Abstract
Recent evidence has linked exposure to addictive drugs to an inability to employ information about adverse consequences, or outcomes, to control behavior. For instance, addicts and drug-experienced animals fail to adapt their behavior to avoid adverse outcomes in gambling and reversal tasks or after changes in the value of expected rewards. These deficits are similar to those caused by damage to the orbitofrontal cortex, suggesting that addictive drugs may cause long-lasting changes in the representation of outcome associations in a circuit that includes the orbitofrontal cortex. Here we test this hypothesis by recording from orbitofrontal neurons in a discrimination task in rats previously exposed to cocaine (30 mg/kg i.p. for 14 days). We found that orbitofrontal neurons recorded in cocaine-experienced rats failed to signal the adverse outcome at the time a decision was made in the task. The loss of this signal was associated with abnormal changes in response latencies on aversive trials. Furthermore, upon reversal of the cue–outcome associations, orbitofrontal neurons in cocaine-treated rats with enduring reversal impairments failed to reverse their cue-selectivity, while orbitofrontal neurons in cocaine-treated rats with normal performance showed an increase in the plasticity of cue-selective firing after reversal. These results provide direct neurophysiological evidence that exposure to cocaine can cause behaviorally relevant changes in the processing of associative information in a circuit that includes the orbitofrontal cortex.
Keywords: neurophysiology, orbitofrontal, psychostimulant, rat, reversal
Introduction
Recent evidence has linked exposure to addictive drugs to an inability to employ information about adverse consequences, or outcomes, to control behavior. For instance, addicts fail to adapt their behavior to avoid adverse outcomes in gambling and reversal tasks (Grant et al., 2000; Bechara et al., 2001; Clark & Robbins, 2002). These results suggest that some drugs of abuse may cause changes in information processing in brain circuits devoted to representing and using anticipated outcomes to guide behavior. This proposal is supported by work in animal models showing a causal relationship between drug exposure and behavioral impairments in outcome-guided behavior. Rats previously sensitized to cocaine and primates previously trained to self-administer cocaine are slower to respond to changes in the predicted outcome in reversal tasks (Jentsch et al., 2002; Schoenbaum et al., 2004), and rats previously exposed to cocaine fail to modify conditioned responding when the predicted outcome is devalued in a Pavlovian setting (Schoenbaum & Setlow, 2005). These deficits are similar to those reported after damage to the orbitofrontal cortex (OFC), a brain structure critical to signaling expected outcomes (Bechara et al., 1997; Gallagher et al., 1999; Fellows & Farah, 2003; Schoenbaum et al., 2003a; Izquierdo et al., 2004; Schoenbaum & Roesch, 2005; Schoenbaum & Setlow, 2005). Yet drug exposure is not equivalent to a brain lesion. To test the hypothesis that these behavioral effects reflect altered processing in the OFC, we investigated whether simple exposure to cocaine causes chronic changes in the encoding of associative information in the OFC, thought to underlie normal performance in these settings.
Materials and methods
Subjects
Ten male Long-Evans rats (175–200 g; Charles River Laboratories, Wilmington, MA, USA) served as subjects. Rats were tested at the University of Maryland School of Medicine in accordance with a protocol approved by the University Institutional Animal Care and Use Committee and NIH guidelines.
Cocaine exposure
Beginning approximately 6 weeks before recording, rats received daily i.p. injections of 30 mg/kg cocaine HCl or saline vehicle (NIDA, Bethesda, MD, USA) for 14 days. At the completion of recording, all rats received ascending doses of cocaine (0.9% saline and 7.5, 15.0, 30.0 mg/kg cocaine HCl i.p.); locomotor activity was monitored over a 1-h period immediately after each injection, using overhead activity monitors mounted in clear Plexiglas training chambers (Coulbourn Instruments, Allentown, PA, USA).
Surgery and histology
Using aseptic, stereotaxic surgical techniques, under isoflurane gas anesthesia a driveable bundle of ten 25-μm-diameter FeNiCr wires (Stablohm 675, California Fine Wire, Grover Beach, CA, USA) was chronically implanted dorsal to the OFC in the left hemisphere at 3.0 mm anterior to bregma, 3.2 mm laterally, and 4.0 mm ventral to the surface of the brain in each rat. Immediately prior to implantation, these wires were freshly cut with surgical scissors to extend ∼1 mm beyond the cannula and electroplated with platinum (H2PtCl6, Aldrich, Milwaukee, WI, USA) to an impedance of ∼300 kΩ. Details of the electrode construction and plating procedure are available (Schoenbaum, 2001). At the end of the study, the final electrode position was marked by passing a 15-μA current through each electrode for ∼10 s to create a small iron deposit. The rats were then killed with an overdose of isoflurane and perfused, and the brains were removed from the skulls and processed for histology using standard techniques, which included treatment with 3% potassium ferrocyanide to visualize the iron deposit (Gallagher et al., 1999; Schoenbaum et al., 1999). Sections were mounted on slides and examined by light microscopy to identify the final electrode position. The location of the final electrode position was mapped to the standardized brain sections in Paxinos & Watson (1997) by comparing the morphology of the rhinal sulcus and the underlying piriform cell layer. By using the distance moved and calculating back from this final electrode position, we were able to reconstruct the recording sites illustrated below in Fig. 2.
Fig. 2.
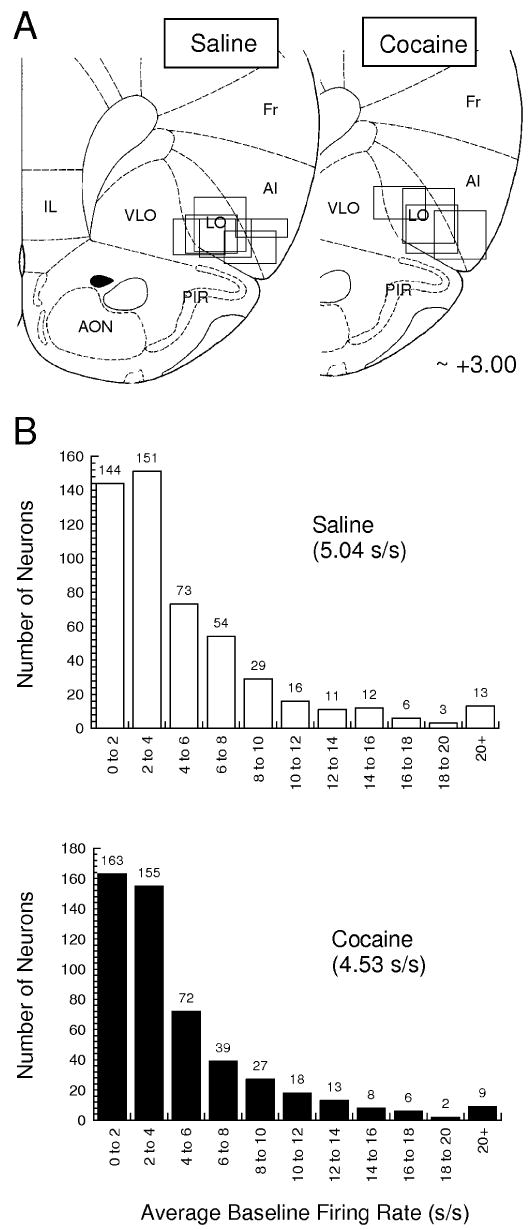
Location of recording sites and distribution of baseline firing rates for OFC neurons recorded in saline- and cocaine-treated rats. (A) Recording sites in the OFC. Boxes indicate the approximate location of recordings in each rat. The vertical distance was determined by calculating back from the histologically verified final electrode position; the horizontal spread reflects the size of the electrode bundle. (B) Average baseline firing rate and distribution of baseline firing rates for neurons recorded in each group. Baseline firing rats were calculated from activity in the intertrial intervals.
