PLD cyst-lining epithelia. Liver sections from a normal subject were immunostained with antibodies that specifically recognize mTOR downstream effector, p-S6rp (A) and p-AKT (B). Consecutive PLD sections from an ADPKD patient (C, E, G, and I) were immunostained with antibodies against Ser2448 p-mTOR (C), Ser240/244 p-S6rp (E), Ser473 p-AKT (G), and Thr202/Tyr204 p-ERK (I). D, F, H, and J are enlarged views of C, E, G, and I, respectively. PLD cyst-lining epithelia show a high level of staining for activated mTOR, S6rp, AKT, and ERK, whereas the normal biliary epithelia show nondetectable p-S6rp (A) and p-AKT (B).
DISCUSSION
This study demonstrates that a sirolimus-containing immunosuppressive regimen is associated with a reduction in polycystic liver volume, presumably through inhibition of mTOR. Consistent with this interpretation, we show that PLD cyst-lining epithelia express high levels of activated mTOR and its downstream effector, p-S6rp.
mTOR is a serine/threonine protein kinase, activated by a Ras-related GTPase, Rheb (Ras homolog enriched in the brain). Rheb is deactivated by a Rheb-GTPase activating protein (RhebGAP) function of the tuberin-hamartin complex.28 Tuberin is known to be phosphorylated by ERK and AKT at multiple sites, with a resultant dissociation from the tuberin-hamartin complex. This dissociation interrupts the tuberin-hamartin complex–mediated Rheb inhibition, leading to an augmented mTOR signaling.29,30 The finding of highly activated ERK and AKT in the PLD cyst-lining epithelium (Figure 6, G to J) is consistent with the possibility of ERK/AKT-mediated mTOR activation.
mTOR forms two distinct protein complexes within mammalian cells, regulatory-associated protein of TOR (raptor) and rapamycin-insensitive companion of TOR (rictor).31,32 mTOR-Raptor complex, inhibited by sirolimus, promotes cell growth by at least two mechanisms. First, mTOR-raptor complex phosphorylates the S6rp kinase and S6rp, thereby augmenting protein translation and ribosomal biogenesis.33 Second, it inactivates (by phosphorylation) the eukaryotic initiating factor 4E (eIF4E)-binding protein (4E-BP1), dissociating 4E-BP1 from the RNA cap-binding protein eIF4E, promoting cap-dependent mRNA translation.34 Sirolimus has been shown to bind the FKBP-rapamycin-binding domain of mTOR. This binding destabilizes the association between mTOR and raptor, preventing the downstream phosphorylation of S6rp kinase/S6rp and 4E-BP1.35
Although previous studies suggest that mTOR-rictor complex is insensitive to sirolimus, recent reports raise the possibility that sirolimus may also affect mTOR-rictor signaling. mTOR-Rictor complex modulates cellular proliferation by phosphorylating the survival factor AKT at Ser 473.36,37 In breast cancer and anaplastic large-cell lymphoma cells, sirolimus treatment reduces the number of viable cells and promotes chemotherapy-induced apoptosis. These effects are associated with reductions in both phospho-mTOR (Ser2448p-mTOR) and phospho-AKT (Ser473p-AKT), possibly via direct and indirect mechanisms.38–41
The role of aberrant mTOR activation in promoting cystic liver enlargement is supported by the observation of a uniform reduction in liver volume in patients receiving sirolimus-containing regimen. Because female ADPKD patients tend to have more progressive PLD growth associated with estrogen exposure, the higher number of females in the nonsirolimus group might potentially have contributed to the difference detected between the two groups. However, this is unlikely because male patients rather than female patients in the nonsirolimus group had the most pronounced liver growth. As shown in Figure 2B, the two patients with the largest percentage liver volume increase were males. One female patient with a liver volume of 9.07 L before renal transplantation actually showed a reduction, possibly as a result of a nearly maximal degree of abdominal distension. Furthermore, a uniform liver volume reduction, observed in the sirolimus group (Figure 2A), would be highly atypical and defy the natural course of PLD. Taken together, the gender distribution could not explain the observations in this study.
