Abstract
Angiotensin II and its type 1 receptor (AT1R) play important roles in the pathogenesis of renal disease and diabetic nephropathy. The 12/15-lipoxygenase pathway of arachidonate metabolism and its lipid products have also been implicated in diabetic nephropathy. However, it is unclear whether 12/15-lipoxygenase regulates expression of AT1R. In cultured rat mesangial cells, we found that the 12/15-lipoxygenase product 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE) increased AT1R mRNA and protein expression, primarily by stabilizing AT1R mRNA. Pretreatment with 12(S)-HETE also amplified the signaling effects of angiotensin II, likely due to the increased AT1R expression. Levels of AT1R protein expression decreased when 12/15-lipoxygenase was knocked down with specific short hairpin RNA (shRNA) compared with control cells. Similarly, levels of the AT1 receptor, but not the AT2 receptor, were significantly lower in mesangial cells and glomeruli derived from 12/15-lipoxygenase knockout mice compared with control mice. Reciprocally, stable overexpression of 12/15-lipoxygenase increased AT1R expression in cultured mesangial cells. In vivo, modified siRNA targeting 12/15-lipoxygenase reduced glomerular AT1R expression in a diabetic mouse model. Interestingly, angiotensin II induced greater levels of 12/15-lipoxygenase, TGF-β1, and fibronectin (FN) in AT1R-overexpressing mesangial cells compared with control cells. Therefore, oxidized lipids generated by the 12/15-lipoxygenase-mediated metabolism of arachidonic acid can enhance AT1R expression in mesangial cells and augment the profibrotic effects of angiotensin II.
Angiotensin II (Ang II) induces mesangial cell (MC) hypertrophy and promotes synthesis of extracellular matrix (ECM) proteins, key processes that are linked to the progression of renal disease and diabetic nephropathy (DN).1,2 The effects of Ang II in most tissues, including kidney, are mediated by two plasma membrane receptors, AT1R and Ang II type 2 receptor (AT2R) subtypes.3,4 AT1R can be further classified as AT1a and AT1b, and cannot be distinguished pharmacologically.3–5 AT1a is the major receptor subtype in cardiovascular and renal systems.4,6 Most of the known effects of Ang II in adult tissues are attributable to AT1R.3–7 Thus, AT1R expression levels dictate the biologic efficacy of Ang II to a large extent. In both clinical and experimental studies, angiotensin-converting enzyme (ACE) inhibitors and AT1R blockers (ARB) have renoprotective effects that cannot be explained entirely by their hemodynamic effects.7–9
Lipoxygenases (LO) are a family of enzymes that insert molecular oxygen into polyunsaturated fatty acids such as arachidonic and linoleic acids.10,11 They are classified as 5-, 8-, 12-, and 15-LO according to the carbon atom of arachidonic acid at which the oxygen is inserted. 12-LO action can produce predominantly 12(S)-HETE as product from arachidonic acid.10 Human and rabbit 15-LO as well as the leukocyte-type 12-LO have high homology and are classified as 12/15-LO (especially in rat and mouse) because they have similar substrate specificities and can form both 12(S)-HETE and 15(S)-HETE from arachidonic acid.10,11 Recently there has been heightened interest in the 12/15-LO pathway because of key data implicating it in the pathogenesis of atherosclerosis, restenosis, hypertension, and diabetes.12–14
12/15-LO is involved in multiple events related to the development of renal disease and DN with potency similar to that of Ang II.15–20 We previously showed that Ang II can induce the activity and expression of 12/15-LO in vascular smooth muscle cells (VSMC) and MC.19,21–23 The growth-promoting and matrix-inducing effects of Ang II in MC were blocked with pharmacologic 12-LO inhibitors and 12-LO ribozymes.19 Furthermore, Ang II-induced DNA and protein syntheses as well as activation of key signals such as p38 mitogen-activated protein kinase (MAPK) were significantly attenuated in MC or VSMC derived from 12/15-LO knockout (12/15-LOKO) mice.20,24 Because such responses of Ang II are mainly mediated via AT1R, in this study we hypothesized that AT1R expression may be influenced by the 12/15-LO pathway.
Several lines of studies indicate that AT1R expression is modulated by Ang II itself as well as various factors such as cytokines, growth factors, nitric oxide, insulin, and LDL.25–27 However, its regulation by 12/15-LO or its oxidized lipid products is not known. Active elements of the renin-angiotensin system (RAS) are present in MC and glomeruli, and changes in AT1R expression levels can influence the activity of the entire RAS. Because MCs are key targets of Ang II actions in the glomeruli, and Ang II responses are attenuated in MC from 12/15-LOKO mice, in this study we investigated the effects of 12/15-LO and its products on AT1R expression in MC. We demonstrate for the first time a novel mechanism by which oxidized lipids can amplify Ang II-induced renal dysfunction by augmenting AT1R expression.
RESULTS
12(S)-HETE Induces AT1R mRNA and Protein Expression in MC
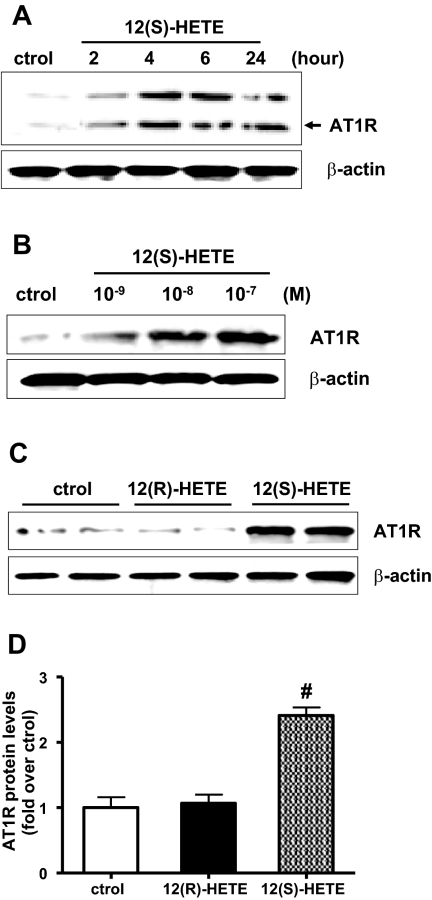
We first examined whether the 12/15-LO product 12(S)-HETE can induce AT1R expression in rat MC (RMC). Western blot results showed that 12(S)-HETE could induce AT1R protein expression in a time- (Figure 1A) and dose- (Figure 1B) dependent manner, with optimal effects at 10−7 M and 24 h. This effect was specific to 12(S)-HETE because a stereoisomer, 12(R)-HETE, which is not a LO product, did not significantly increase AT1R protein expression (Figure 1, C and D). AT1R antibody specificity was determined with negative control lysates from 3T3 fibroblasts (Supplement Figure S1)
Figure 1.