Odor discrimination training
Discrimination training was conducted in aluminium chambers approximately 18′ on each side with sloping walls narrowing to an area of 12′ × 12′ at the bottom. These chambers were not the same chambers in which cocaine sensitization occurred. A single odor port and a single fluid well were located on a panel in the right wall of each chamber below two panel lights; the odor port was connected to an air flow dilution olfactometer to allow the rapid delivery of olfactory cues. The fluid well received three lines through which could be delivered water or solutions containing sucrose or quinine. A fourth line in the bottom of the well was connected to a vacuum to allow the well to be rapidly drained. Task control was implemented via computer. Odor discrimination problems were composed of odor pairs chosen from compounds obtained from International Flavors and Fragrances (New York, NY, USA), as in prior studies (Schoenbaum et al., 1998, 2003b). During training, rats were maintained on water restriction.
Trials were signaled by illumination of the panel lights inside the box. When these lights were on, nosepoke into the odor port resulted in delivery of the preselected odor cue to a small hemicylinder located behind this opening. The rat terminated odor sampling by leaving the odor port, and then had 3 s to make a go response at the fluid well located below the port. If a response was made after sampling a positive odor, then a 0.05-mL bolus of a 10% sucrose solution was delivered to the well after a variable delay (500–1500 ms). If the same response was made after sampling a negative odor, then a 0.05-mL bolus of a 0.02 m (0.722%) quinine solution was delivered after a similar delay. If the rat did not respond within 3 s, the trial was counted as a no-go. Between trials, any remaining fluid was removed via the vacuum-assisted drain line, and the fluid well was flushed with water to remove any trace solution. Rats typically began each session with a new odor pair by responding on every trial, and then learned to withhold responding after sampling the negative odor. Rats rarely failed to respond on positive trials. A behavioral criterion was defined as 18 correct responses (go on positive trials, no-go on negative trials) in a moving block of 20 trials. Rats were trained for 60–100 trials after meeting this criterion in order to acquire neural data after learning for subsequent analyses; then the odor–outcome associations were reversed. Training continued until the rats met the criterion on the reversal. Performance phases were defined based on go, no-go performance to include a pre-criterion phase, before the rat met the criterion on the discrimination, a post-criterion phase, after the rat met the criterion, and a reversal phase, after the odor–outcome associations were switched. The pre-criterion phase was further divided into an early period and a late period, based on the rat's sixth error. The rats received training on several problems prior to surgery, and then neural data were collected as the rats acquired novel discriminations in sessions after surgery.
Single-unit recording
For each recording session, the rat was placed in the training chamber, and the electrode wires were screened for neural activity while the rat explored the open chamber. Active wires were selected for recording, and a training session was begun. If no activity was detected, the rat was removed, and the electrode assembly was advanced 40 or 80 μm. Otherwise the electrode was advanced at the end of the session, so that neural activity was never acquired from the same location twice. Neural activity was recorded using two identical Plexon Multichannel Acquisition Processor systems (Dallas, TX, USA), interfaced with odor discrimination training chambers described above. Signals from the electrode wires were amplified 20× by an op-amp headstage (Plexon Inc., HST/8o50-G20-GR), located on the electrode array. Immediately outside the training chamber, the signals were passed through a differential pre-amplifier (Plexon Inc., PBX2/16sp-r-G50/16fp-G50), where the single unit signals were amplified 50× and filtered at 150–9000 Hz. The single-unit signals were then sent to the Multichannel Acquisition Processor box, where they were further filtered at 250–8000 Hz, digitized at 40 kHz and amplified at 1–32×. Waveforms (> 2.5 : 1 signal-to-noise) were extracted from active channels and recorded to disk by an associated workstation with event timestamps from the behavior computer.
Statistical data analysis
Units were sorted using Offline Sorter software from Plexon Inc., using a template matching algorithm and notes regarding the waveforms were made during the session. Sorted files were then processed in Neuroexplorer to extract unit timestamps and relevant event markers. These data were subsequently analysed using statistical routines in Matlab (Natick, MA, USA) to examine activity during odor sampling (from 50 ms after odor onset to 50 ms after odor offset), during the variable delay after a response at the fluid well (from 50 ms before the response until fluid delivery), and after fluid delivery (first 500 ms). Firing activity (spikes/s) in each time window was compared on positive and negative trials during pre- and post-criterion trial blocks using anova (P < 0.05), and neurons with a significant difference in activity were categorized as ‘selective’ or ‘active’ for the odor with the higher firing rate in that time window and phase (NB: anova was used for consistency with prior datasets; however, the results were also similar with non-parametric tests). Note that by using this terminology we do not mean to imply that the neuron is inactive to the other odor. Pearson chi-squared tests (P < 0.05) were used to compare the proportions of neurons with different firing properties in saline- and cocaine-treated rats. In addition, we computed a cue-selectivity index for outcome-expectant neurons. This index was calculated, for a given neuron, as (frs − frq)/(frs + frq), where frs was the firing rate during sampling of the sucrose-predicting odor cue, and frq was the firing rate during sampling of the quinine-predicting odor cue. The distribution of this measure in different populations was evaluated using the Wilcoxon signed rank test. Finally, population histograms were constructed by averaging across histograms from each neuron.
The results of statistical analyses are reported in the text. We have also provided the results of power analyses wherever the resultant P-value was nearly significant (P ≤ 0.3). Power was calculated using G*power, a freely available shareware program.
Results
We recorded neural activity during performance of a go, no-go two-odor discrimination task employed in previous studies (Schoenbaum et al., 1998, 1999, 2003b; Setlow et al., 2003; Saddoris et al., 2005). Rats were trained to sample an odor on each trial and then execute a response at a nearby fluid well. A response after the ‘positive’ odor was considered correct and resulted in delivery of 0.05 mL of a 10% sucrose solution; the same response after the ‘negative’ odor was considered incorrect and resulted in delivery of the same amount of a 0.02 m quinine solution. In each session, rats learned to discriminate a pair of novel odor cues. The rats began each session by responding on every trial but rapidly learned to go to the well after sampling the odor cue paired with sucrose and to withhold that response (no-go) after sampling the odor cue paired with quinine.