Another potential explanation for these results is that the detected difference is related to the avoidance of calcineurin inhibitors rather than the exposure to sirolimus. Indeed, one of the functions of PC1 is to activate calcineurin/NFAT (nuclear factor of activated T cells) signaling.42 The use of a calcineurin inhibitor (cyclosporine) has been associated with a higher frequency of acquired cystic kidney diseases after renal transplantation.43 However, because PC1-mediated calcineurin/NFAT activation is presumably disrupted in ADPKD, further inhibition with calcineurin inhibitor may exert lesser effect. The consistent reduction in liver volume with sirolimus also renders this explanation unlikely.
We have examined the volumes of native polycystic kidneys after renal transplantation in our patients. Consistent with a previous report,9 the reduction in the native kidney volume tends to be more pronounced in the patients treated with sirolimus. However, the difference did not reach a statistical significance (Figure 4). This could possibly result from the small number of patients. It is also possible that sirolimus given orally is more efficacious in treating polycystic liver than polycystic kidney disease. Sirolimus is absorbed in the small intestine and undergoes an extensive presystemic metabolism by the intestinal and hepatic cytochrome P-450 system (CYP3A4), followed by biliary excretion of its metabolites. At least two of the six identified metabolites have retained immunosuppressive activity.44,45 This presystemic biliary exposure to sirolimus/its active metabolites, combined with a reduced drug delivery to end-stage polycystic kidneys as a result of compromised renal blood flow, may in part account for the observed differential efficacy of sirolimus in the polycystic liver and kidneys.
Because liver volume, not derangement of liver function, is the major source of morbidity and mortality in PLD, the observation of a significant reduction in polycystic liver volume with sirolimus-containing regimen is encouraging. Although a prospective, confirmatory study is necessary, sirolimus shows promise as a potential treatment option for severe PLD.
CONCISE METHODS
Clinical Data Collection
The study was approved by the Institutional Review Board of the Mayo Clinic College of Medicine. The records of ADPKD patients, diagnosed by clinical criteria, who participated in a prospective, randomized trial in kidney transplantation, including ADPKD and non-ADPKD renal transplant recipients, comparing sirolimus-mycophenalate mofetil-prednisone to tacrolimus-mycophenalate mofetil-prednisone at Mayo Clinic in Rochester, Minnesota, between April 2001 and January 2006 were reviewed.12 Of 116 ADPKD patients, 16 patients met the following criteria: (a) PLD evident on imaging studies; (b) on sirolimus-containing or calcineurin inhibitor–based immunosuppression initiated at the time of renal transplantation and not switched from one group to the other during the period of observation; (c) on the same immunosuppressive for >6 mo; (d) abdominal imaging study (CT or MRI) obtained within 12 mo before the renal transplantation and, for the patients on sirolimus-containing regimen, the repeated imaging while on sirolimus or within 11 mo after the termination of sirolimus (in cases where sirolimus was terminated); and (e) absence of cyst reductive procedures or liver transplant. Seven patients on sirolimus-containing and nine on nonsirolimus regimen met these criteria and were included. Their characteristics are summarized in Table 1. Laboratory parameters at the times of the first and second scans are summarized in Table 2.
Volumetric Determination of the Liver and Native Kidney
The total volumes of the cystic livers and kidneys were measured by a nephrologist (Q.Q.) and a radiologist (B.F.K.) blinded to the patients’ immunosuppressive regimens. Single breath-hold CT scans, which demonstrated negligible artifacts from respiration, were used for 29 of the 32 acquisitions with or without intravenous contrast. The remaining three were obtained with MRI. CT scanning parameters were 120 kVp, 380 mA, 512 × 512 acquisition matrix, 5 to 7 mm collimation, 1 pitch, and 5 to 7 mm increment with no overlap. MRI parameters were repetition time 2150 ms, echo time 30.0 ms, nex 1.0, 256/128 acquisition matrix, 8 or 15 mm slice thickness. Digital images were reviewed on PC workstation. The cross-sectional areas of each cut-slice were measured using a standard software system (QREADS) at Mayo Clinic, Rochester. Each cross-sectional area was outlined and the software generated a numeric number of the area (in mm2). The volume of each cut-slice (in mm3) was calculated by multiplying the cross-sectional area by the thickness of the slice. Two separate measurements were performed. The variation was minimal, and the average was used for analysis.