12(S)-hydroxyeicosatetranoic acid (12(S)-HETE) enhances angiotensin II (Ang II) type I receptor (AT1R) protein expression in rat mesangial cells (RMC). (A) Quiescent RMC were treated with 12(S)-HETE (10−7 M) for various time periods and AT1R protein expression determined by Western blotting. (B) Quiescent RMC were treated with 12(S)-HETE (10−9 to 10−7 M) for 24 h and AT1R protein expression determined by Western blotting. (C) Quiescent RMC were left alone or treated with 12(R)-HETE (10−7 M) or 12 (S)-HETE (10−7 M) for 24 h and AT1R protein expression determined. (D) Bar graph represents mean ± SEM of four independent experiments (#P < 0.01 versus control).
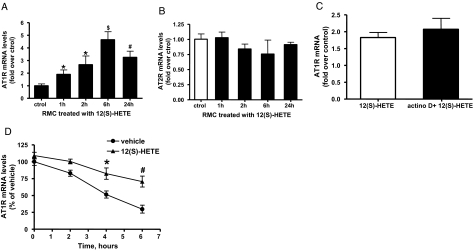
To determine whether there is a parallel increase in AT1R mRNA expression, we performed quantitative real-time PCR with AT1R-specific primers and β-actin internal standard. AT1R mRNA expression was significantly increased in RMC treated with 12(S)-HETE from 1 to 24 h (Figure 2A), indicating that 12(S)-HETE can enhance the expression of both AT1R mRNA and protein. On the other hand, 12(S)-HETE did not significantly change AT2R mRNA expression (Figure 2B).
Figure 2.
Effect of 12(S)-HETE on AT1R or angiotensin II type II receptor (AT2R) mRNA expression in RMC. (A) 12(S)-HETE-induced AT1R expression: RMC were serum starved for 48 h, then treated with 12(S)-HETE (10−7 M) for 1 to 24 h. Expression of AT1R (A) was determined by quantitative reverse transcriptase PCR (QRT-PCR) using β-actin as an internal control. Data represent mean ± SEM of three independent experiments (*P < 0.05; #P < 0.01; $P < 0.001 versus control). (B) No change in AT2R expression seen under the same conditions. (C). RMC were pretreated with vehicle or with 2 μg/ml actinomycin D for 1 h and then stimulated with 12(S)-HETE (10−7 M) for 2 h and QRT-PCR with gene-specific primers performed with total RNA isolated from these cells. Bar graph represents fold over control AT1R mRNA induction by 12(S)-HETE in cells treated without or with actinomycin D. (D) Quiescent RMC were pretreated with either vehicle or 12(S)-HETE (10−7 M) for 6 h, then exposed to 2 μg/ml actinomycin D. Total RNA was then extracted at the indicate time points and real time QRT-PCR performed. The mRNA level of AT1R in vehicle-treated RMC before addition of actinomycin D was set as 100%. Each point represents relative AT1R mRNA level (mean ± SEM) of three independent experiments (*P < 0.05; #P < 0.01 versus vehicle by two-way ANOVA) and shows decreased rate of disappearance of AT1R mRNA in 12(S)-HETE-treated cells relative to vehicle control.
Pretreatment of RMC for 1 h with actinomycin D (2 μg/ml), an inhibitor of transcription, did not significantly alter 12(S)-HETE-induced increase in AT1R mRNA expression (Figure 2C), suggesting that 12(S)-HETE-induced AT1R mRNA was not due to increases in transcription.
Reports show that AT1R can also be regulated by posttranscriptional and mRNA stability mechanisms.26–28 To test this, quiescent RMC were preincubated with 12(S)-HETE or vehicle for 6 h, and then gene transcription inhibited by actinomycin D treatment, and total RNA extracted at various time points thereafter. Real-time PCR data (Figure 2D) showed that the rate of AT1R mRNA decay, a key parameter of mRNA stability, was significantly slower in cells treated with 12(S)-HETE compared with vehicle. These results demonstrate that 12(S)-HETE induces AT1R via increasing mRNA stability.
12(S)-HETE Enhances Ang II Binding
Radioligand binding assays were performed to determine whether 12(S)-HETE can enhance Ang II binding to MC. RMC were treated with either 12(S)-HETE or vehicle for different times and binding of radioactive Ang II measured. Nonlinear regression analysis of [125I]-labeled Ang II saturation binding revealed that Ang II binding was greater in 12(S)-HETE treated RMC (Figure 3A). 12(S)-HETE treatment increased the Bmax value to 975 ± 108.5 fmol/mg protein compared with vehicle Bmax of 403.1 ± 47.3, without changes in affinity for the radioligand (Kd value 0.54 ± 0.15 versus 0.47 ± 0.14 nmol/L). Scatchard plots of binding data revealed no change in slope with increase in x intercept (Figure 3B), indicating that 12(S)-HETE-enhanced surface receptor density rather than binding affinity. Figure 3C shows that 12(S)-HETE-induced increase in Ang II binding was evident from 2 to 24 h (P < 0.05).
Figure 3.
Effects of 12(S)-HETE on Ang II binding. RMC were treated with 12(S)-HETE (10−7 M) for 24 h and (A) saturation binding assays with increasing amounts of [125I]-Ang II were performed on intact cells cultured in 24-well dishes. Unlabeled-Ang II (10−6 M) was used to define nonspecific binding. Samples were incubated for 90 min at 22°C, followed by 3 washes with PBS. Cells were lysed with NaOH and counted in a gamma counter. (B) Specific binding affinities were calculated from Scatchard plots using Prism software. (C) Ang II binding examined in cells treated with or without 12(S)-HETE for 2, 6, or 24 h. Bar graph represents mean ± SEM of three independent experiments (*P < 0.05 versus control).
AT1R Expression is Lower in 12/15-LO-Deficient MC
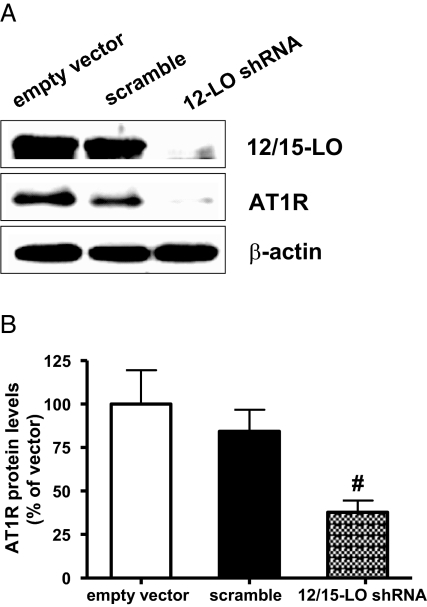
We next evaluated loss-of-function approaches by examining the effects of 12/15-LO knockdown with specific shRNA. RMC were transfected with empty vector or vector expressing U6 promoter-driven rat 12/15-LO shRNA or scrambled shRNA as described.17,29 The 12/15-LO shRNA effectively reduced 12/15-LO protein expression (Figure 4A). Furthermore, AT1R protein expression was also significantly reduced in 12/15-LO shRNA-treated MC compared with scrambled control (Figure 4, A and B).