Neural activity was recorded in this task in rats that had been previously exposed to non-contingent injections of saline or cocaine (30 mg/kg i.p. every 14 days). As expected, locomotor activity increased significantly in rats treated with cocaine relative to their pre-exposure levels and in comparison with activity in saline-treated controls. A two-factor anova (group × day) revealed significant main effects of group (F1,12 = 19.4, P < 0.001) and day (F14,168 = 3.29, P < 0.001) and a significant interaction (F14,168 = 4.40, P < 0.001). Recording began 4 weeks after the end of drug treatment and continued for approximately 8 weeks. At the end of recording, all rats were challenged with ascending doses of cocaine. As illustrated in Fig. 1, rats with a history of cocaine exposure exhibited greater locomotor activity than saline-treated controls to saline and the lowest dose of cocaine. A two-factor anova (groups × dose) revealed a significant interaction (F3,27 = 3.97, P = 0.018). Note that cocaine exposure took place in a different training environment from the behavioral recording. As a result, the cocaine-treated rats did not exhibit increased levels of gross locomotor activity during the recording sessions. Locomotor activity scores are not available to confirm this; however, examination of latencies to initiate trials, odor sampling periods and initial response latencies show no differences between groups.
Fig. 1.
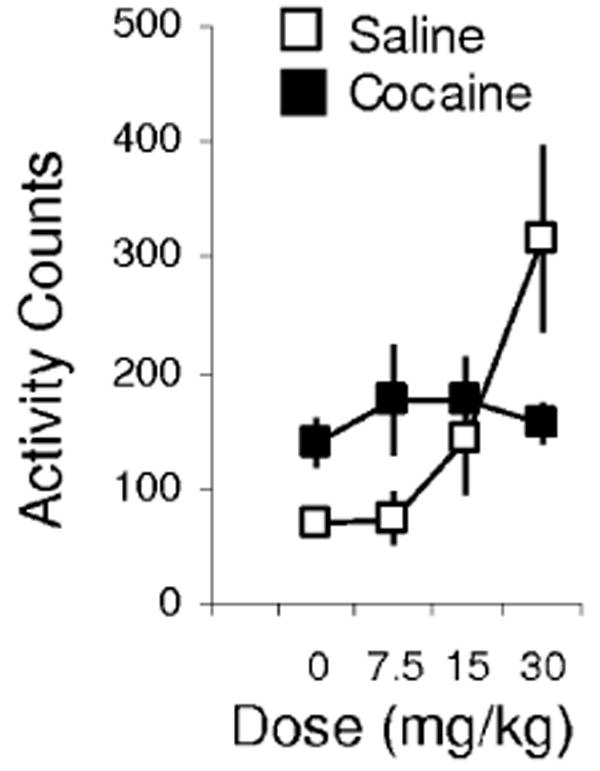
Locomotor activity in response to ascending doses of cocaine during a 4-h session at the conclusion of recording. Cocaine-treated rats exhibited more activity in response to saline and the lowest dose of cocaine, indicating a lasting effect of cocaine treatment on the rats from which neural data were obtained.
Recordings were made in the lateral orbital and ventral agranular insular regions within the OFC (Fig. 2A). We recorded a total of 538 neurons in the saline-treated rats (n = 6) and 529 neurons in the cocaine-treated rats (n = 4). As in prior studies, neural data were acquired primarily (> 95%) from regular spiking cells (Connors & Gutnick, 1990; Schoenbaum & Eichenbaum, 1995). The distribution of the average firing rates for the neurons in each group is given in Fig. 2B. These neurons were recorded in 44 sessions in saline-treated rats and 45 sessions in cocaine-treated rats. In all sessions, the rats learned a novel odor problem and successfully acquired a reversal of that problem, meeting a criterion of 18 correct responses in a moving block of 20 trials both before and again after reversal. For our analyses, each session was divided into a pre-criterion phase, a post-criterion phase and a reversal phase. In addition, the pre-criterion phase was also divided into an early block of trials, consisting of trials before the rat's sixth error, and a late block of trials, consisting of the remaining trials before the rat met the behavioral criterion. The average number of trials in each phase for these sessions is shown in Table 1; they did not differ between cocaine- and saline-treated rats.
Table 1.
Average number of trials in each performance block
| Pre-criterion | ||||
|---|---|---|---|---|
| Early | Late | Post-criterion | Reversal | |
| Saline (n = 44) | 13 | 37 | 80 | 127 |
| Cocaine (n = 45) | 13 | 35 | 76 | 143 |
anova indicated no significant differences at P < 0.05.
Effect of cocaine on behavior during recording
As illustrated in Fig. 3C, there was no effect of cocaine treatment on the number of trials the rats required to meet the go, no-go behavioral criterion on the discrimination problems (F1,87 = 0.17, P = 0.68). However there was an effect on the rats' latencies to respond at the fluid well after sampling sucrose- or quinine-predicting odors during learning. In saline-treated rats, these response latencies changed as they learned which outcome was predicted by each odor; the rats made faster responses after sampling the positive odor, as they came to expect the appetitive sucrose outcome, and slower responses after sampling the negative odor, as they came to expect the aversive quinine outcome. As illustrated in Fig. 3B, cocaine-treated rats failed to slow their responses after sampling the quinine-predicting odors. anovas revealed a significant effect of treatment on response latency on negative (F1,87 = 9.85, P = 0.0023) but not positive trials (F1,87 = 2.16, P = 0.14, power = 0.64). Thus, even though the cocaine-treated rats were able ultimately to withhold responding on negative trials like saline-treated controls, cocaine-treated rats failed to change the speed or vigor of responding to reflect the motivational value of the expected outcome when they did respond.
Fig. 3.
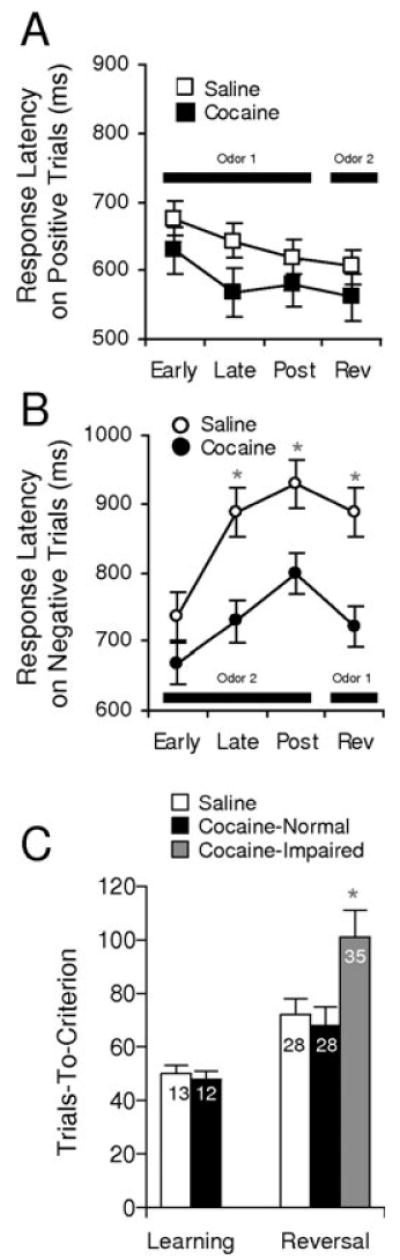
Behavioral performance in saline- and cocaine-treated rats. (A and B) Latencies to enter the fluid well when a decision was made to respond on positive (A) and negative (B) trials. Response latencies were calculated as the time from leaving the odor port until entry into the fluid well and are shown separately for trials early and late in the pre-criterion phase and post-criterion. This calculation did not consider no-go trials. Cocaine-treated rats failed to develop the normal increase in latency to respond on negative trials during learning and also after reversal. (C) Bars show trials required to attain the go, no-go performance criterion of 18 correct responses in a moving block of 20 trials, during initial learning and after reversal. Numbers on the bars indicate errors to criterion. For reversal, data are shown separately for sessions from cocaine-treated rats that performed poorly compared with saline-treated controls. (*, significant difference at P < 0.05 or better)
After the rats learned the initial discriminations, they also learned reversals of these problems in all sessions analysed in the current report. Unlike our previous behavioral study (Schoenbaum et al., 2002), there was not a significant main effect of cocaine treatment on the reversal learning performance of the rats during these recording sessions [72 trials to criterion (TTC) for saline and 83 TTC for cocaine; F1,87 = 1.27, P = 0.26, power = 0.62]. There are several possible reasons for this difference. First, it may reflect the training on the task that the rats received before sensitization or that a reversal was not introduced unless the rat had learned the original discrimination relatively rapidly in the recording session. Unsuccessful reversal sessions were often aborted. Thus, the dataset is biased to encourage accurate performance. These procedures would obviously minimize any impairment in the cocaine-treated rats. Indeed, cocaine-treated rats have a lower proportion of successful recording sessions.