Immunohistochemistry
Five-micrometer sections from formalin-fixed, paraffin-embedded blocks were deparaffinized in xylene and hydrated with 100%, 95%, 70% ethanol serially; then the sections were exposed to 1.5% H2O2 (Sigma-Aldrich, St. Louis, MO) to quench endogenous peroxidases (×30min) and incubated in proteinase K (Chemicon, Temecula, CA) (×30min, 37°C) for antigen retrieval. Nonspecific binding was blocked by normal (rabbit) blocking serum (Vector Laboratories, Burlingame, CA). The sections were incubated with rabbit anti-phospho-mTOR (Ser2448) (Abcam, Cambridge, MA), anti-phospho-Akt (Ser473), anti-phospho-ERK (Thr202/Tyr204), and anti-phospho-S6rp (Ser240/244) antibody (Cell Signaling Technology, Beverly, MA) in normal blocking serum (overnight at room temperature) in a humidified chamber. The sections were then incubated with appropriate biotinylated secondary antibody and with VECTASTAIN elite ABC reagent (Vector laboratories). To enhance nuclear detail, all slides were counterstained with hematoxylin and then mounted with mounting media (Fisher Scientific, Kalamazoo, MI).
Statistical Analyses
The numerical values of the liver and kidney volumes for each patient were compared. The data from sirolimus and nonsirolimus groups were compared using the t test. Results were expressed as mean ± SEM. P < 0.05 was considered significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by National Institutes of Health grants DK63064 (Q.Q.), DK DK073567 (Q.Q.), and DK44863 (V.E.T.), and by the FUTR, career development award, Mayo Foundation (Q.Q.).
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Bae KT, Zhu F, Chapman AB, Torres VE, Grantham JJ, Guay-Woodford LM, Baumgarten DA, King BF Jr, Wetzel LH, Kenney PJ, Brummer ME, Bennett WM, Klahr S, Meyers CM, Zhang X, Thompson PA, Miller JP: Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol 1: 64–69, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Chauveau D, Fakhouri F, Grunfeld JP: Liver involvement in autosomal-dominant polycystic kidney disease: Therapeutic dilemma. J Am Soc Nephrol 11: 1767–1775, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Torres VE, Harris PC: Mechanisms of disease: Autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2: 40–55; quiz 55, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG: PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell 109: 157–168, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ: Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983–1994, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, Rome LA, Sullivan LP, Grantham JJ: cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int 57: 1460–1471, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Nagao S, Yamaguchi T, Kusaka M, Maser RL, Takahashi H, Cowley BD, Grantham JJ: Renal activation of extracellular signal-regulated kinase in rats with autosomal-dominant polycystic kidney disease. Kidney Int 63: 427–437, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Nadasdy T, Laszik Z, Lajoie G, Blick KE, Wheeler DE, Silva FG: Proliferative activity of cyst epithelium in human renal cystic diseases. J Am Soc Nephrol 5: 1462–1468, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T: The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103: 5466–5471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haughey BH, Gates GA, Arfken CL, Harvey J: Meta-analysis of second malignant tumors in head and neck cancer: The case for an endoscopic screening protocol. Ann Otol Rhinol Laryngol 101: 105–112, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM: mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, Schwab TR, Gloor JM, Cosio FG, Lund WJ, Kremers WK, Nyberg SL, Ishitani MB, Prieto M, Velosa JA: Complete avoidance of calcineurin inhibitors in renal transplantation: A randomized trial comparing sirolimus and tacrolimus. Am J Transplant 6: 514–522, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Flechner SM, Goldfarb D, Solez K, Modlin CS, Mastroianni B, Savas K, Babineau D, Kurian S, Salomon D, Novick AC, Cook DJ: Kidney transplantation with sirolimus and mycophenolate mofetil-based immunosuppression: 5-year results of a randomized prospective trial compared to calcineurin inhibitor drugs. Transplantation 83: 883–892, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Alperovich G, Rama I, Lloberas N, Franquesa M, Poveda R, Goma M, Herrero-Fresneda I, Cruzado JM, Bolanos N, Carrera M, Grinyo JM, Torras J: New immunosuppresor strategies in the treatment of murine lupus nephritis. Lupus 16: 18–24, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Douros J, Suffness M: New antitumor substances of natural origin. Cancer Treat Rev 8: 63–87, 1981 [DOI] [PubMed] [Google Scholar]