Figure 4.
12/15 lipoxygenase (12/15-LO) short hairpin RNA (shRNA) can reduce AT1R expression in mesangial cells (MC). (A) RMC were transfected with empty vectors and vectors expressing 12/15-LO or scrambled shRNA sequence. 12/15-LO and AT1R protein expression were determined by Western blotting. (B) Bar graph quantification showing significant reduction in relative AT1R expression in 12/15-LO shRNA-transfected RMC compared with scrambled shRNA or empty vector. Data represent mean ± SEM of four independent experiments (#P < 0.01 versus scrambled control).
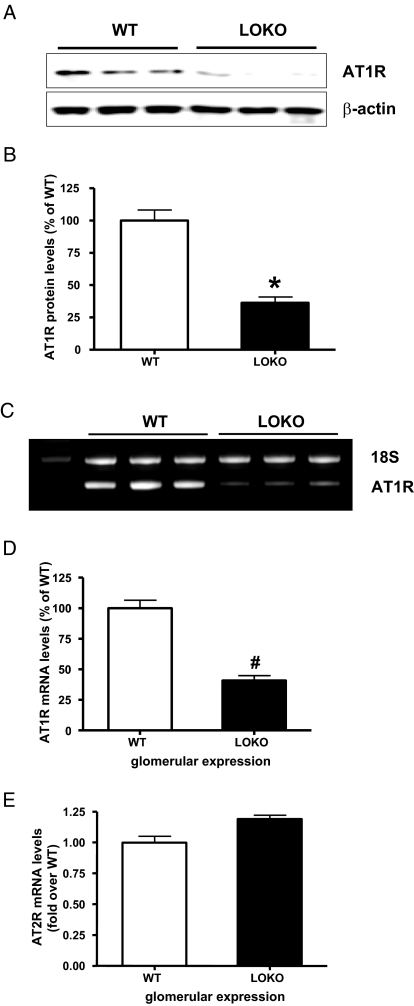
We next reasoned that AT1R levels would be lower in mouse MC (MMC) derived from 12/15-LOKO mice relative to control. Figure 5, A and B show that AT1R protein levels were indeed significantly lower in MMC derived from 12/15-LOKO mice. Furthermore, AT1R mRNA expression was also lower in glomeruli isolated from 12/15-LOKO mice relative to wild-type mice (Figure 5, C and D). However, glomerular AT2R mRNA levels were unaltered (Figure 5E).
Figure 5.
AT1R, but not AT2R, levels are reduced in 12/15-LO gene knockout mice. (A) Mouse MC (MMC) from wild type and 12/15 lipoxygenase knockout (12/15-LOKO) mice were lysed and total protein extracts prepared for AT1R determination by Western blots. (B). Significant reduction in AT1R protein levels in MMC from LOKO mice relative to wild type was noted (*P < 0.05 versus wild type). (C) Glomeruli isolated from wild-type and 12/15-LOKO mice were used for RNA extraction and AT1R mRNA expression determined by reverse transcriptase PCR (RT-PCR) using 18S as internal control. (D) Bar graph representation of data shows significant decrease in AT1R mRNA (#P < 0.01 versus wild type). (E) AT2R mRNA expression not altered in glomeruli from LOKO mice.
12/15-LO Overexpression in MC Increases AT1R Expression
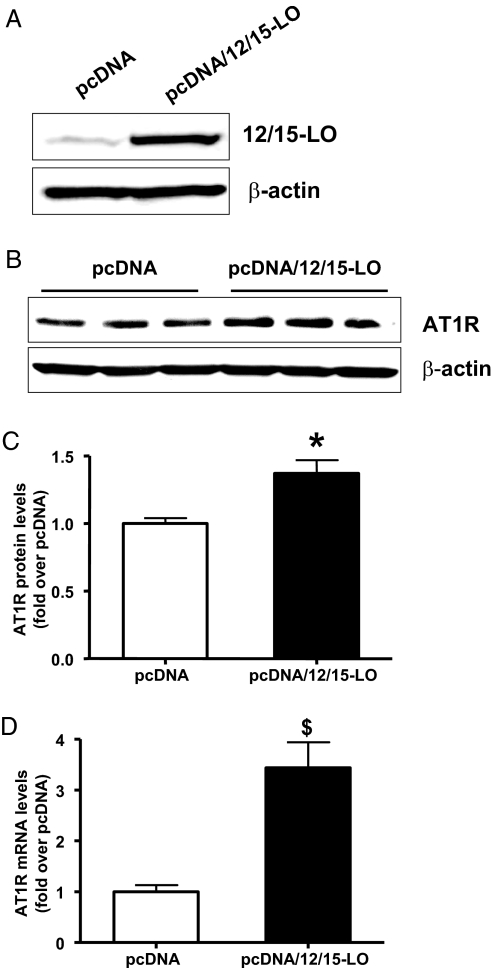
Because 12(S)-HETE increased AT1R expression in MC, we hypothesized that AT1R levels should be elevated in MC that overexpress 12/15-LO (gain of function). We stably overexpressed mouse 12/15-LO in an immortalized MMC line that retains MC characteristics (Figure 6A). AT1R protein (Figure 6, B and C) and mRNA levels (Figure 6D) were enhanced in these MMC stably overexpressing 12/15-LO cDNA (pcDNA/12/15-LO) versus cells transfected with control vector (pcDNA). AT2R expression was not altered (data not shown).
Figure 6.
Expression of AT1R in MMC stably overexpressing 12/15-LO. (A) MMC stably overexpressing 12/15-LO (MMC-pcDNA/12/15-LO) and control (MMC-pcDNA) cells were serum-depleted for 48 h and 12/15-LO protein expression determined by Western blotting. (B) A representative Western blot showing increased AT1R protein expression in 12/15-LO overexpressing MC relative to control cells. (C) Bar graph quantification showing a significant increase in AT1R protein in 12/15-LO-overexpressing cells relative to pcDNA; *P < 0.05. (D) Total RNA was isolated from both control and 12/15-LO-overexpressing cells and AT1R mRNA levels quantified by real-time PCR. A significant increase in AT1R mRNA expression was observed in MMC stably overexpressing 12/15-LO versus control pcDNA. Data represent mean ± SEM of four independent experiments ($P < 0.001 versus pcDNA).