Second, rats in the current experiment had extensive practice on reversals even after sensitization because multiple sessions were required for neuronal recordings. This may have played a role, as we have found that even OFC-lesioned rats are able to improve reversal performance to normal levels after acquiring several reversal problems (Schoenbaum et al., 2002). A two-factor anova comparing the performance of these rats on the initial reversal problems vs. their performance on the last problem revealed a main effect of reversal number (F5,40 = 2.79, P = 0.029), indicating an effect of practice, and a weak trend towards reversal impairment in the cocaine-treated rats on the initial problems (80 TTC for saline and 97 TTC for cocaine; F1,8 = 3.65, P = 0.09, power = 0.17).
Although there was no overall effect, there were individual variations in the performance of the cocaine-treated rats on the reversals. Specifically, whereas two of the four rats performed like controls (69 and 68 TTC; average TTC shown in Fig. 3C; F1,86 = 0.12, P = 0.72), the other two rats displayed persistently and significantly impaired reversal performance (105 and 96 TTC; average TTC shown in Fig. 3C; F1,86 = 5.64, P = 0.019). A two-factor anova comparing performance in these groups on the initial reversal problems vs. their performance on the last problem revealed a significant main effect of group (F1,7 = 6.68, P = 0.02) and of reversal number (F5,35 = 2.78, P = 0.03). In the subsequent analyses, we will use the presence of normal and impaired rats as a tool with which to explore the neurophysiological correlates of reversal performance in the cocaine-treated group.
Neurons that fire in anticipation of adverse outcomes fail to become activated by predictive cues in cocaine-experienced rats
As we were interested in the extent to which OFC neurons signaled expected outcomes, we began by examining whether they became activated in anticipation of the outcomes after the rat had made a response at the fluid well. To do so, we analysed firing activity during a delay period after responding but before delivery of sucrose or quinine, thereby comparing neural activity on correct, positive go trials vs. activity on incorrect, negative go trials. This comparison was made using data from pre-criterion trials, in order to identify differential activity that occurred early in learning, as we have previously reported in the OFC (Schoenbaum et al., 1998, 2003b).
In the current dataset, approximately 19% of the OFC neurons recorded in saline-treated rats exhibited differential firing during this delay period by anova; as illustrated in Table 2, this population included neurons that fired significantly more in anticipation of sucrose and others that fired significantly more in anticipation of quinine. Figure 4A shows an example of this correlate in a single unit recorded in a saline-treated rat. This neuron fired after the response, in anticipation of and during presentation of sucrose but not quinine. The outcome-expectant response developed in the pre-criterion phase, essentially signaling the expected outcome after a response had been made, early in learning. The population response of sucrose- or quinine-expectant neurons is shown in Fig. 4B.
Table 2.
Outcome-expectant neurons in the OFC in saline- and cocaine-treated rats
| Sucrose-expectant | Quinine-expectant | |
|---|---|---|
| Saline (n = 538) | 43 | 57 |
| Cocaine (n = 529) | 37 | 61 |
χ2 tests indicated no significant differences at P < 0.05.
Fig. 4.
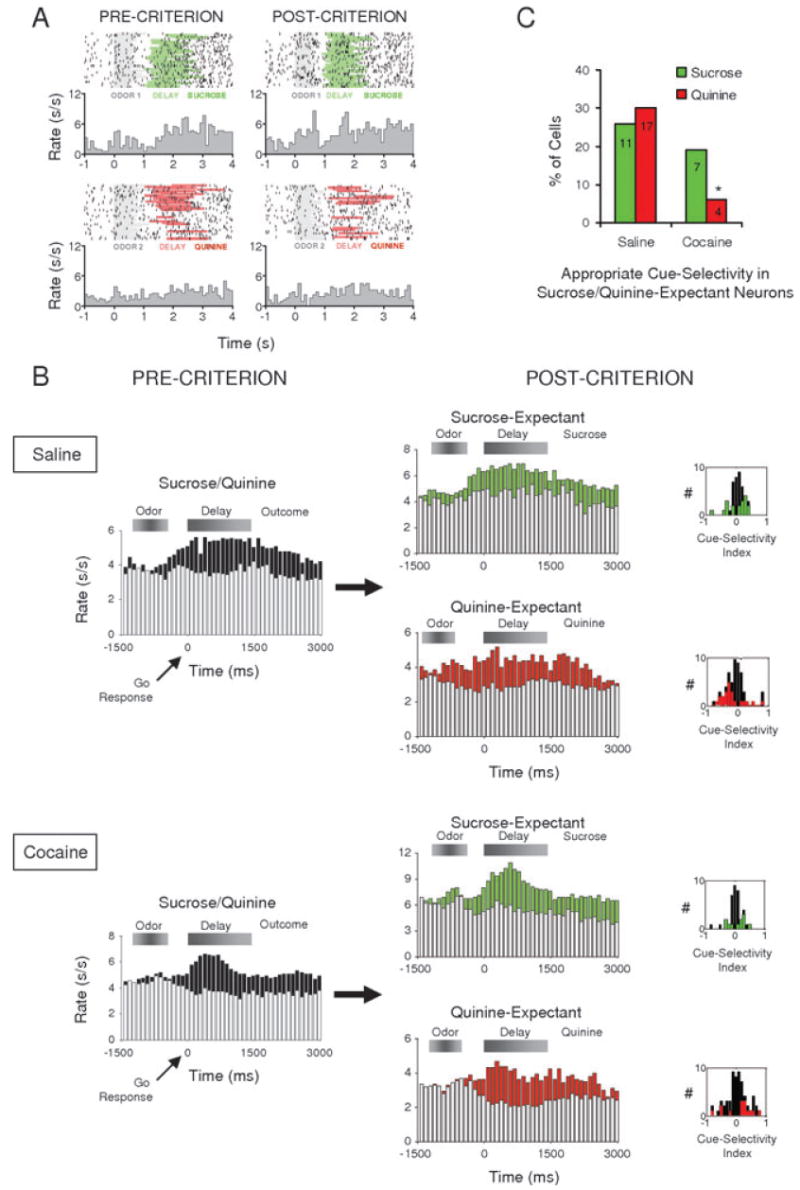
Cue-selectivity in outcome-expectant OFC neurons in saline- and cocaine-treated rats. (A) Single-unit with outcome-expectant activity recorded in a saline-treated rat. In the pre-criterion trials, this neuron fires selectively during a delay in anticipation of sucrose (green shading), as compared with quinine (red shading). In the post-criterion trials, the neuron becomes selective for the sucrose-predicting odor cue (grey shading, top panel), while still firing selectively in immediate anticipation of the sucrose. Raster displays show neural activity on individual trials, synchronized to odor onset, and each histogram shows average activity in spikes/s in 100-ms bins. (B) Population responses for outcome-expectant neurons. Shown are average firing rates, in spikes/s in 100-ms bins, on trials with the neurons' preferred outcomes (green bars, sucrose trials; red bars, quinine trials; black bars, preferred outcome trials) and non-preferred outcomes (white bars), synchronized to the response at the fluid well. Shaded boxes above the histograms indicate the approximate timing of odor sampling and the delay period. In saline-treated rats, both sucrose- and quinine-expectant neurons developed higher firing to the cue that predicted the neurons' preferred outcomes. By contrast, in cocaine-treated rats, only the sucrose-expectant neurons showed this correlate; the quinine-expectant population failed to become cue-selective after learning. An index of cue-selectivity, shown for each neuron in the population in insets next to each histogram, also demonstrates this difference. This index will be positive for neurons that fire more to the sucrose-predicting cue and negative for neurons that fire more to the quinine-predicting cue; green/red bars indicate scores for single-units that were significantly cue-selective by anova. The quinine-expectant population in cocaine-treated rats was unique in that these neurons were as likely to become selective for the odor that predicted sucrose as for the odor that predicted quinine. Note that because the histograms show neural activity synchronized on the behavioral response, only data from go trials are shown; however, statistical analyses of cue-selectivity shown in the insets considered activity on all trials, irrespective of whether a response was made. (C) Percentage of sucrose- (green) and quinine-expectant (red) single-units that developed significantly selective activity for the odor cue predicting sucrose and quinine, respectively. Cocaine-treated rats had significantly fewer neurons with these correlates overall, largely due to the near complete failure of quinine-expectant neurons to become selective for the quinine-predicting odor cue. (*, significant difference at P < 0.05 or better)