- 16.Eng CP, Sehgal SN, Vezina C: Activity of rapamycin (AY-22,989) against transplanted tumors. J Antibiot (Tokyo) 37: 1231–1237, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Witzig TE, Geyer SM, Ghobrial I, Inwards DJ, Fonseca R, Kurtin P, Ansell SM, Luyun R, Flynn PJ, Morton RF, Dakhil SR, Gross H, Kaufmann SH: Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol 23: 5347–5356, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Popma JJ, Leon MB, Moses JW, Holmes DR Jr, Cox N, Fitzpatrick M, Douglas J, Lambert C, Mooney M, Yakubov S, Kuntz RE: Quantitative assessment of angiographic restenosis after sirolimus-eluting stent implantation in native coronary arteries. Circulation 110: 3773–3780, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE: Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349: 1315–1323, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP: Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant 21: 598–604, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Tao Y, Kim J, Schrier RW, Edelstein CL: Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 16: 46–51, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Masyuk T, LaRusso N: Polycystic liver disease: New insights into disease pathogenesis. Hepatology 43: 906–908, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Nichols MT, Gidey E, Matzakos T, Dahl R, Stiegmann G, Shah RJ, Grantham JJ, Fitz JG, Doctor RB: Secretion of cytokines and growth factors into autosomal dominant polycystic kidney disease liver cyst fluid. Hepatology 40: 836–846, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Fabris L, Cadamuro M, Fiorotto R, Roskams T, Spirli C, Melero S, Sonzogni A, Joplin RE, Okolicsanyi L, Strazzabosco M: Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology 43: 1001–1012, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, Meininger CJ, Franchitto A, Onori P, Marzioni M, Taffetani S, Fava G, Stoica G, Venter J, Reichenbach R, De Morrow S, Summers R, Alpini G: Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 130: 1270–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Arnold HL, Harrison SA: New advances in evaluation and management of patients with polycystic liver disease. Am J Gastroenterol 100: 2569–2582, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Ravine D, Walker RG, Gibson RN, Forrest SM, Richards RI, Friend K, Sheffield LJ, Kincaid-Smith P, Danks DM: Phenotype and genotype heterogeneity in autosomal dominant polycystic kidney disease. Lancet 340: 1330–1333, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Manning BD, Cantley LC: Rheb fills a GAP between TSC and TOR. Trends Biochem Sci 28: 573–576, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Manning BD, Cantley LC: United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans 31: 573–578, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J: Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A 101: 13489–13494, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K: Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN: Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10: 457–468, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM: RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A 95: 1432–1437, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonenberg N, Gingras AC: The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol 10: 268–275, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Oshiro N, Yoshino K, Hidayat S, Tokunaga C, Hara K, Eguchi S, Avruch J, Yonezawa K: Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells 9: 359–366, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM: Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM: Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Chang SB, Miron P, Miron A, Iglehart JD: Rapamycin inhibits proliferation of estrogen-receptor-positive breast cancer cells. J Surg Res 138: 37–44, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Chiang GG, Abraham RT: Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem 280: 25485–25490, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM: Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22: 159–168, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Vega F, Medeiros LJ, Leventaki V, Atwell C, Cho-Vega JH, Tian L, Claret FX, Rassidakis GZ: Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Cancer Res 66: 6589–6597, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puri S, Magenheimer BS, Maser RL, Ryan EM, Zien CA, Walker DD, Wallace DP, Hempson SJ, Calvet JP: Polycystin-1 activates the calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway. J Biol Chem 279: 55455–55464, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Lien YH, Hunt KR, Siskind MS, Zukoski C: Association of cyclosporin A with acquired cystic kidney disease of the native kidneys in renal transplant recipients. Kidney Int 44: 613–616, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Ferron GM, Mishina EV, Zimmerman JJ, Jusko WJ: Population pharmacokinetics of sirolimus in kidney transplant patients. Clin Pharmacol Ther 61: 416–428, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Christians U, Sattler M, Schiebel HM, Kruse C, Radeke HH, Linck A, Sewing KF: Isolation of two immunosuppressive metabolites after in vitro metabolism of rapamycin. Drug Metab Dispos 20: 186–191, 1992 [PubMed] [Google Scholar]