Role of MAPK Activation in 12(S)-HETE-Induced AT1R
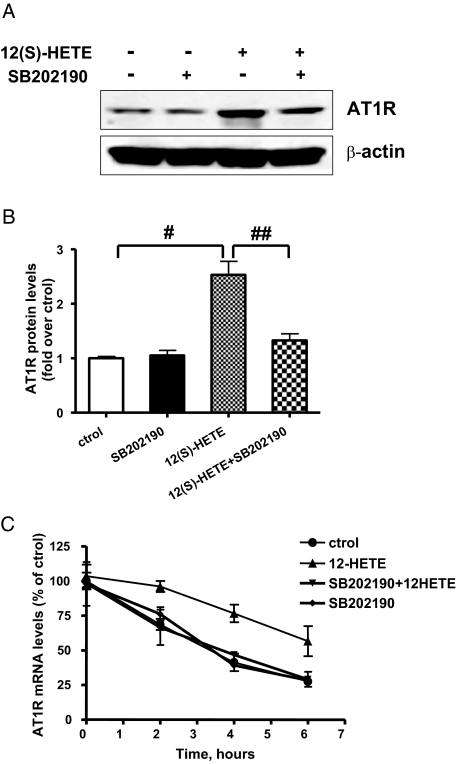
We next examined the role of specific signal transduction mechanisms by which 12(S)-HETE may regulate AT1R expression. Because our recent data showed that 12(S)-HETE primarily activates p38MAPK in MC,19 we evaluated the role of this kinase. Serum-depleted RMC were pretreated with the p38MAPK inhibitor SB202190 (10−6 M) for 30 min and then stimulated with 12(S)-HETE for 6 h. In the presence of SB202190, 12(S)-HETE-induced AT1R protein expression was significantly attenuated (Figure 7, A and B), thereby demonstrating the involvement of p38MAPK.
Figure 7.
Role of p38MAPK in 12(S)-HETE-induced AT1R. (A) Quiescent RMC were pretreated with p38MAPK inhibitor SB202190 (10−6 M for 30 min), then stimulated with 0.1 μM 12(S)-HETE for 6 h, and AT1R protein expression was determined by Western blotting. (B) Bar graph representation of data in A. Results shown are representative of three experiments (#P < 0.01 versus control; ##P < 0.01 versus 12(S)-HETE). (C) RMC were preincubated with SB202190, then with 12(S)-HETE for 6 h, followed by actinomycin D and then total mRNA extracted at the indicated time periods. AT1R mRNA levels remaining at each time point were quantified by real-time PCR (mean ± SEM, n = 3).
Interestingly, the p38MAPK pathway has been implicated in the regulation of protein translation and mRNA stability of other genes.30,31 Because our data show that 12(S)-HETE can enhance AT1R mRNA stability and involvement of p38MAPK, we hypothesized that p38MAPK may also mediate 12(S)-HETE-induced AT1R mRNA stability. Quiescent RMC were pretreated with SB202190 (10−6 M) for 30 min before addition of 12(S)-HETE and 6 h later actinomycin D was used to block transcription. Then AT1R mRNA levels were quantified at the indicated time points. Figure 7C shows that AT1R mRNA stability was increased in 12(S)-HETE-treated cells relative to control group and this was clearly blocked by the p38MAPK inhibitor, suggesting that p38MAPK plays a key role in 12/15-LO mediated AT1R mRNA stabilization in MC.
12(S)-HETE Pretreatment Amplifies Ang II Downstream Signaling
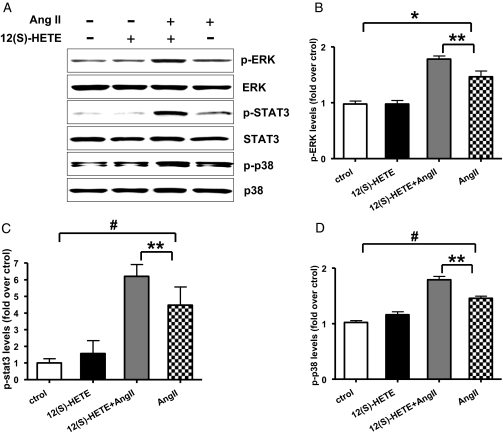
Evidence shows that Ang II can activate p38MAPK, extracellular signal-regulated kinase (ERK) and signal transducer and activator of transcription 3 (STAT3) signaling cascades via AT1R. We therefore hypothesized that 12(S)-HETE-induced enhancement in AT1R expression can augment the signaling and functional responses of Ang II. RMC were pretreated with 12(S)-HETE (10−7 M) for 24 h (at which time period 12-HETE induction of p38MAPK is no longer evident) and then stimulated with Ang II (10−7 M for 5 min). Figure 8, A through D indicate that Ang II can induce p38MAPK, ERK1/2, and STAT3 phosphorylation in control cells, whereas in cells pretreated with 12(S)-HETE, Ang II-induced MAPK and STAT-3 activations were significantly amplified, presumably due to augmented AT1R levels.
Figure 8.
12(S)-HETE pretreatment can amplify Ang II signaling responses in RMC. (A) Quiescent RMC pretreated with 12(S)-HETE (10−7 M) for 24 h, and then stimulated with Ang II (10−7 M) for 5 min. Cell lysates were immunoblotted with specific antibodies to phosphorylated and nonphosphorylated forms of ERK1/2, p38MAPK and STAT3. (B through D) Densities of protein bands were quantified and presented as fold over control. Ang II can induce ERK1/2, p38MAPK, and STAT3 signaling activation, and 12(S)-HETE pretreatment enhances these effects of Ang II (*P < 0.05; #P < 0.01 versus control; **P < 0.05 versus Ang II, n = 3).