Signaling the expected outcome after responding, although critical for some learning functions (Rescorla & Wagner, 1972; Dickinson, 1989), cannot help guide the response. To investigate whether outcome-expectant neurons also became activated during presentation of the odor cues, we compared neural activity during sampling of the sucrose- vs. the quinine-predicting odor cue, in the pre-criterion and the post-criterion training phase. We were particularly interested in whether outcome-expectant neurons would become activated for the odor cue that predicted their preferred outcome, as is illustrated by the example in Fig. 4A. This neuron fires in anticipation of sucrose in the pre-criterion phase and then becomes activated for the sucrose-predicting odor cue in the post-criterion phase, as if activating a representation of sucrose during sampling of the odor cue.
Inspection of the population histograms in Fig. 4B indicates that outcome preference did appear to have influenced the odor preference of the populations. That is, after learning (Fig. 4B, POST-CRITERION), the sucrose-expectant population fired more to the sucrose-predictive odor cue, whereas the quinine-expectant population fired more to the quinine-predictive odor cue. However, because of the variability in the timing of odor onset/offset, relative to the response, among the large number of trials that contribute to these data, it is impossible visually to isolate the period of odor sampling in these histograms. Furthermore, the population histograms in Fig. 4 do not show data on all trials. Instead, because they are constructed to show activity during the delay period, they can only show data from trials in which a rat made a response to the fluid well. To confirm the qualitative impression from the population histograms, we analysed cue selectivity in the individual neurons in each population (i.e. sucrose-expectant and quinine-expectant), using data from all trials in the post-criterion phase (positive go and negative go/no-go). The results of this analysis are illustrated in the insets next to each population histogram in Fig. 4B. These insets show an index of odor selectivity for each sucrose- or quinine-expectant neuron. This index ranges from 1 to −1, with positive values indicating greater firing to the sucrose-predicting odor cue and negative values indicating greater firing to the quinine-predicting odor cue. The number of neurons that developed significant odor selectivity in the post-criterion phase, by anova, is shown by green bars for the sucrose-expectant population and by red bars for the quinine-expectant population. As the insets show, the cue-selective, sucrose-expectant neurons exhibited indices > 1 and the cue-selective, quinine-expectant neurons exhibited indices < 1; statistical testing of these distributions indicated that these neurons were significantly more likely to become active for the odor predicting their preferred outcome (Wilcoxon signed rank = 256, zval = −2.62, P = 0.0088). This pattern was most striking for quinine-expectant neurons, although the two populations were not significantly different in the degree of divergence from zero (χ2 = 1.23, P = 0.27, power = 0.19). Notably this pattern was similar for the neurons that fired in anticipation of quinine whether the analysis used data from negative go or negative no-go trials (NB: this analysis cannot be done for sucrose-expectant neurons because rats in this task do not make no-go responses on positive trials). Thus, after learning, the quinine-expectant population was significantly more active for the quinine-predictive odor during cue-sampling, irrespective of whether the rat responded on a trial. This pattern of firing is consistent with signaling of the expected outcome rather than signaling of the response or the correctness of the response.
OFC neurons in cocaine-treated rats differed from neurons in controls in their ability to signal the expected outcome during performance of this task. In particular, although 19% of neurons in cocaine-treated rats signaled the expected outcome after a response was made, just as with neurons in control rats (Table 2 and Fig. 4B), this population largely failed to become active for the appropriate odor during cue sampling. This was particularly evident for the neurons that fired in anticipation of the aversive quinine outcome. These quinine-expectant neurons did not show any difference, after learning (Fig. 4B, POST-CRITERION), in firing to the predictive odor cues. This was not because these neurons failed to become cue-selective, but rather because they were equally likely to become active for either odor cue. This is illustrated by the distribution of the cue-selectivity index for the neurons in this population, shown in the inset next to the population histogram in Fig. 4B. This distribution is symmetrically distributed around zero (Wilcoxon signed rank = 262, zval = −0.61, P = 0.54), in sharp contrast to the distribution in the saline-treated controls just above (χ2 = 8.35, P = 0.004). A direct comparison of the proportion of neurons in saline- and cocaine-treated rats that developed statistically significant firing to the appropriate predictive cue, shown in Fig. 4C, confirms that cocaine exposure essentially abolished activation of quinine-expectant neurons during sampling of the quinine-predicting cue (vs. controls: χ2 = 11.42, P = 0.007; vs. chance: χ2 = 0.7, P = 0.40) but had little effect on the activation of sucrose-expectant neurons during sampling of the sucrose-predicting cue (vs. controls: χ2 = 0.51, P = 0.48; vs. chance: χ2 = 5.05, P = 0.024). Indeed, the effect of cocaine treatment on the development of cue selectivity in the quinine-expectant population was quite specific inasmuch as cocaine treatment did not affect the development of cue-selective firing in neurons that did not fire in anticipation of the outcomes (Table 3). The lack of an effect of cocaine exposure on these correlates is reflected in the population responses shown in Fig. 5A. In both saline- and cocaine-treated rats, cue-selective activity in these neurons increased with learning (Fig. 5A, PRE-CRITERION to POST-CRITERION). Failure to activate the quinine-expectant neurons during cue sampling was accompanied by abnormal behavior on the quinine trials (Fig. 3B).