siRNA Oligonucleotides Targeted to 12/15-LO Reduce AT1R Expression In Vivo in the Glomeruli of Diabetic Mice
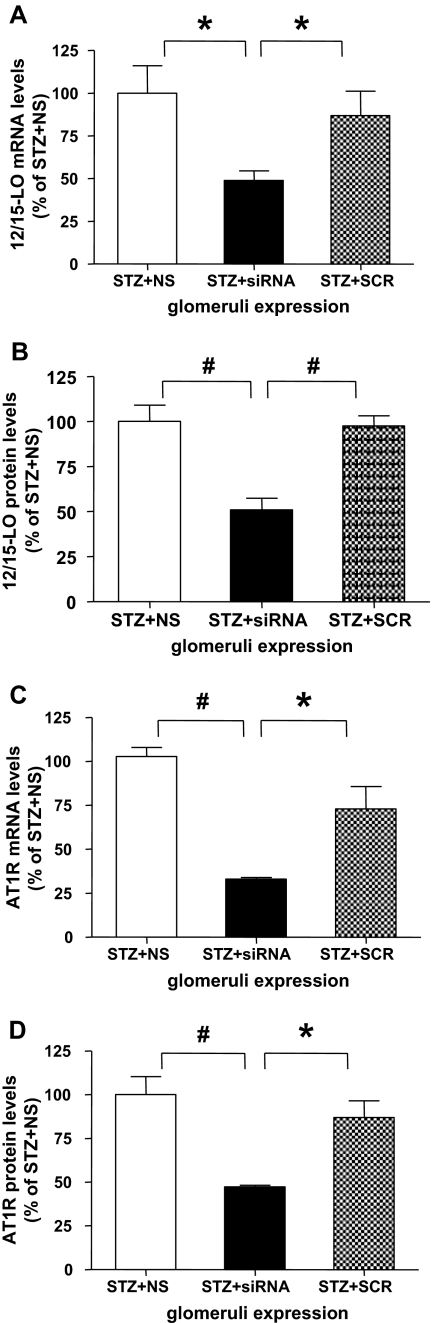
We next tested the in vivo relevance by examining whether siRNA targeting 12/15-LO can block AT1R expression in vivo in a mouse model of type 1 diabetes. DBA/2J mice were made diabetic with streptozotocin and then injected twice weekly with cholesterol-tagged, double-stranded siRNA oligonucleotides targeting 12/15-LO or scrambled sequences as described in figure legends and Supplemental Methods. Mice were sacrificed after 7 wk, glomeruli isolated, and expression of 12/15-LO and AT1R examined. Figure 9, A and B show that 12/15-LO-specific siRNA could efficiently knockdown 12/15-LO mRNA and protein expression, respectively, in vivo in the renal glomeruli. Interestingly, in parallel, AT1R mRNA (Figure 9C) and protein (Figure 9D) expression in these glomeruli were also attenuated by 12/15-LO siRNA by over 50% relative to scrambled oligonucleotides. These results provide additional in vivo supportive evidence for the involvement of 12/15-LO in AT1R expression. Furthermore, they demonstrate the efficacy of cholesterol tagged siRNA to target genes in the glomeruli.
Figure 9.
12/15-LO siRNA injection reduces AT1R expression in mouse glomeruli. 12 DBA/2J mice received 50 mg/kg of streptozotocin intraperitoneally on 5 consecutive days to induce diabetes (glucoses >300 mg/dl).46 Synthetic cholesterol-tagged siRNA oligonucleotides 233 and 826 were dissolved in PBS (total 400 μg) before injections. Three mouse groups (four diabetic mice/group) were injected (subcutaneously) twice weekly per mouse with vehicle, 12/15-LO chol-siRNA, or the scrambled mismatch oligonucleotides, and sacrificed 7 wk after the first siRNA injection. Glomeruli were isolated from renal cortical tissues by sequential sieving as described previously46 and pooled from four mice in each group. The siRNA dose and injection frequency were based on previous reports 40,47 as well as our initial efficacy study demonstrating 12/15-LO knockdown up to 4 d after one injection. Total mRNA and protein were isolated from glomeruli and samples were analyzed in triplicate. (A) 12/15-LO and (C) AT1R mRNA levels were quantified by real-time PCR. Data represent mean ± SEM (* P < 0.05; #P < 0.01). 12/15-LO and AT1R mRNA in glomeruli were significantly decreased by 12/15-LO siRNA injection, but not scrambled oligonucleotides. Protein levels of (B) 12/15-LO and (D) AT1R in isolated glomeruli were determined by Western blotting. Bar graphs represent protein density as mean ± SEM (*P < 0.05; #P < 0.01).
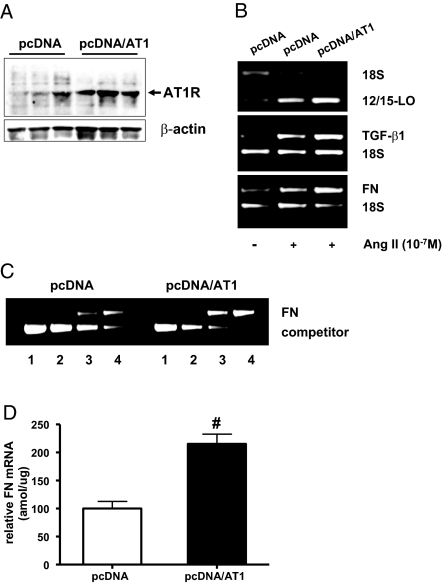
Overexpression of AT1R Enhances Ang II-Induced Pro-Fibrotic Gene Expression in MC
Because evidence shows that Ang II can induce the expression of 12/15-LO as well as the profibrotic markers TGF-β1 and FN in MC,19,20,32,33 we hypothesized that a crosstalk mechanism may operate by which these parameters may be elevated in MC that overexpress AT1R. We used RMC that stably overexpress the rat AT1a receptor (pcDNA/AT1) and retain MC characteristics. Immunoblotting confirmed that AT1R expression was elevated in pcDNA/AT1 cells compared with those expressing control pcDNA (Figure 10A). Furthermore, Ang II-induced expression of 12/15-LO, TGF-β1, and FN mRNA was markedly higher in AT1R overexpressing cells (pcDNA/AT1) relative to pcDNA control (Figure 10B). Quantitative competitive reverse transcriptase PCR (RT-PCR) further confirmed that Ang II-induced FN expression is significantly enhanced in pcDNA/AT1 cells (Figure 10, C and D). These results are consistent with our hypothesis that AT1R overexpression can amplify Ang II effects related to ECM accumulation and renal dysfunction and further underscore the significance of this novel crosstalk between 12/15-LO and AT1R.
Figure 10.
12/15-LO, TGF-β1, and fibronectin (FN) expression in RMC stably overexpressing AT1R. (A) RMC stably overexpressing AT1R (pcDNA/AT1) and control (pcDNA) cells were serum-depleted for 48 h, and AT1R protein expression was determined by Western blotting. (B) RMC stably overexpressing AT1R and control cells were serum-depleted for 48 h and treated with or without Ang II (10−7 M) for 24 h. 12/15-LO, TGF-β1, and FN mRNA levels were determined by relative RT-PCR. (C) Representative gel showing quantitative competitive RT-PCR46 of FN in control and RMC stably overexpressing AT1R stimulated with Ang II for 24 h. Each lane represents a competitive RT-PCR reaction with fixed amount of FN WT cDNA and a variable amount of AT1R competitor cDNA as follows: lane 1, 50 amol/μl; lane 2, 10 amol/μl; lane 3, 5 amol/μl; lane 4, 1 amol/μl. (D) A significant increase in Ang II-induced FN mRNA expression was observed in RMC stably overexpressing AT1R versus control pcDNA. Data represents mean ± SEM of three independent experiments (#P < 0.01 versus pcDNA).
DISCUSSION
Ang II acts mainly via AT1R to mediate its profibrotic and growth-promoting effects, and various factors regulate AT1R expression.25–27,34 However, although Ang II can induce the formation of several eicosanoid-oxidized lipids that affect renal function, it is not known whether such lipids directly modulate AT1R expression. In this study, we demonstrate that bioactive lipids of the 12/15-LO pathway can directly regulate AT1R expression and downstream signaling.
Evidence shows that the LO product 12(S)-HETE mimics the actions of Ang II and high glucose related to the development of diabetic renal disease.17–20 Furthermore, cellular growth, matrix production, activation of oxidant stress, specific signaling pathways, and transcriptional factors were attenuated in MMC derived from 12/15-LOKO mice relative to wild type.20 Importantly, the growth-promoting and matrix-inducing effects of Ang II in MC were blocked by 12-LO inhibitors and were also attenuated in MMC derived from 12/15-LOKO mice.19,20 We therefore reasoned that AT1R expression may be regulated by 12/15-LO and this was supported by several lines of evidence obtained in this study.
The 12/15-LO product 12(S)-HETE directly increased both AT1R mRNA and protein expression, but not AT2R. 12(S)-HETE also enhanced Ang II binding in MC. In addition, shRNA targeting 12/15-LO reduced AT1R expression in MC. Furthermore, MMC and glomeruli from LOKO mice had lower AT1R expression relative to wild type. Reciprocally, MMC overexpressing mouse 12/15-LO had increased AT1R levels compared with mock transfected cells.
Although there are conflicting reports regarding AT1R expression in kidneys of streptozotocin-induced diabetic rodents,35–37 nonetheless, the importance of AT1R activation in progressive diabetic glomerulosclerosis is evident because AT1R blockade with ARB significantly reduced proteinuria and glomerulosclerosis in clinical and experimental diabetes.38,39 Because our recent studies demonstrated that 12/15-LO is overexpressed in experimental models of type 1 and 2 diabetes18,39 and administration of the ARB losartan to Zucker obese, insulin-resistant rats abrogated the increment of 12/15-LO in the renal cortex of obese rats toward levels seen in lean controls,39 we hypothesized that AT1R expression in vivo can be reduced by silencing 12/15-LO expression. Thus we administered 12/15-LO-specific synthetic siRNA tagged with cholesterol (for improved bioavailability and renal expression as reported40) to diabetic mice. Along with a reduction in renal glomerular 12/15-LO expression, the 12/15-LO siRNA, but not control scrambled shRNA significantly diminished AT1R expression in vivo in the glomeruli. These results demonstrate a novel molecular strategy based on siRNA to downregulate AT1R in vivo in the kidney. These siRNA also reduced key indices of DN in the mice (Yuan and Natarajan, unpublished results). These in vitro and in vivo results support the concept that AT1R expression can be regulated, at least in part, by activation of the 12/15-LO pathway and its bioactive oxidized lipid products.
There has been heightened interest in 12/15-LO because of its reported association with the pathogenesis of atherosclerosis,12,41 restenosis,42 hypertension,13,22,43 and DN.17–20 12(S)-HETE is involved in multiple events related to the development of these diseases with potency similar to the biofunction of Ang II,14,17,19–22 Crossbreeding 12/15-LOKO mice with atherosclerosis-prone ApoE −/− mice greatly reduced atherosclerosis development.12 Our data presented here suggest a close relationship between 12/15-LO and AT1R upregulation. Hence, the reduced response to Ang II in MMC from 12/15-LOKO mice and attenuated atherosclerosis in 12/15-LOKO mice may be due to downregulated AT1R expression caused at least in part by the 12/15-LO deficiency.
Because agents that interfere with Ang II action may decrease glomerular injury without altering glomerular pressure, Ang II has direct effects via AT1R receptor to induce sclerosis independent of its hemodynamic action.7–9,32,33 At present, it is not certain that increased activity of other local components of RAS such as ACE), angiotensinogen and Ang II are concomitantly required, or AT1R overexpression itself is sufficient to propagate renal injury. In our study, 12(S)-HETE did not affect angiotensinogen mRNA or protein levels in MC (data not shown).
We noted that 12(S)-HETE induced AT1R can functionally enhance Ang II signaling because pretreatment of MC with 12(S)-HETE significantly amplified Ang II-induced ERK1/2, p38MAPK, and STAT3 activations. We previously reported that 12/15-LO and its products can increase TGF-β1 expression and signaling in MC and vice versa.17 Taken together with our present observations, it appears that 12/15-LO can crosstalk and amplify the effects of two key potent profibrotic and renal dysfunction-induced agents, TGF-β1 and Ang II.
To further ascertain the functional relevance of 12/15-LO induced AT1R, we demonstrated that Ang II effects on key pathologic targets such as 12/15-LO, TGF-β1, and FN were markedly enhanced in AT1R overexpressing MC compared with control cells. Thus, increased AT1R expression by factors such as 12/15-LO can accelerate disease progression by augmenting Ang II signaling. This is further supported by the fact that 12/15-LO is implicated in the pathogenesis of DN, obesity, atherosclerosis, and hypertension; all disorders associated with augmented Ang II actions.
AT1R is regulated by transcriptional, posttranscriptional, and translational mechanisms.26–28,44,45 Our data suggests that 12(S)-HETE increases AT1R expression via posttranscriptional mRNA stability rather than enhanced transcription. Evidence shows that the 3′-untranslated region of AT1R regulates mRNA stabilization.26–28 Furthermore, the p38MAPK pathway has been implicated in mRNA stabilization of several genes.30,31 We noted that a specific inhibitor of p38MAPK blocked 12(S)-HETE-induced AT1R protein expression, suggesting that p38MAPK plays an important role in mediating 12(S)-HETE induced AT1R mRNA stability and AT1R expression.
In summary, 12/15-LO and its product 12(S)-HETE can augment AT1R and Ang II signaling in MC. Furthermore, overexpression of AT1R in MC augments the Ang II bioactivity related to ECM deposition. This novel crosstalk suggests that new therapeutic strategies based on 12/15-LO inhibition could prove beneficial in renal diseases associated with enhanced Ang II actions.
CONCISE METHODS
Materials
12(S)-HETE and its stereoisomer 12(R)-HETE were from Biomol (Plymouth Meeting, Pennsylvania); Actinomycin D was from Sigma-Aldrich Chemicals (St. Louis, Missouri); AT1R antibody was from Santa Cruz Biotechnology (Santa Cruz, California); p38 MAPK inhibitor (SB202190) was from Calbiochem (La Jolla, California). Antibodies for phosphospecific and nonphospho-p38MAPK, STAT3, and ERK1/2, and horseradish peroxidase-conjugated secondary antibodies were from Cell Signaling (Beverly, Massachusetts); β-actin antibody was from Sigma (St. Louis, Missouri); and supersignal chemiluminescence reagent was from Pierce (Rockford, Illinois); 12/15-LO antibody has been described previously.18 RT-PCR kits were from Applied Biosystems (Foster City, California) and primers for Quantum RNA 18S and β-actin internal standards from Ambion Inc. (Austin, Texas); Lipofectamine 2000 transfection reagent was from Invitrogen (Carlsbad, California), RNA-STAT60 reagent from Tel-Test (Friendswood, Texas), and [125I]-Ang II from Amersham (Piscataway, New Jersey).