Table 3.
Cue-selective neurons in the OFC in saline- and cocaine-treated rats
| Positive cue | Negative cue | |
|---|---|---|
| Saline (n = 538) | 73 | 64 |
| Pre- + post-criterion | 12 | 11 |
| Post-criterion only | 61 | 53 |
| Cocaine (n = 529) | 94 | 56 |
| Pre- + post-criterion | 22 | 13 |
| Post-criterion only | 72 | 43 |
χ2 tests indicated no significant differences at P < 0.05; the table does not include cue- and outcome-selective neurons shown in Fig. 4C.
Fig. 5.
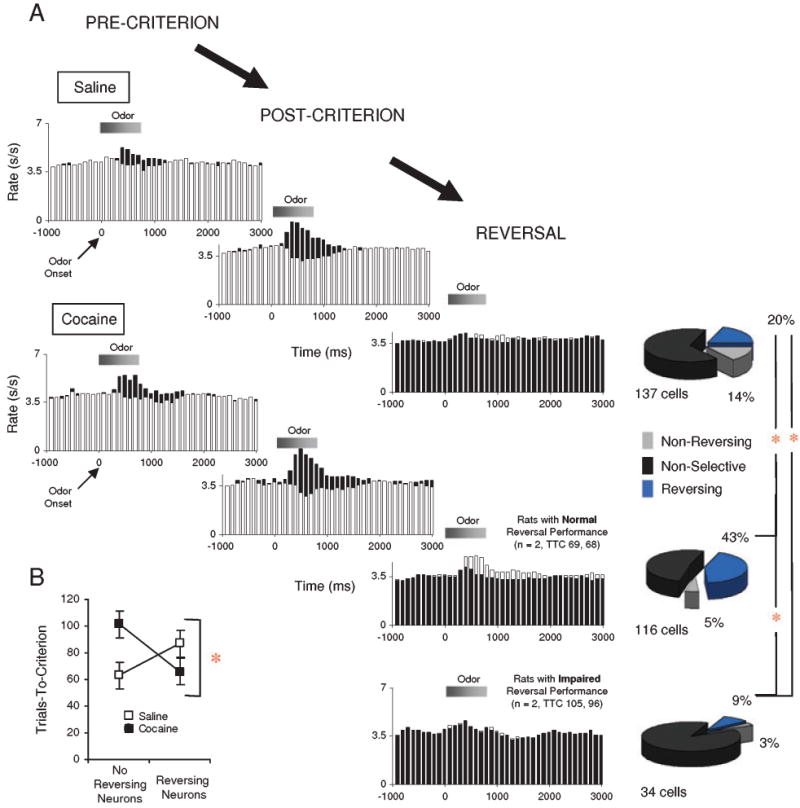
Cue-selectivity in OFC neurons that did not fire in anticipation of the outcome in saline- and cocaine-treated rats. (A) Population responses for cue-selective neurons during pre-criterion, post-criterion, and reversal. Shown are average firing rates, in spikes/s in 100-ms bins, for the neurons' preferred (black bars) and non-preferred cue (white bars), based on selective neural activity in the post-criterion phase. Shaded boxes above the histograms indicate the approximate timing of the odor sampling period. Pie-charts to the right of the reversal histograms show how many of the single units in the populations continued to be significantly cue-selective by anova after reversal, and whether the cue-preference in these neurons reversed or not. Neurons developed cue-selectivity during learning in both saline- and cocaine-treated rats, and changed that firing selectivity during reversal. In controls, the proportion of these neurons that reversed was similar to that reported in prior studies. In cocaine-treated rats, the proportion was significantly lower in rats that performed poorly on reversals, and significantly higher in rats that performed like controls. This was evident also in the population responses, which did not distinguish between the odor cues after reversal, except in the cocaine-treated rats that performed normally. (B) Average trials required to attain the go, no-go performance criterion of 18 correct responses in a moving block of 20 trials during reversal, shown for sessions in which reversing cue-selective neurons were recorded, and sessions in which cue-selective neurons were recorded but failed to reverse. Cocaine-treated rats performed significantly better in sessions in which reversing neurons were found; normal rats exhibited the opposite trend. (*, significant difference at P < 0.05 or better).
Cue-selective neuronal firing is inflexible during reversal learning in reversal-impaired, cocaine-experienced rats
Reversal of odor–outcome associations occurred in the same sessions in which rats learned the original problems, so that we were able to examine neural encoding in the same populations described earlier. Our analysis focused on the effect of reversal on cue selectivity in the two populations identified during learning described above: (1) those neurons with outcome-expectant activity that had become selective for the appropriate odor cue after learning (Fig. 4) and (2) the remaining population of cue-selective neurons that did not fire selectively to the outcome (Fig. 5).
Previously we have reported that cue-selective neurons in the OFC typically reverse or become non-selective during reversal learning in this task (Schoenbaum et al., 1999, 2003b, 2006). Here we again found that to be the case, for each of the cue-selective populations we have identified. Of the 28 outcome-expectant neurons that had appropriate cue-selectivity (Fig. 4C), 45% (13 neurons) reversed, and 50% (14 neurons) became non-selective. Cue-selective neurons that did not fire to the outcome also often reversed their selectivity, as illustrated by the pie charts in Fig. 5A; however, the population response of these neurons no longer distinguished between the two odor cues after reversal (Fig. 5A, REVERSAL; NB: other OFC neurons develop selectivity, so a total population response would show a reversal of selectivity). Interestingly, in control rats, these neurons were significantly less likely to reverse than cue-selective, outcome-expectant neurons (χ2 = 9.04, P = 0.0026). This novel finding is consistent with the proposal that cue-selective firing in the outcome-expectant neurons directly incorporates information about the predicted outcome, which changes across reversal.
In cocaine-treated rats, the effect of reversal on cue-selective firing differed from the effect in controls. To begin with, only two of the 11 outcome-expectant, cue-selective neurons reversed in cocaine-treated rats. Of course the power of this analysis is limited (χ2 = 2.66, P = 0.10, power = 0.33), as much of the quinine-expectant population failed to become selective to the quinine-predicting cue during learning (as described earlier; Fig. 4C). However, it is perhaps notable that none of the four neurons that did fire to the quinine-predicting odor cue reversed selectivity, in contrast to controls in which 6/17 of these neurons did so. This failure was again accompanied by an inability to modulate the speed of responding on negative trials after reversal (now for a different odor cue; Fig. 3).