MC Culture
Primary cultures of RMC from Sprague-Dawley rats and MMC from genetic control (C57BL/6, WT) or leukocyte-type 12/15-LOKO mice (Jackson Laboratories, Strain: B6.129S2-Alox15tm1Fun, Stock Number 002778) were cultured as described previously.18–20 Cells between five to nine passages were used. RMC stably overexpressing AT1a receptor and corresponding mock transfected cells were a generous gift from Dr. Hanna Abboud (University of Texas Health Science Center, San Antonio, Texas). Stable overexpression of mouse 12/15-LO was performed as described previously17 in an immortalized MMC cell line46 obtained from Dr. Kumar Sharma (Thomas Jefferson University, Philadelphia, Pennsylvania).
RNA Interference and Transfection with shRNA
Design, construction, and transfections of shRNA and control vectors were performed as described.17,29
In Vivo Experiments with Mice
See Supplemental Information.
Relative Multiplex PCR, Quantitative Competitive RT-PCR, Real time-RT-PCR (QRT-PCR), and Western Blots.
These were performed as described previously18,19,47 and also described in Supplemental Information. Primers used are summarized in Table 1.
Table 1.
Primer sequences for amplification of various transcripts
| Target | Product (bp) | |
|---|---|---|
| Angiotensin II type I receptor (AT1R) (R) | 223 | |
| Sense | AAGGCTGGCAGGCACAGTTAC | |
| Antisense | CGCTACAGCGGTACTCCATAGCG | |
| AT1R (M) | 317 | |
| Sense | GAAGCTGAAGACTGTGGC | |
| Antisense | GCTGGCAAACTGGCCAAG | |
| Angiotensin II type II receptor (R/M) | 163 | |
| Sense | CGGGTGGAAGAACAGAATTACCCG | |
| Antisense | TGCCAGGTCAATGACTGCTATAA | |
| 12/15-Lipoxygnease (R/M) | 139 | |
| Sense | CGCTGGCACTCTGTTTGAAGCG | |
| Antisense | TGGATGGCTATGGGCAAGA | |
| TGF-β1 (R) | 403 | |
| Sense | CGAGGTGACCTGGGCACCATCC | |
| Antisense | CTGTGCTCATTGCCTTGTC | |
| Fibronectin (R) | 446 | |
| Sense | GCAAGCCTGAACCTGAAGAGACC | |
| Antisense | CCTGGTGTCCTGATCATTGCATC |
R, rat sequence; M, mouse sequence
Radioligand Binding Assay
See Supplemental Information.
Statistical Analyses
Data are expressed as mean ± SEM of multiple experiments. Paired t tests were used to compare two groups or ANOVA with Turkey's post test for multiple groups using PRISM software (Graph Pad, San Diego, California). Rate of degradation of AT1R mRNA was analyzed by two-way ANOVA. Statistical significance was detected at the 0.05 level.
DISCLOSURES
None.
Acknowledgments
These studies were supported by grants from the National Institutes of Health (R01 DK058191 and R21 DK076669). We thank Dr. Young-Sook Kim and Janine Lapage for their help, Dr. Hanna Abboud for RMC-overexpressing AT1a receptor, and Dr. Kumar Sharma for the MMC line.
Published online ahead of print. Publication date available at www.jasn.org.
Z.-G.X. and H.Y. contributed equally to this work.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Matsusaka T, Hymes J, Ichikawa I: Angiotensin in progressive renal diseases: Theory and practice. J Am Soc Nephrol 7: 2025–2043, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Wolf G, Ziyadeh FN: The role of angiotensin II in diabetic nephropathy: emphasis on non hemodynamic mechanisms. Am J Kidney Dis 29: 153–163, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Timmermans PB, Benfield P, Chiu AT, Herblin WF, Wong PC, Smith RD: Angiotensin II receptors and functional correlates. Am J Hypertens 5: 221S–235S, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Siragy HM: AT1 and AT2 receptor in the kidney: Role in health and disease. Semin Nephrol 24: 93–100, 2004. ′ [DOI] [PubMed] [Google Scholar]
- 5.Chiu AT, Dunscomb J, Kosierowski J, Burton CR, Santomenna LD, Corjay MH, Benfield P: The ligand binding signatures of the rat AT1A, AT1B, and the human AT1 receptors are essentially identical. Biochem Biophys Res Commun 197: 440–449, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Llorens-Cortes C, Greenberg B, Huang H, Corvol P: Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension 24: 538–548, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Anderson S, Rennke HG, Garcia DL, Brenner BM: Short and long term effects of antihypertensive therapy in the diabetic rat. Kidney Int 36: 526–536, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Volpini RA, da Silva CG, Costa RS, Coimbra TM: Effect of enalapril and losartan on the events that precede diabetic nephropathy in rats. Diabetes Metab 19: 43–51, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto S: Mammalian lipoxygenase: Molecular structures and functions. Biochim Biophys Acta 1128: 117–131, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Funk CD: The molecular biology of mammalian lipoxygenase and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim Biophys Acta 1304: 65–68, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD: Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest 103: 1597–1604, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nozawa K, Tuck ML, Golub M, Eggena P, Nadler JL, Stern N: Inhibition of lipoxygenase pathway reduces blood pressure in renovascular hypertensive rats. Am J Physiol 2592: H1774–H1780, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Natarajan R, Nadler JL: Lipoxygenases and lipid signaling in diabetes. Frontiers in Biosciences 8: S783–S795, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Antonipillai I, Nadler J, Vu EJ: A 12-lipoxygenase product, 12-hydroxyeicosatetraenoic acid, is increased in diabetics with incipient and early renal disease. J Clin Endocrinol Metab 81: 1940–1945, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Katoh T, Lakkis FG, Makita N, Badr KF: Co-regulated expression of glomerular 12/15-lipoxygenase and interleukin-4 mRNAs in rat nephrotoxic nephritis. Kidney Int 46: 341–349, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Kim YS, Xu ZG, Reddy MA, Li SL, Lanting L, Sharma K, Adler SG, Natarajan R: Novel interactions between TGF-β1 actions and the 12/15-lipoxygenase pathway in mesangial cells. J Am Soc Nephrol 16: 352–362, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Kang SW, Adler SG, Nast CC, LaPage J, Gu JL, Nadler JL, Natarajan R: 12-lipoxygenase expression is increased in diabetic nephropathy. Kidney Int 59: 1354–1362, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Reddy MA, Adler SG, Kim YS, Lanting L, Rossi JJ, Kang SW, Nadler JL, Shahed A, Natarajan R: Interaction of MAPK and 12-lipoxygenase pathways in growth and matrix protein expression in mesangial cells. Am J Physiol Renal Physiol 283: F985–F994, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Kim YS, Reddy MA, Lanting L, Adler SG, Natarajan R: Differential behavior of mesangial cells derived from 12/15-lipoxygenase knockout mice relative to control mice. Kidney Int 64: 1702–1714, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Natarajan R, Gu JL, Rossi J, Gonzales N, Lanting L, Xu L, Nadler J: Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc Natl Acad Sci U S A 90: 4947–4951, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natarajan R, Gonzales N, Lanting L, Nadler J: Role of the lipoxygenase pathway in angiotensin II-induced vascular smooth muscle cell hypertrophy. Hypertension 23: 142–147, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Reddy MA, Thimmalapura PR, Lanting L, Nadler JL, Fatima S, Natarajan R: The oxidized lipid and lipoxygenase product 12(S)-hydroxyeicosatetraenoic acid induces hypertrophy and fibronectin transcription in vascular smooth muscle cells via p38 MAPK and cAMP response element binding protein activation. Mediation of angiotensin II effects. J Biol Chem 277: 9920–9928, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Reddy MA, Kim YS, Lanting L, Natarajan R: Reduced growth responses in 12/15-lipoxygenase deficient smooth muscle cells. Hypertension 41: 1294–1300, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Nickenig G, Harrison DG: The AT1-type angiotensin receptor in oxidative stress and atherogenesis. Part II: AT1 receptor regulation. Circulation 105: 530–536, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Nickenig G, Sachinidis A, Michaelsen F, Bohm M, Seewald S, Vetter H: Upregulation of vascular angiotensin II receptor gene expression by low-density lipoprotein in vascular smooth muscle cells. Circulation 95: 473–478, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Nickenig G, Roling J, Strehlow K, Schnabel P, Bohm M: Insulin induces upregulation of vascular AT1 receptor gene expression by posttranscriptional mechanisms. Circulation 98: 2453–2460, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Thekkumkara TJ, Thomas WG, Motel TJ, Baker KM: Functional role for the angiotensin II receptor (AT1A) 3′-untranslated region in determining cellular responses to agonist: Evidence for recognition by RNA binding proteins. Biochem J 329: 255–264, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li SL, Dwarakanath RS, Cai Q, Lanting L, Natarajan R: Effects of silencing leukocyte-type 12/15-lipoxygenase using short interfering RNAs. J Lipid Res 46: 220–229, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Frevel MA, Bakheet T, Silva AM, Hissong JG, Khabar KS, Williams BR: p38 mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol Cell Biol 23: 425–436, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonathan LED, Gareth S, Andrew RC, Jeremy S: The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilization. Cell. Signal. 16: 1113–1121, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Mezzano SA, Ruiz-Ortega M, Egido J: Angiotensin II and renal fibrosis. Hypertension 38: 635–638, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Leehey DJ, Singh AK, Alavi N, Singh R: Role of angiotensin II in diabetic nephropathy. Kidney Int Suppl 77: S93–S98, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Park SY, Song CY, Kim BC, Hong HK, Lee HS: Angiotensin II mediates LDL-induced superoxide generation in mesangial cells. Am J Physiol Renal Physiol 285: 909–915, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Harrison-Bernard LM, Imig JD, Carmines PK: Renal AT1 receptor protein expression during the early stage of diabetes mellitus. Int J Exp Diabetes Res 3: 97–108, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ballermann BJ, Skorecki KL, Brenner BM: Reduced glomerular angiotensin II receptor density in early untreated diabetes mellitus in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 247: 110–116, 1984 [DOI] [PubMed] [Google Scholar]
- 37.Kalinyak JE, Sechi LA, Griffin CA, Don BR, Tavangar K, Kraemer FB, Hoffman AR, Schambelan M: The reninangiotensin system in streptozotocin-induced diabetes mellitus in the rat. J Am Soc Nephrol 4: 1337–1345, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Remuzzi A, Perico N, Amuchastegui CS, Malanchini B, Mazerska M, Battaglia C, Bertani T, Remuzzi G: Short- and long-term effect of angiotensin II receptor blockade in rats with experimental diabetes. J Am Soc Nephrol 4: 40–49, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Xu ZG, Lanting L, Vaziri ND, Li Z, Sepassi L, Rodriguez-Iturbe B, Natarajan R: Upregulation of angiotensin II type 1 receptor, inflammatory mediators and enzymes of arachidonate metabolism in obese zucker rat kidney: Reversal by angiotensin II type 1 receptor blockade. Circulation 111: 1962–1999, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP: Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432: 173–178, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Natarajan R, Gerrity RG, Gu JL, Lanting L, Thomas L, Nadler JL: Role of 12-lipoxygenase and oxidant stress in hyperglycaemia-induced acceleration of atherosclerosis in a diabetic pig model. Diabetologia 45: 125–133, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Gu JL, Pei H, Thomas L, Nadler JL, Rossi JJ, Lanting L, Natarajan R: Ribozyme-mediated inhibition of rat leukocyte-type 12-lipoxygenase prevents intimal hyperplasia in balloon injured rat carotid arteries. Circulation 103: 1446–1452, 2001 [DOI] [PubMed] [Google Scholar]
- 43.DelliPizzi A, Guan H, Tong X, Takizawa H, Nasjletti A: Lipoxygenase-dependent mechanisms in hypertension. Clin Exp Hypertens 22: 181–192, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Sugawara A, Takeuchi K, Uruno A, Ikeda Y, Arima S, Kudo M, Sado K, Taniyama Y, Ito S: Transcriptional suppression of type 1 angiotensin II receptor gene expression by peroxisome proliferator-activated receptor-gamma in vascular smooth muscle cells. Endocrinology 142: 3125–3134, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Ji H, Fabucci ME, Falconetti C, Zheng W, Sandberg K: Translational control of the rat angiotensin type 1a receptor by alternative splicing. Gene 341: 93–100, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Wolf G, Haberstroh U, Neilson EG: Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol 140: 95–107, 1992 [PMC free article] [PubMed] [Google Scholar]
- 47.Xu ZG, Li SL, Lantin L, Kim YS, Shanmugam N, Reddy MA, Natarajan R: Relationship between 12/15-lipoxygenase and COX-2 in mesangial cells: Potential role in diabetic nephropathy. Kidney Int 69: 512–519, 2006 [DOI] [PubMed] [Google Scholar]