Furthermore, the outcome-expectant neurons that did reverse cue selectivity in cocaine-treated rats were recorded in the two rats with reversal performance that was indistinguishable from controls. Cue-selective neurons in the two cocaine-treated rats with poor reversal performance rarely reversed. This was evident in the cue-selective, outcome-expectant neurons discussed above, but there were too few of them to allow us to draw any conclusions. However, consideration of the remaining cue-selective neurons revealed that these neurons were significantly less likely to reverse cue-selectivity in cocaine-treated rats with impaired reversal performance than in rats with normal performance (χ2 = 13.52, P = 0.0002). This failure was evident irrespective of whether the neuron was originally selective for the positive or the negative odor cue. By contrast, cue-selective neurons in cocaine-treated rats with normal reversal performance were significantly more likely to reverse than cue-selective neurons in saline-treated controls (χ2 = 16.24, P = 0.0001), including neurons selective for both the positive and the negative odor cue. Note that the proportion of reversing neurons in controls was similar to that in several prior studies (Schoenbaum et al., 1999, 2003b, 2006).
We also compared performance in sessions in which we observed reversal of cue-selective activity with performance in sessions in which we observed cue-selective activity that did not reverse. As illustrated in Fig. 5B, cocaine-treated rats performed better in sessions in which reversing, cue-selective neurons were recorded (n = 15) than in sessions in which cue-selective neurons were recorded but no reversals were observed (n = 20) (F1,33 = 4.65, P = 0.038). Thus, whether we analysed according to the performance of each rat or according to the performance within each session, across rats there seemed to be a strong relationship between reversal performance and the strength of an OFC neuronal signal that reflects reversal in cocaine-treated rats.
Of course this relationship could simply reflect a general relationship between plasticity in the OFC and reversal performance. To investigate this possibility, we examined reversal performance and reversal of cue-selectivity in saline-treated control rats. This analysis indicated that saline-treated rats actually performed slightly worse in sessions in which cue-selective activity reversed (n = 16) than in sessions in which cue-selective activity was recorded but did not reverse (n = 26) (Fig. 5B). A two-factor anova comparing these data to findings in cocaine-treated rats revealed a significant interaction between group and performance in sessions with and without reversing neurons (F1,70 = 7.28, P = 0.0087). Thus, in cocaine-treated rats, a greater reversal signal was observed in the OFC neurons when rats successfully changed their behavior, whereas, in saline-treated rats, a greater reversal signal was observed when rats had more difficulty changing their behavior.
Notably, cocaine-treated rats with normal reversal performance also had more cue-selective neurons initially than either saline-treated controls (116/327 vs. 137/538; χ2 = 9.85, P = 0.0017) or their impaired counterparts (116/327 vs. 34/202; χ2 = 21.36, P = 0.0001). Meanwhile, rats with poor performance had significantly fewer cue-selective neurons initially than their counterparts or the control rats (137/538 vs. 34/202; χ2 = 6.16, P = 0.013). Thus, normal reversal performance after cocaine exposure is associated with a general increase in plasticity of cue-selective activity in the OFC.
Discussion
A growing body of evidence indicates that exposure to addictive drugs in general, and psychostimulants in particular, causes persistent changes in OFC function. For example, addicts show metabolic changes in the OFC at rest and during craving episodes induced by exposure to drug-associated cues, even after periods of abstinence (Breiter et al., 1997; Maas et al., 1998; Childress et al., 1999; Rogers et al., 1999; London et al., 2000; Volkow & Fowler, 2000; Bolla et al., 2003). In addition, rats sensitized to amphetamine exhibit progressive increases in spontaneous neural activity during passive drug administration (Homayoun & Moghaddam, 2006) and declines in the density of dendritic spines in the OFC a month after the last drug exposure (Crombag et al., 2004). These changes are associated with impaired performance on OFC-dependent tasks (Grant et al., 2000; Bechara et al., 2001; Clark & Robbins, 2002; Jentsch et al., 2002; Schoenbaum & Setlow, 2004; Schoenbaum et al., 2004; Kantak et al., 2005).
Here we have identified potential neurophysiological substrates of these performance deficits. We found that OFC neurons in cocaine-treated rats failed to signal adverse outcomes properly during learning; neurons in cocaine-experienced rats responded normally in anticipation of quinine once the decision had been made to respond, but they failed to become active during consideration of the quinine-predicting cue, even after learning. Along with this deficit, we also found that after reversal of cue–outcome contingencies, cue-selective neural responses in the OFC were generally less flexible in cocaine-treated rats than in controls.
These neurophysiological deficits were associated with behavioral impairments. Cocaine-impaired rats failed to modify their response latencies on negative trials during learning and a subset with altered encoding exhibited reversal impairments. These deficits are similar to those we have reported previously in OFC-lesioned or cocaine-treated rats (Schoenbaum et al., 2003a, 2004). As in those studies, these deficits were observed despite apparently normal choice performance on the initial discrimination problems. As we and others have pointed out (Roberts, 2006; Schoenbaum & Roesch, 2005), intact choice performance on discriminations probably reflects the fact that choices can be driven by multiple associative mechanisms, whereas the speed or vigor of a responding (and reversal learning) seems to be more sensitive to information about the expected outcome. This is illustrated by studies that demonstrate that changes in the outcome value affect latencies to respond while leaving choice performance intact (Sage & Knowlton, 2000).
Evidence implicates the OFC in the ability to use information about expected outcomes to guide behavior (Schoenbaum & Roesch, 2005). This is particularly evident in settings that involve reversals or reinforcer devaluation (Bechara et al., 1997; Gallagher et al., 1999; Fellows & Farah, 2003; Schoenbaum et al., 2003a; Izquierdo et al., 2004). The OFC may be critical in these settings because changing contingencies force animals to rely more heavily on OFC-dependent signaling of expected outcomes (Fellows & Farah, 2003; Izquierdo et al., 2004; Schoenbaum & Roesch, 2005). Under this hypothesis, outcome expectancies generated by the OFC would allow animals to recognize violations of those expectations when contingencies change, thereby facilitating flexible neural encoding and new learning. Data consistent with this proposal include our report that rats with OFC lesions show persistently inflexible encoding after reversal in downstream regions of the basolateral amygdala (Saddoris et al., 2005), and the observation both here, as well as in a re-analysis of a previously published dataset (Schoenbaum et al., 2006), that the level of plasticity of encoding in the OFC in saline-treated controls is inversely related to the rapidity of reversal learning. Thus, in normal rats, reversal of cue-selective correlates in the OFC increases the more errors the rats make, as if under the control of feedback regarding deviations from the expected outcomes.
In such a model of OFC function, the two categories of neurophysiological changes in cocaine-treated rats reported here would be related. That is, the inability of cocaine-exposed rats to signal the adverse outcome properly would disrupt their ability to recognize violations of expectations after reversal of the reinforcement contingencies in the task. This lack of feedback would result in inflexible neural correlates, as well as in inflexible behavior. Consistent with this idea, impaired reversal performance in the cocaine-treated rats was associated with reduced plasticity of cue-selective correlates in the OFC after reversal.
The inability to signal adverse outcomes and consequently to use feedback to modify behavior and associative learning when contingencies are changing might account for the failure of cocaine-experienced animals to modify responding after reversal or reinforcer devaluation (Jentsch et al., 2002; Schoenbaum & Setlow, 2004; Schoenbaum et al., 2004; Nelson & Killcross, 2006). A similar deficit could also explain addicts' inability to make appropriate decisions in analogous testing situations (Rogers et al., 1999; Bechara et al., 2001). A failure to signal adverse outcomes during decision-making would also have obvious implications for drug-seeking behavior of addicts, as well as for recent findings in a number of animal models of addiction in which rats are unable to withhold drug-seeking behavior in the face of adverse outcomes (Miles et al., 2003; Deroche-Gamonet et al., 2004; Vanderschuren & Everitt, 2004).
Interestingly, some of the cocaine-treated rats in this study exhibited normal reversal performance despite their failure to signal costs appropriately during initial learning. Although reversal performance was apparently normal in these rats, it was in fact associated with abnormally high levels of plasticity in the OFC. That is, these rats exhibited greater plasticity in the OFC than controls performing at a similar level. Improved function in these rats both confirms the relationship between poor reversal performance and the loss of flexible encoding in the impaired rats and also indicates that cocaine-related changes in the OFC can be mitigated by other factors to re-establish normal responding. Interestingly, normal function in these rats was not associated with a correction of deficient encoding in the outcome-expectant population.
The improved function in these rats highlights the important caveat that our results should not be interpreted to demonstrate an isolated deficit in the OFC. Instead, these findings provide a marker for dysfunction in one part of a larger corticolimbic circuit that is critical for processing information about outcomes. Changes in neural correlates in the OFC could reflect effects of cocaine (or compensation) in other parts of this circuit. Indeed, we have found marked changes in OFC correlates in rats performing this same task after neurotoxic lesions of basolateral amygdala (Schoenbaum et al., 2003b). These lesions caused a reduction in plasticity and also completely eliminated the activation of outcome-expectant neurons during cue-sampling, effects which are similar in some respects to those reported here. This similarity illustrates the general principle that changes in structures afferent to the OFC, many of which are affected by cocaine, can have a dramatic impact on encoding in the OFC.
Finally, it is also notable that the effect of cocaine on neural encoding and behavior reported here did not require long-access self-administration. Although somewhat contrary to recent studies suggesting that extended periods of self-administration are critical for the transition to addiction (Deroche-Gamonet et al., 2004; Vanderschuren & Everitt, 2004), the fact that neural correlates (and behavior) did exhibit long-lasting changes after simple drug exposure shows that there are important effects of drugs that do not depend upon extensive and contingent drug-taking (although they may depend on relatively high doses). This conclusion is also supported by other reports of long-lasting and theoretically significant electrophysiological and structural changes in limbic circuits after short-term, non-contingent psychostimulant exposure (Trantham et al., 2002; Robinson & Kolb, 2004; Brady et al., 2005), some of which are similar to those seen after self-administration protocols (Trantham et al., 2002; Robinson & Kolb, 2004). In this context, it is intriguing that one recent study reported that changes in OFC-dependent behavior were not observed in rats receiving cocaine non-contingently when testing was conducted without any withdrawal period (Kantak et al., 2005). Thus, perhaps the cocaine-induced changes to the OFC function reported here and in previous studies in which testing began weeks after cocaine exposure (Schoenbaum et al., 2004; Schoenbaum & Setlow, 2005) may ‘incubate’ over time, as has been reported for craving-induced relapse in addicts and in animal models of addiction (Grimm et al., 2001; Kosten et al., 2005; Lu et al., 2005). If this were the case, then the effects of psychostimulant exposure on the OFC might be particularly critical in supporting drug-taking in the early stages of addiction, when drugs are taken intermittently, and also during periods of abstinence, when there is a risk of relapse. Consistent with this idea, recent reports have linked the OFC to cue-induced relapse to cocaine seeking (Hutcheson & Everitt, 2003; Fuchs et al., 2004). Clearly it will be of interest to test whether the changes in associative encoding in the OFC reported here in cocaine-exposed rats are affected by these critical variables.
Acknowledgments
We are grateful to Dr Stephen Warrenburg at International Flavors and Fragrances for his assistance in obtaining odor compounds. This work was supported by grants from the NIDA (R01-DA015718, G.S.), NINDS (T32-NS07375, M.R.R.) and NIDCD (T32-DC00054, T.A.S.).
Abbreviations
- OFC
orbitofrontal cortex
- TTC
trials to criterion
References
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1294. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Andersen SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Keihl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, Glick SD, O'Donnell P. Selective disruption of nucleus accumbens gating mechanisms in rats behaviorally sensitized to methamphetamine. J Neurosci. 2005;25:6687–6695. doi: 10.1523/JNEUROSCI.0643-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Robbins TW. Decision-making deficits in drug addiction. Trends Cognitive Sci. 2002;6:361–363. doi: 10.1016/s1364-6613(02)01960-5. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrisic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2004;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addictionlike behavior in the rat. Science. 2004;305:951–953. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Dickinson A. Expectancy theory in animal conditioning. In: Klein SB, Mowrer RR, editors. Contemporary Learning Theories: Pavlovian Conditioning and the Status of Traditional Learning Theory. Erlbaum; Hillsdale, NJ: 1989. pp. 279–308. [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ. The effects of selective orbitofrontal cortex lesions on the acquisition and performance of cue-controlled cocaine seeking in rats. Ann NY Acad Sci. 2003;1003:410–411. doi: 10.1196/annals.1300.038. [DOI] [PubMed] [Google Scholar]
- Izquierdo AD, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Udo T, Ugalde F, Luzzo C, Di Pietro N, Eichenbaum HB. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacology. 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, Poling J, Oliveto A. ‘Incubation’ of cocaine relapse during a disulfiram clinical trial. College on Problems of Drug Dependence Annual Meeting; Orlando, Florida, USA. June 18–23, 2005; 2005. p. 90. College on Problems of Drug Dependence (W.K. Bickel, President). Abstracts. [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nature Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Miles FJ, Everitt BJ, Dickinson A. Oral cocaine seeking by rats: action or habit? Behav Neurosci. 2003;117:927–938. doi: 10.1037/0735-7044.117.5.927. [DOI] [PubMed] [Google Scholar]
- Nelson A, Killcross S. Amphetamine exposure enhances habit formation. J Neurosci. 2006;26:3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1997. [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Roberts AC. Primate orbitofrontol cortex and adaptive behavior. Trends Cogn Sci. 2006;10:83–90. doi: 10.1016/j.tics.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Sage JR, Knowlton BJ. Effects of US devaluation on win-stay and win-shift radial maze performance in rats. Behav Neurosci. 2000;114:295–306. doi: 10.1037//0735-7044.114.2.295. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G. Olfactory learning and the neurophysiological study of rat prefrontal function. In: Nicolelis MAL, Simon SA, editors. CRC Series: Methods in Chemosensory Research. CRC Press; Boca Raton, FL: 2001. pp. 371–427. [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cereb Cortex. 2004;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cereb Cortex. 2005;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Memory. 2003a;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003b;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. J Neurophysiol. 2006;95:1509–1517. doi: 10.1152/jn.01052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Schoenbaum G, Gallagher M. Neural encoding in ventral striatum during olfactory discrimination learning. Neuron. 2003;38:625–636. doi: 10.1016/s0896-6273(03)00264-2. [DOI] [PubMed] [Google Scholar]
- Trantham H, Szumlinski KK, McFarland K, Kalivas PW, Lavin A. Repeated cocaine administration alters the electrophysiological properties of prefrontal cortical neurons. Neuroscience. 2002;113:749–753. doi: 10.1016/s0306-4522(02)00246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]


